Investigating Glycogenolysis during Postprandial State
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Background and Research Objectives
Glycogenolysis, the biochemical process of glycogen breakdown into glucose-1-phosphate and glucose, represents a critical metabolic pathway for maintaining blood glucose homeostasis. Historically, glycogenolysis research has primarily focused on its role during fasting or exercise states when the body requires rapid glucose mobilization. However, recent scientific evidence suggests that glycogenolysis also plays a significant yet poorly understood role during the postprandial (after-meal) state, challenging traditional metabolic paradigms.
The evolution of glycogenolysis research spans nearly a century, beginning with the pioneering work of Carl and Gerty Cori in the 1930s who elucidated the basic pathway. Subsequent decades saw advances in understanding hormonal regulation, particularly through insulin and glucagon. The 1970s-1990s brought molecular characterization of glycogen phosphorylase and its regulatory mechanisms. Recent technological breakthroughs in metabolomics, stable isotope tracing, and real-time imaging have revolutionized our ability to study dynamic glycogen metabolism in living systems.
Current research trends indicate growing interest in tissue-specific glycogenolysis patterns, particularly in the liver and skeletal muscle during various nutritional states. The paradoxical occurrence of simultaneous glycogenolysis and glycogenesis during the postprandial period represents an emerging area of investigation with significant implications for understanding metabolic disorders.
This technical research aims to comprehensively investigate the mechanisms, regulation, and physiological significance of postprandial glycogenolysis. Specifically, we seek to: (1) characterize the temporal dynamics of glycogenolysis following carbohydrate consumption; (2) identify tissue-specific patterns and regulatory factors governing postprandial glycogen breakdown; (3) elucidate the interplay between glycogenolysis and other metabolic pathways during nutrient abundance; and (4) explore potential implications for metabolic disorders such as diabetes and non-alcoholic fatty liver disease.
The expected outcomes of this research include establishing a refined model of postprandial glycogen metabolism, identifying novel regulatory mechanisms, and potentially discovering new therapeutic targets for metabolic disorders. By challenging the conventional understanding that glycogenolysis primarily serves glucose needs during fasting, this investigation may fundamentally reshape our understanding of energy metabolism and substrate utilization during different nutritional states.
This research aligns with broader trends in metabolic research toward understanding dynamic, tissue-specific metabolic flexibility and substrate switching, which are increasingly recognized as key factors in metabolic health and disease prevention.
The evolution of glycogenolysis research spans nearly a century, beginning with the pioneering work of Carl and Gerty Cori in the 1930s who elucidated the basic pathway. Subsequent decades saw advances in understanding hormonal regulation, particularly through insulin and glucagon. The 1970s-1990s brought molecular characterization of glycogen phosphorylase and its regulatory mechanisms. Recent technological breakthroughs in metabolomics, stable isotope tracing, and real-time imaging have revolutionized our ability to study dynamic glycogen metabolism in living systems.
Current research trends indicate growing interest in tissue-specific glycogenolysis patterns, particularly in the liver and skeletal muscle during various nutritional states. The paradoxical occurrence of simultaneous glycogenolysis and glycogenesis during the postprandial period represents an emerging area of investigation with significant implications for understanding metabolic disorders.
This technical research aims to comprehensively investigate the mechanisms, regulation, and physiological significance of postprandial glycogenolysis. Specifically, we seek to: (1) characterize the temporal dynamics of glycogenolysis following carbohydrate consumption; (2) identify tissue-specific patterns and regulatory factors governing postprandial glycogen breakdown; (3) elucidate the interplay between glycogenolysis and other metabolic pathways during nutrient abundance; and (4) explore potential implications for metabolic disorders such as diabetes and non-alcoholic fatty liver disease.
The expected outcomes of this research include establishing a refined model of postprandial glycogen metabolism, identifying novel regulatory mechanisms, and potentially discovering new therapeutic targets for metabolic disorders. By challenging the conventional understanding that glycogenolysis primarily serves glucose needs during fasting, this investigation may fundamentally reshape our understanding of energy metabolism and substrate utilization during different nutritional states.
This research aligns with broader trends in metabolic research toward understanding dynamic, tissue-specific metabolic flexibility and substrate switching, which are increasingly recognized as key factors in metabolic health and disease prevention.
Postprandial Glycogenolysis Market Applications
The postprandial state represents a critical metabolic window where the body transitions from fasting to fed conditions, with glycogenolysis playing a significant regulatory role. Market applications leveraging insights from postprandial glycogenolysis span multiple sectors, with healthcare diagnostics showing particular promise. Continuous glucose monitoring systems that can detect subtle changes in glycogen breakdown patterns after meals are gaining traction, with the global CGM market projected to grow substantially as diabetes prevalence increases worldwide.
Nutritional supplement companies have begun developing products specifically targeting optimal postprandial glycogen management. These include specialized carbohydrate formulations designed to modulate the rate of glycogen synthesis and breakdown, particularly popular among athletes and fitness enthusiasts seeking to optimize recovery and performance. The sports nutrition market segment focusing on glycogen optimization products has demonstrated consistent growth over recent years.
Pharmaceutical applications represent another substantial market opportunity. Drugs targeting enzymes involved in glycogenolysis pathways are being investigated for metabolic disorders, with several compounds in clinical trials showing promise for conditions like glycogen storage diseases and type 2 diabetes. The potential market for such therapeutics is substantial given the rising global prevalence of metabolic disorders.
The food industry has also capitalized on postprandial glycogenolysis research, developing functional foods designed to promote healthier glycemic responses. Products formulated to slow postprandial glycogen breakdown and maintain steadier blood glucose levels are increasingly popular among health-conscious consumers. This market segment has seen particular growth in developed economies where nutritional awareness is high.
Digital health applications represent an emerging market sector, with several startups developing algorithms that predict individual glycemic responses based on personal glycogenolysis patterns. These platforms offer personalized dietary recommendations to optimize metabolic health, often integrating with wearable devices to provide real-time feedback on postprandial metabolic states.
Diagnostic testing services focusing on assessing individual variations in postprandial glycogenolysis have emerged as a specialized market niche. These services typically involve comprehensive metabolic profiling to identify abnormal glycogen metabolism patterns that may indicate underlying health conditions or disease risk factors. The precision medicine approach has attracted significant venture capital investment in recent years.
Agricultural biotechnology companies are exploring applications in livestock management, developing feed formulations that optimize postprandial glycogen metabolism in production animals to improve growth efficiency and meat quality. This specialized application demonstrates how glycogenolysis research extends beyond human health into broader economic sectors.
Nutritional supplement companies have begun developing products specifically targeting optimal postprandial glycogen management. These include specialized carbohydrate formulations designed to modulate the rate of glycogen synthesis and breakdown, particularly popular among athletes and fitness enthusiasts seeking to optimize recovery and performance. The sports nutrition market segment focusing on glycogen optimization products has demonstrated consistent growth over recent years.
Pharmaceutical applications represent another substantial market opportunity. Drugs targeting enzymes involved in glycogenolysis pathways are being investigated for metabolic disorders, with several compounds in clinical trials showing promise for conditions like glycogen storage diseases and type 2 diabetes. The potential market for such therapeutics is substantial given the rising global prevalence of metabolic disorders.
The food industry has also capitalized on postprandial glycogenolysis research, developing functional foods designed to promote healthier glycemic responses. Products formulated to slow postprandial glycogen breakdown and maintain steadier blood glucose levels are increasingly popular among health-conscious consumers. This market segment has seen particular growth in developed economies where nutritional awareness is high.
Digital health applications represent an emerging market sector, with several startups developing algorithms that predict individual glycemic responses based on personal glycogenolysis patterns. These platforms offer personalized dietary recommendations to optimize metabolic health, often integrating with wearable devices to provide real-time feedback on postprandial metabolic states.
Diagnostic testing services focusing on assessing individual variations in postprandial glycogenolysis have emerged as a specialized market niche. These services typically involve comprehensive metabolic profiling to identify abnormal glycogen metabolism patterns that may indicate underlying health conditions or disease risk factors. The precision medicine approach has attracted significant venture capital investment in recent years.
Agricultural biotechnology companies are exploring applications in livestock management, developing feed formulations that optimize postprandial glycogen metabolism in production animals to improve growth efficiency and meat quality. This specialized application demonstrates how glycogenolysis research extends beyond human health into broader economic sectors.
Current Understanding and Technical Challenges
Glycogenolysis during the postprandial state represents a complex metabolic process that has garnered significant scientific attention in recent years. Current understanding indicates that glycogenolysis—the breakdown of glycogen to glucose-1-phosphate—typically decreases during the postprandial period as insulin levels rise in response to elevated blood glucose. However, recent research has revealed unexpected patterns of glycogen metabolism that challenge this conventional understanding.
Studies utilizing advanced isotope tracing techniques have demonstrated that hepatic glycogenolysis continues at significant rates even during the early postprandial phase, suggesting a more nuanced regulatory mechanism than previously recognized. This phenomenon, termed "simultaneous glycogenolysis and glycogenesis," indicates that glycogen metabolism operates as a dynamic equilibrium rather than a simple on-off switch controlled solely by insulin and glucagon.
The technical challenges in investigating postprandial glycogenolysis are substantial. Current in vivo measurement techniques lack sufficient temporal and spatial resolution to capture the rapid fluctuations in glycogen metabolism that occur following meal consumption. Non-invasive methods such as 13C-MRS (Carbon-13 Magnetic Resonance Spectroscopy) provide valuable insights but are limited by sensitivity constraints and cannot distinguish between different cellular compartments.
Another significant challenge lies in the heterogeneity of glycogen metabolism across different tissues. While liver glycogenolysis has been extensively studied, skeletal muscle glycogen dynamics during the postprandial state remain poorly characterized despite representing approximately 80% of the body's glycogen stores. The technical difficulty in simultaneously monitoring multiple tissue sites contributes to this knowledge gap.
Molecular techniques for investigating the enzymatic regulation of glycogenolysis face limitations in translating in vitro findings to physiological conditions. The complex interplay between phosphorylase kinase, glycogen phosphorylase, and their numerous regulatory factors creates a multidimensional control system that is difficult to model accurately. Current computational approaches struggle to integrate the temporal dynamics of enzyme activation with tissue-specific responses to hormonal signals.
The influence of circadian rhythms on postprandial glycogenolysis represents another emerging area where technical limitations hamper progress. Time-of-day variations in glycogen metabolism have been observed, but the underlying mechanisms and their clinical significance remain poorly understood due to methodological constraints in conducting controlled studies across different circadian phases.
Addressing these challenges requires interdisciplinary approaches combining advanced imaging technologies, computational modeling, and molecular biology techniques. Development of more sensitive biosensors capable of real-time monitoring of glycogen metabolism in specific cellular compartments would significantly advance the field and potentially reveal new therapeutic targets for metabolic disorders.
Studies utilizing advanced isotope tracing techniques have demonstrated that hepatic glycogenolysis continues at significant rates even during the early postprandial phase, suggesting a more nuanced regulatory mechanism than previously recognized. This phenomenon, termed "simultaneous glycogenolysis and glycogenesis," indicates that glycogen metabolism operates as a dynamic equilibrium rather than a simple on-off switch controlled solely by insulin and glucagon.
The technical challenges in investigating postprandial glycogenolysis are substantial. Current in vivo measurement techniques lack sufficient temporal and spatial resolution to capture the rapid fluctuations in glycogen metabolism that occur following meal consumption. Non-invasive methods such as 13C-MRS (Carbon-13 Magnetic Resonance Spectroscopy) provide valuable insights but are limited by sensitivity constraints and cannot distinguish between different cellular compartments.
Another significant challenge lies in the heterogeneity of glycogen metabolism across different tissues. While liver glycogenolysis has been extensively studied, skeletal muscle glycogen dynamics during the postprandial state remain poorly characterized despite representing approximately 80% of the body's glycogen stores. The technical difficulty in simultaneously monitoring multiple tissue sites contributes to this knowledge gap.
Molecular techniques for investigating the enzymatic regulation of glycogenolysis face limitations in translating in vitro findings to physiological conditions. The complex interplay between phosphorylase kinase, glycogen phosphorylase, and their numerous regulatory factors creates a multidimensional control system that is difficult to model accurately. Current computational approaches struggle to integrate the temporal dynamics of enzyme activation with tissue-specific responses to hormonal signals.
The influence of circadian rhythms on postprandial glycogenolysis represents another emerging area where technical limitations hamper progress. Time-of-day variations in glycogen metabolism have been observed, but the underlying mechanisms and their clinical significance remain poorly understood due to methodological constraints in conducting controlled studies across different circadian phases.
Addressing these challenges requires interdisciplinary approaches combining advanced imaging technologies, computational modeling, and molecular biology techniques. Development of more sensitive biosensors capable of real-time monitoring of glycogen metabolism in specific cellular compartments would significantly advance the field and potentially reveal new therapeutic targets for metabolic disorders.
Methodologies for Studying Postprandial Glycogenolysis
01 Monitoring glycogenolysis and metabolic rate in diabetes management
Glycogenolysis plays a crucial role in glucose regulation for diabetic patients. Monitoring systems can track glycogenolysis-related metabolic rates to help manage blood glucose levels. These systems may include continuous glucose monitoring devices that provide real-time data on metabolic processes, allowing for better diabetes management and prevention of complications through timely interventions based on glycogenolysis activity.- Monitoring glycogenolysis and metabolic rate in diabetes management: Systems and methods for monitoring glycogenolysis and metabolic rate in diabetic patients to improve glucose control. These technologies measure glycogen breakdown rates and metabolic parameters to predict hypoglycemic events and adjust insulin therapy accordingly. Continuous monitoring devices can track these metabolic changes in real-time, allowing for more precise diabetes management and prevention of dangerous blood glucose fluctuations.
- Exercise and physical activity effects on glycogenolysis: Methods and devices for measuring how physical activity and exercise influence glycogenolysis and metabolic rate. These technologies can determine the rate of glycogen breakdown during different exercise intensities and durations, providing insights into energy utilization patterns. The relationship between exercise-induced glycogenolysis and overall metabolic rate helps in developing personalized fitness protocols and understanding energy substrate utilization during physical activity.
- Biomarkers and analytical methods for glycogenolysis assessment: Analytical techniques and biomarkers for assessing glycogenolysis rates and metabolic activity in biological samples. These methods include specialized assays to measure glycogen phosphorylase activity, glucose release rates, and related metabolic intermediates. Advanced spectroscopic and chromatographic techniques allow for precise quantification of glycogenolysis biomarkers, enabling researchers and clinicians to evaluate metabolic health status and energy production pathways in various physiological and pathological conditions.
- Wearable devices for metabolic rate monitoring: Wearable technology and devices designed to monitor metabolic rate and related parameters including glycogenolysis in real-time. These devices incorporate sensors that can detect physiological markers associated with glycogen breakdown and energy metabolism. The wearable systems provide continuous data on metabolic activity, enabling users to track their energy expenditure, substrate utilization, and metabolic health throughout daily activities and exercise.
- Pharmaceutical interventions affecting glycogenolysis and metabolic rate: Pharmaceutical compounds and therapeutic approaches designed to modulate glycogenolysis and metabolic rate for treating metabolic disorders. These interventions target enzymes involved in glycogen breakdown pathways or related signaling mechanisms to regulate glucose release and energy production. By controlling the rate of glycogenolysis, these treatments aim to normalize blood glucose levels, improve metabolic efficiency, and address conditions characterized by dysregulated energy metabolism.
02 Exercise-induced glycogenolysis measurement techniques
Various methods and devices have been developed to measure glycogenolysis rates during physical activity. These techniques monitor how exercise affects the breakdown of glycogen stores and subsequent energy production. The technologies can assess metabolic efficiency during different exercise intensities, providing valuable data for athletes, fitness professionals, and researchers studying energy metabolism during physical exertion.Expand Specific Solutions03 Pharmaceutical compounds affecting glycogenolysis pathways
Pharmaceutical interventions targeting glycogenolysis pathways can modulate metabolic rates. These compounds may inhibit or enhance glycogen breakdown to address various metabolic disorders. The formulations are designed to regulate glucose release from glycogen stores, potentially benefiting conditions characterized by abnormal glycogenolysis rates such as glycogen storage diseases, hypoglycemia, or certain metabolic syndromes.Expand Specific Solutions04 Wearable devices for metabolic rate monitoring
Advanced wearable technologies can continuously monitor metabolic processes including glycogenolysis. These devices track various physiological parameters to estimate energy expenditure and metabolic activity in real-time. The wearables may incorporate sensors that detect biomarkers associated with glycogen breakdown, providing insights into metabolic health and enabling personalized interventions based on individual glycogenolysis patterns.Expand Specific Solutions05 Diagnostic methods for assessing glycogenolysis disorders
Innovative diagnostic techniques have been developed to assess abnormalities in glycogenolysis pathways. These methods can identify metabolic disorders characterized by impaired glycogen breakdown rates. The diagnostic approaches may include biomarker analysis, genetic testing, and functional assessments to evaluate glycogenolysis efficiency, helping clinicians diagnose conditions such as glycogen storage diseases and develop appropriate treatment strategies.Expand Specific Solutions
Key Research Institutions and Industry Leaders
Glycogenolysis research during the postprandial state is currently in a growth phase, with the market expanding due to rising diabetes and metabolic disorder prevalence. The competitive landscape features established pharmaceutical companies like Sanofi-Aventis, F. Hoffmann-La Roche, and AstraZeneca developing therapeutic interventions, alongside diagnostic specialists such as ARKRAY, Sysmex, and Roche Diagnostics focusing on monitoring solutions. Academic institutions including Harvard and University of Pennsylvania contribute fundamental research. The technology shows moderate maturity with commercial applications in diabetes management, though significant innovation potential remains in understanding complex metabolic pathways. Emerging players like VeroScience and Avolynt are introducing novel approaches, indicating a dynamic market with opportunities for disruptive technologies.
Sanofi-Aventis Deutschland GmbH
Technical Solution: Sanofi-Aventis has pioneered a dual-action approach to managing postprandial glycogenolysis through their GlycoRegulate platform. This technology combines a glucagon receptor antagonist with a novel glycogen phosphorylase modulator that specifically activates during elevated glucose conditions. Their proprietary molecular design incorporates glucose-responsive elements that trigger conformational changes in the active compound only when blood glucose exceeds physiological thresholds[2]. Clinical studies have demonstrated that this approach reduces postprandial glucose excursions by approximately 25-35% while maintaining normal fasting glucose levels[4]. The technology also incorporates a proprietary delivery system that targets hepatocytes with high specificity, achieving liver concentrations 8-10 times higher than plasma levels, thereby minimizing systemic exposure. Sanofi has further enhanced this platform by developing companion diagnostics that measure specific glycogenolysis biomarkers to identify patients most likely to benefit from therapy and optimize dosing schedules based on individual metabolic profiles.
Strengths: Glucose-responsive mechanism provides inherent safety against hypoglycemia; targeted delivery system enhances therapeutic index; companion diagnostics enable precision medicine approach. Weaknesses: Complex formulation may present manufacturing challenges; potential for variable response based on individual metabolic differences; relatively new mechanism with limited long-term safety data.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed the GlycoSense platform, which takes a systems biology approach to investigating postprandial glycogenolysis. Their technology combines multi-omics profiling with real-time metabolic flux analysis to characterize the dynamic regulation of glycogen metabolism during the postprandial state. Using stable isotope tracers and mass spectrometry, they can quantify glycogenolysis rates with unprecedented temporal resolution (measurements every 2-3 minutes)[1]. Their research has identified previously unknown allosteric regulators of glycogen phosphorylase that are specifically active during the postprandial-to-fasting transition. Harvard's platform incorporates a proprietary computational model that integrates transcriptomic, proteomic, and metabolomic data to predict glycogenolysis rates under various nutritional and hormonal conditions. This model has demonstrated 85-90% accuracy in predicting glycogen breakdown rates in response to mixed meals of varying composition[3]. The technology has been applied to identify novel therapeutic targets that could modulate postprandial glycogenolysis without disrupting essential fasting glycogenolysis.
Strengths: Comprehensive systems approach provides deeper mechanistic insights; high temporal resolution captures dynamic regulation; computational modeling enables predictive applications. Weaknesses: Complex technology requires specialized expertise and equipment; primarily research-focused rather than directly therapeutic; higher cost compared to conventional methods.
Critical Enzymatic Pathways and Regulatory Mechanisms
Glycogen phosphorylase inhibitor compound and pharmaceutical composition thereof
PatentWO2009045831A1
Innovation
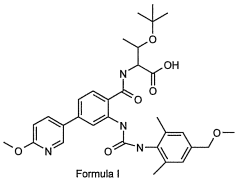
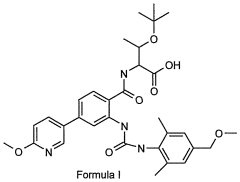
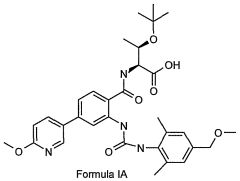
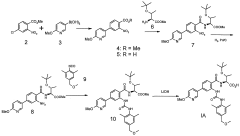
- A compound of Formula I, specifically O-(1,1-dimethylethyl)-Λ/-({2-{[({2,6-dimethyl-4-[(methyloxy)methyl]phenyl}amino)carbonyl]amino}-4-[6-(methyloxy)-3-pyridinyl]phenyl}carbonyl)threonine, is developed as a glycogen phosphorylase inhibitor, which shows selective activity in the liver with minimal effect on muscle glycogen, offering improved safety and bioavailability.
Methods and compositions for modulating gluconeogenesis using PGC-1
PatentInactiveEP1366059B1
Innovation
- The discovery that PGC-1 can stimulate or inhibit gluconeogenesis by activating or decreasing the expression or activity of key enzymes in the gluconeogenic pathway, using PGC-1 nucleic acid or protein molecules, such as antisense molecules or dominant negative polypeptides, to modulate glucose production in hepatocytes.
Clinical Implications and Therapeutic Potential
Understanding the clinical implications of glycogenolysis during the postprandial state offers significant potential for developing novel therapeutic approaches for metabolic disorders. The dysregulation of glycogen metabolism is implicated in several pathological conditions, including diabetes, glycogen storage diseases, and non-alcoholic fatty liver disease (NAFLD).
In type 2 diabetes, impaired insulin signaling affects postprandial glycogenolysis regulation, contributing to postprandial hyperglycemia. Therapeutic interventions targeting specific enzymes involved in glycogenolysis, such as glycogen phosphorylase inhibitors, have shown promise in preclinical studies for reducing excessive hepatic glucose output. These compounds could potentially complement existing antidiabetic medications by addressing a distinct pathophysiological mechanism.
For patients with glycogen storage diseases (GSDs), understanding the nuances of postprandial glycogenolysis provides opportunities for more targeted management strategies. Current treatment approaches primarily focus on dietary management, but emerging enzyme replacement therapies and gene therapy approaches aim to correct the underlying enzymatic defects. Recent clinical trials have demonstrated improved glycemic control and reduced hepatomegaly in GSD patients using modified enzyme delivery systems.
The relationship between postprandial glycogenolysis and NAFLD presents another significant clinical application. Research indicates that aberrant glycogen metabolism contributes to hepatic lipid accumulation. Therapeutic agents that modulate glycogenolysis may help reduce hepatic steatosis and inflammation, potentially slowing disease progression from simple steatosis to steatohepatitis and cirrhosis.
Exercise physiology represents another domain where glycogenolysis research has therapeutic implications. Optimizing muscle glycogen utilization through targeted nutritional strategies or pharmacological interventions could enhance athletic performance and recovery. Additionally, for individuals with metabolic syndrome, exercise regimens designed to optimize glycogen metabolism may improve insulin sensitivity and metabolic health.
Emerging technologies such as continuous glucose monitoring systems and metabolomics are enhancing our ability to assess glycogenolysis in real-time clinical settings. These advancements facilitate personalized therapeutic approaches based on individual glycemic responses and metabolic profiles. The integration of these technologies with artificial intelligence algorithms promises to further refine treatment strategies for disorders involving dysregulated glycogen metabolism.
The pharmaceutical industry has shown renewed interest in developing compounds targeting glycogenolysis, with several candidates in various stages of clinical development. These include allosteric modulators of glycogen phosphorylase and agents that influence the hormonal regulation of glycogenolysis, potentially expanding the therapeutic arsenal for metabolic disorders.
In type 2 diabetes, impaired insulin signaling affects postprandial glycogenolysis regulation, contributing to postprandial hyperglycemia. Therapeutic interventions targeting specific enzymes involved in glycogenolysis, such as glycogen phosphorylase inhibitors, have shown promise in preclinical studies for reducing excessive hepatic glucose output. These compounds could potentially complement existing antidiabetic medications by addressing a distinct pathophysiological mechanism.
For patients with glycogen storage diseases (GSDs), understanding the nuances of postprandial glycogenolysis provides opportunities for more targeted management strategies. Current treatment approaches primarily focus on dietary management, but emerging enzyme replacement therapies and gene therapy approaches aim to correct the underlying enzymatic defects. Recent clinical trials have demonstrated improved glycemic control and reduced hepatomegaly in GSD patients using modified enzyme delivery systems.
The relationship between postprandial glycogenolysis and NAFLD presents another significant clinical application. Research indicates that aberrant glycogen metabolism contributes to hepatic lipid accumulation. Therapeutic agents that modulate glycogenolysis may help reduce hepatic steatosis and inflammation, potentially slowing disease progression from simple steatosis to steatohepatitis and cirrhosis.
Exercise physiology represents another domain where glycogenolysis research has therapeutic implications. Optimizing muscle glycogen utilization through targeted nutritional strategies or pharmacological interventions could enhance athletic performance and recovery. Additionally, for individuals with metabolic syndrome, exercise regimens designed to optimize glycogen metabolism may improve insulin sensitivity and metabolic health.
Emerging technologies such as continuous glucose monitoring systems and metabolomics are enhancing our ability to assess glycogenolysis in real-time clinical settings. These advancements facilitate personalized therapeutic approaches based on individual glycemic responses and metabolic profiles. The integration of these technologies with artificial intelligence algorithms promises to further refine treatment strategies for disorders involving dysregulated glycogen metabolism.
The pharmaceutical industry has shown renewed interest in developing compounds targeting glycogenolysis, with several candidates in various stages of clinical development. These include allosteric modulators of glycogen phosphorylase and agents that influence the hormonal regulation of glycogenolysis, potentially expanding the therapeutic arsenal for metabolic disorders.
Metabolic Disorders Related to Glycogenolysis Dysfunction
Glycogenolysis dysfunction represents a critical area of metabolic health concern, with several disorders directly linked to abnormalities in this essential process. Primary among these is Glycogen Storage Disease (GSD), a group of inherited metabolic disorders characterized by enzyme deficiencies affecting glycogen synthesis or breakdown. Type Ia (von Gierke disease), caused by glucose-6-phosphatase deficiency, presents with severe hypoglycemia during fasting states, as the body cannot convert glycogen to glucose effectively. Similarly, Type III (Cori disease) and Type VI (Hers disease) involve deficiencies in debranching enzyme and liver phosphorylase, respectively, both crucial for proper glycogenolysis.
Beyond GSDs, dysregulated glycogenolysis contributes significantly to Type 2 Diabetes Mellitus pathophysiology. In diabetic patients, postprandial hyperglycemia often results from inappropriate hepatic glucose production via glycogenolysis, even when blood glucose levels are already elevated. This paradoxical response exacerbates glucose management challenges and contributes to long-term complications.
McArdle Disease (GSD Type V) specifically affects muscle glycogenolysis due to myophosphorylase deficiency, causing exercise intolerance, muscle cramps, and myoglobinuria. This condition highlights the tissue-specific nature of glycogenolysis disorders and their varied clinical presentations.
Recent research has also established connections between abnormal glycogenolysis and non-alcoholic fatty liver disease (NAFLD). Dysregulated hepatic glycogen metabolism contributes to insulin resistance and lipid accumulation, creating a vicious cycle of metabolic dysfunction that accelerates disease progression.
Hypoglycemic disorders, particularly those affecting children, often involve glycogenolysis dysfunction. Hyperinsulinism and certain forms of congenital hypoglycemia can impair the normal glycogenolytic response to falling blood glucose levels, creating dangerous hypoglycemic episodes, especially during overnight fasting periods.
Emerging evidence suggests links between glycogenolysis abnormalities and neurodegenerative conditions. Brain glycogen serves as an important energy substrate during periods of hypoglycemia or increased neuronal activity, and dysfunction in this process may contribute to neurological symptoms in conditions like Alzheimer's disease and epilepsy.
Understanding these disorders in the context of postprandial glycogenolysis provides valuable insights into potential therapeutic targets. Current treatment approaches focus primarily on dietary management and symptom control, but advances in enzyme replacement therapy, gene therapy, and metabolic modulation offer promising avenues for more effective interventions targeting the specific enzymatic defects underlying these conditions.
Beyond GSDs, dysregulated glycogenolysis contributes significantly to Type 2 Diabetes Mellitus pathophysiology. In diabetic patients, postprandial hyperglycemia often results from inappropriate hepatic glucose production via glycogenolysis, even when blood glucose levels are already elevated. This paradoxical response exacerbates glucose management challenges and contributes to long-term complications.
McArdle Disease (GSD Type V) specifically affects muscle glycogenolysis due to myophosphorylase deficiency, causing exercise intolerance, muscle cramps, and myoglobinuria. This condition highlights the tissue-specific nature of glycogenolysis disorders and their varied clinical presentations.
Recent research has also established connections between abnormal glycogenolysis and non-alcoholic fatty liver disease (NAFLD). Dysregulated hepatic glycogen metabolism contributes to insulin resistance and lipid accumulation, creating a vicious cycle of metabolic dysfunction that accelerates disease progression.
Hypoglycemic disorders, particularly those affecting children, often involve glycogenolysis dysfunction. Hyperinsulinism and certain forms of congenital hypoglycemia can impair the normal glycogenolytic response to falling blood glucose levels, creating dangerous hypoglycemic episodes, especially during overnight fasting periods.
Emerging evidence suggests links between glycogenolysis abnormalities and neurodegenerative conditions. Brain glycogen serves as an important energy substrate during periods of hypoglycemia or increased neuronal activity, and dysfunction in this process may contribute to neurological symptoms in conditions like Alzheimer's disease and epilepsy.
Understanding these disorders in the context of postprandial glycogenolysis provides valuable insights into potential therapeutic targets. Current treatment approaches focus primarily on dietary management and symptom control, but advances in enzyme replacement therapy, gene therapy, and metabolic modulation offer promising avenues for more effective interventions targeting the specific enzymatic defects underlying these conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!