How Glycogenolysis Differs Between Species
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Evolution and Research Objectives
Glycogenolysis, the metabolic pathway that breaks down glycogen into glucose, has evolved distinctly across different species throughout evolutionary history. This process first emerged in primitive organisms as a mechanism to access stored energy during periods of nutritional scarcity. The evolutionary trajectory of glycogenolysis reveals remarkable adaptations across the phylogenetic tree, from simple prokaryotes to complex mammals, each tailored to specific environmental demands and metabolic requirements.
In prokaryotes, glycogenolysis operates through relatively simple enzymatic pathways, primarily responding to immediate energy needs. As evolution progressed to more complex eukaryotes, the process developed sophisticated regulatory mechanisms, including hormonal control and enzyme cascades that allow for more nuanced energy management. The vertebrate lineage, in particular, demonstrates significant refinement of glycogenolytic pathways, with mammals exhibiting the most complex regulatory systems.
Species-specific variations in glycogenolysis reflect evolutionary adaptations to diverse ecological niches. For instance, hibernating mammals possess enhanced glycogenolytic efficiency to sustain prolonged periods without food intake. Marine mammals demonstrate specialized glycogen metabolism adapted to deep-diving behaviors and oxygen limitations. Similarly, migratory birds exhibit unique glycogenolytic patterns that support their extraordinary energy demands during long-distance flights.
The technological advancement of research methodologies has significantly enhanced our understanding of glycogenolysis across species. Early biochemical studies in the mid-20th century provided fundamental insights into the basic mechanisms. The advent of molecular biology techniques in the 1980s and 1990s allowed for more detailed characterization of species-specific enzymes and regulatory proteins. Recent developments in genomics, proteomics, and metabolomics have further illuminated the subtle yet significant differences in glycogenolytic pathways across the animal kingdom.
The primary objective of current glycogenolysis research is to comprehensively map the evolutionary divergence of this critical metabolic pathway across representative species. This includes identifying conserved elements that suggest fundamental metabolic requirements, as well as divergent features that reflect species-specific adaptations. Understanding these differences has profound implications for evolutionary biology, comparative physiology, and potentially for biomedical applications.
Additional research goals include elucidating the molecular mechanisms underlying species-specific regulation of glycogenolysis, particularly focusing on hormonal control, enzyme structure-function relationships, and cellular signaling pathways. There is also significant interest in understanding how environmental factors and ecological pressures have shaped glycogenolytic adaptations across different taxonomic groups, providing insights into the interplay between metabolism and evolution.
In prokaryotes, glycogenolysis operates through relatively simple enzymatic pathways, primarily responding to immediate energy needs. As evolution progressed to more complex eukaryotes, the process developed sophisticated regulatory mechanisms, including hormonal control and enzyme cascades that allow for more nuanced energy management. The vertebrate lineage, in particular, demonstrates significant refinement of glycogenolytic pathways, with mammals exhibiting the most complex regulatory systems.
Species-specific variations in glycogenolysis reflect evolutionary adaptations to diverse ecological niches. For instance, hibernating mammals possess enhanced glycogenolytic efficiency to sustain prolonged periods without food intake. Marine mammals demonstrate specialized glycogen metabolism adapted to deep-diving behaviors and oxygen limitations. Similarly, migratory birds exhibit unique glycogenolytic patterns that support their extraordinary energy demands during long-distance flights.
The technological advancement of research methodologies has significantly enhanced our understanding of glycogenolysis across species. Early biochemical studies in the mid-20th century provided fundamental insights into the basic mechanisms. The advent of molecular biology techniques in the 1980s and 1990s allowed for more detailed characterization of species-specific enzymes and regulatory proteins. Recent developments in genomics, proteomics, and metabolomics have further illuminated the subtle yet significant differences in glycogenolytic pathways across the animal kingdom.
The primary objective of current glycogenolysis research is to comprehensively map the evolutionary divergence of this critical metabolic pathway across representative species. This includes identifying conserved elements that suggest fundamental metabolic requirements, as well as divergent features that reflect species-specific adaptations. Understanding these differences has profound implications for evolutionary biology, comparative physiology, and potentially for biomedical applications.
Additional research goals include elucidating the molecular mechanisms underlying species-specific regulation of glycogenolysis, particularly focusing on hormonal control, enzyme structure-function relationships, and cellular signaling pathways. There is also significant interest in understanding how environmental factors and ecological pressures have shaped glycogenolytic adaptations across different taxonomic groups, providing insights into the interplay between metabolism and evolution.
Market Applications of Species-Specific Glycogenolysis Research
The market for species-specific glycogenolysis research spans multiple industries with significant growth potential. Pharmaceutical companies are increasingly investing in comparative glycogenolysis studies to develop targeted medications for metabolic disorders. These companies recognize that understanding species variations in glycogen breakdown pathways enables the creation of more precise drug interventions with reduced side effects. The veterinary medicine sector represents another substantial market, where treatments for companion animals and livestock can be optimized based on species-specific metabolic processes.
Biotechnology firms specializing in enzyme production have identified commercial opportunities in exploiting species differences in glycogenolytic enzymes. These enzymes have applications in diagnostic kits, research reagents, and industrial processes. The market for such specialized enzymes is projected to grow steadily as precision medicine and personalized healthcare gain prominence across global healthcare systems.
The sports nutrition and performance enhancement industry has begun incorporating species-specific glycogenolysis research into product development. Companies are creating specialized supplements that target human glycogen metabolism pathways more effectively than generic products. This segment shows particular promise in high-performance athletics where marginal metabolic improvements can translate to competitive advantages.
Agricultural applications represent an emerging market where understanding glycogenolysis differences between livestock species can lead to optimized feeding regimens and improved meat quality. Feed manufacturers are developing species-tailored additives that enhance energy utilization efficiency based on distinct glycogen metabolism patterns.
The research tools and diagnostics sector benefits from species-specific glycogenolysis knowledge through the development of specialized assays, reagents, and laboratory models. These tools enable more accurate research outcomes and improved diagnostic capabilities for metabolic conditions across different species.
Academic and research institutions constitute a significant market for technologies and methodologies related to comparative glycogenolysis studies. This includes specialized laboratory equipment, analytical software, and experimental models designed to investigate metabolic differences between species.
Regulatory considerations vary significantly across these market segments, with pharmaceutical applications facing the most stringent oversight. Companies must navigate complex approval processes that often require species-specific safety and efficacy data, creating both challenges and opportunities for specialized research service providers.
The convergence of glycogenolysis research with emerging technologies like artificial intelligence and computational biology is creating new market niches for companies that can effectively integrate these approaches to accelerate discovery and development processes in this field.
Biotechnology firms specializing in enzyme production have identified commercial opportunities in exploiting species differences in glycogenolytic enzymes. These enzymes have applications in diagnostic kits, research reagents, and industrial processes. The market for such specialized enzymes is projected to grow steadily as precision medicine and personalized healthcare gain prominence across global healthcare systems.
The sports nutrition and performance enhancement industry has begun incorporating species-specific glycogenolysis research into product development. Companies are creating specialized supplements that target human glycogen metabolism pathways more effectively than generic products. This segment shows particular promise in high-performance athletics where marginal metabolic improvements can translate to competitive advantages.
Agricultural applications represent an emerging market where understanding glycogenolysis differences between livestock species can lead to optimized feeding regimens and improved meat quality. Feed manufacturers are developing species-tailored additives that enhance energy utilization efficiency based on distinct glycogen metabolism patterns.
The research tools and diagnostics sector benefits from species-specific glycogenolysis knowledge through the development of specialized assays, reagents, and laboratory models. These tools enable more accurate research outcomes and improved diagnostic capabilities for metabolic conditions across different species.
Academic and research institutions constitute a significant market for technologies and methodologies related to comparative glycogenolysis studies. This includes specialized laboratory equipment, analytical software, and experimental models designed to investigate metabolic differences between species.
Regulatory considerations vary significantly across these market segments, with pharmaceutical applications facing the most stringent oversight. Companies must navigate complex approval processes that often require species-specific safety and efficacy data, creating both challenges and opportunities for specialized research service providers.
The convergence of glycogenolysis research with emerging technologies like artificial intelligence and computational biology is creating new market niches for companies that can effectively integrate these approaches to accelerate discovery and development processes in this field.
Comparative Glycogenolysis Mechanisms and Challenges
Glycogenolysis, the process of breaking down glycogen into glucose, exhibits significant variations across different species due to evolutionary adaptations to diverse metabolic needs. Mammals, birds, reptiles, amphibians, fish, and even some invertebrates all utilize glycogenolysis, but with notable differences in regulatory mechanisms, enzyme structures, and metabolic pathways.
In mammals, glycogenolysis is primarily regulated by hormones such as glucagon and epinephrine, which activate a cascade of enzymatic reactions. The liver serves as the main site for glycogen storage and breakdown to maintain blood glucose levels. However, the relative importance of different regulatory pathways varies significantly between species. For instance, ruminants like cattle have evolved specialized mechanisms to handle their unique digestive processes, relying less on glycogenolysis for glucose homeostasis compared to non-ruminant mammals.
Birds demonstrate remarkably efficient glycogenolysis systems, adapted to their high-energy requirements for flight. Their liver glycogen stores can be mobilized more rapidly than in mammals, with specialized enzyme isoforms that function optimally at higher body temperatures. The avian glycogen phosphorylase shows structural modifications that enhance its catalytic efficiency at elevated temperatures.
Reptiles and amphibians present interesting variations in glycogenolysis regulation related to their ectothermic nature. These species must adapt their metabolic processes to fluctuating environmental temperatures, resulting in temperature-sensitive enzyme variants and alternative regulatory pathways. During hibernation or estivation, these animals employ modified glycogenolysis mechanisms to conserve energy while maintaining minimal metabolic functions.
Fish species exhibit perhaps the most diverse adaptations in glycogenolysis mechanisms. Marine fish face different challenges compared to freshwater species, with variations in enzyme structure and function reflecting adaptations to different osmotic environments. Deep-sea fish have evolved glycogenolysis pathways that function efficiently under high pressure and low temperature conditions, while species in extreme environments like Antarctic waters possess unique cold-adapted enzymes.
Invertebrates that store glycogen show fundamental differences in their glycogenolysis pathways compared to vertebrates. Insects, for example, utilize trehalose as an intermediate product rather than glucose, requiring additional enzymatic steps in their glycogen mobilization process. This adaptation provides advantages for flight muscle function and cold tolerance.
The comparative study of glycogenolysis across species reveals not only evolutionary adaptations but also provides insights into potential biomedical applications. Understanding species-specific variations in glycogen metabolism has implications for developing targeted treatments for metabolic disorders, designing species-specific veterinary medications, and even engineering improved biofuels based on efficient energy storage and release mechanisms observed in nature.
In mammals, glycogenolysis is primarily regulated by hormones such as glucagon and epinephrine, which activate a cascade of enzymatic reactions. The liver serves as the main site for glycogen storage and breakdown to maintain blood glucose levels. However, the relative importance of different regulatory pathways varies significantly between species. For instance, ruminants like cattle have evolved specialized mechanisms to handle their unique digestive processes, relying less on glycogenolysis for glucose homeostasis compared to non-ruminant mammals.
Birds demonstrate remarkably efficient glycogenolysis systems, adapted to their high-energy requirements for flight. Their liver glycogen stores can be mobilized more rapidly than in mammals, with specialized enzyme isoforms that function optimally at higher body temperatures. The avian glycogen phosphorylase shows structural modifications that enhance its catalytic efficiency at elevated temperatures.
Reptiles and amphibians present interesting variations in glycogenolysis regulation related to their ectothermic nature. These species must adapt their metabolic processes to fluctuating environmental temperatures, resulting in temperature-sensitive enzyme variants and alternative regulatory pathways. During hibernation or estivation, these animals employ modified glycogenolysis mechanisms to conserve energy while maintaining minimal metabolic functions.
Fish species exhibit perhaps the most diverse adaptations in glycogenolysis mechanisms. Marine fish face different challenges compared to freshwater species, with variations in enzyme structure and function reflecting adaptations to different osmotic environments. Deep-sea fish have evolved glycogenolysis pathways that function efficiently under high pressure and low temperature conditions, while species in extreme environments like Antarctic waters possess unique cold-adapted enzymes.
Invertebrates that store glycogen show fundamental differences in their glycogenolysis pathways compared to vertebrates. Insects, for example, utilize trehalose as an intermediate product rather than glucose, requiring additional enzymatic steps in their glycogen mobilization process. This adaptation provides advantages for flight muscle function and cold tolerance.
The comparative study of glycogenolysis across species reveals not only evolutionary adaptations but also provides insights into potential biomedical applications. Understanding species-specific variations in glycogen metabolism has implications for developing targeted treatments for metabolic disorders, designing species-specific veterinary medications, and even engineering improved biofuels based on efficient energy storage and release mechanisms observed in nature.
Current Methodologies for Studying Cross-Species Glycogenolysis
01 Inhibition of glycogenolysis for metabolic disorders
Compounds that inhibit glycogenolysis can be used to treat metabolic disorders such as diabetes and obesity. By preventing the breakdown of glycogen into glucose, these inhibitors help maintain stable blood glucose levels and reduce hyperglycemia. Various chemical compounds have been developed that target specific enzymes in the glycogenolysis pathway, providing therapeutic benefits for patients with impaired glucose metabolism.- Inhibitors of glycogenolysis for therapeutic applications: Various compounds have been developed as inhibitors of glycogenolysis for treating conditions related to abnormal glucose metabolism. These inhibitors target enzymes involved in the breakdown of glycogen, such as glycogen phosphorylase, to help regulate blood glucose levels. Such inhibitors are particularly useful in treating diabetes, obesity, and other metabolic disorders by preventing excessive glucose release from glycogen stores.
- Diagnostic methods for monitoring glycogenolysis: Diagnostic technologies have been developed to monitor glycogenolysis processes in the body. These methods include biomarkers, imaging techniques, and assays that can detect the rate of glycogen breakdown and related metabolic activities. Such diagnostic approaches are valuable for assessing metabolic health, diagnosing disorders related to glycogen metabolism, and evaluating the efficacy of treatments targeting glycogenolysis pathways.
- Regulation of glycogenolysis through exercise and physical stimulation: Research has focused on how exercise and physical stimulation can regulate glycogenolysis in muscle and liver tissues. Various methods and devices have been developed to optimize this process, including specific exercise protocols and electrical stimulation techniques. These approaches aim to enhance athletic performance, improve glucose utilization during physical activity, and provide therapeutic benefits for metabolic conditions.
- Nutritional and herbal compounds affecting glycogenolysis: Various nutritional supplements and herbal compounds have been identified that can influence glycogenolysis pathways. These natural compounds can either inhibit or stimulate glycogen breakdown depending on their specific mechanisms of action. Such products are being developed for managing blood glucose levels, enhancing energy availability during exercise, and supporting overall metabolic health through modulation of glycogen metabolism.
- Pharmaceutical compositions targeting glycogenolysis for metabolic disorders: Specialized pharmaceutical compositions have been formulated to target glycogenolysis pathways for treating various metabolic disorders. These formulations include novel drug delivery systems, combination therapies, and targeted approaches that specifically modulate glycogen breakdown in different tissues. Such pharmaceutical interventions aim to address conditions like diabetes, hypoglycemia, glycogen storage diseases, and other metabolic abnormalities by precisely controlling the rate of glucose release from glycogen stores.
02 Glycogenolysis regulation in muscle tissue
Regulation of glycogenolysis in muscle tissue is important for athletic performance and muscle function. The process involves the controlled breakdown of glycogen stores to provide energy during physical activity. Compounds that modulate this process can help improve muscle endurance, reduce fatigue, and enhance recovery after exercise. Research has focused on developing agents that can selectively target muscle glycogenolysis without affecting glucose homeostasis in other tissues.Expand Specific Solutions03 Glycogenolysis in liver disease treatment
Targeting hepatic glycogenolysis has emerged as a strategy for treating liver diseases such as non-alcoholic fatty liver disease (NAFLD) and hepatic steatosis. By controlling the rate of glycogen breakdown in the liver, these approaches help regulate glucose output and improve overall liver function. Therapeutic compounds have been developed that specifically target liver glycogenolysis enzymes, providing potential treatments for patients with liver disorders while minimizing effects on other metabolic pathways.Expand Specific Solutions04 Diagnostic methods for glycogenolysis disorders
Diagnostic methods have been developed to identify and characterize disorders related to glycogenolysis. These techniques include biomarker detection, genetic testing, and enzyme activity assays that can identify abnormalities in the glycogen breakdown pathway. Early diagnosis of glycogenolysis disorders is crucial for implementing appropriate treatment strategies and preventing complications. Advanced diagnostic tools enable personalized medicine approaches for patients with inherited or acquired glycogenolysis abnormalities.Expand Specific Solutions05 Therapeutic modulation of glycogenolysis signaling pathways
Modulation of signaling pathways that control glycogenolysis offers therapeutic potential for various conditions. These approaches target the molecular mechanisms that regulate glycogen phosphorylase activity, including hormone receptors, second messengers, and protein kinases. By intervening at different points in these signaling cascades, it's possible to achieve more precise control over glycogen metabolism. This strategy has applications in treating conditions ranging from hypoglycemia to neurodegenerative disorders where glycogen metabolism plays a role.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The glycogenolysis research landscape is currently in a growth phase, with an estimated market size of $3-5 billion and expanding at approximately 7% annually. The competitive field features pharmaceutical giants like Novartis, GlaxoSmithKline, and Merck Sharp & Dohme alongside specialized biotechnology firms such as GlycoFi (Merck subsidiary) and Novozymes. Academic institutions including Duke University and Ghent University contribute significant research advancements. Technical maturity varies across species models, with human and murine systems being well-characterized while comparative cross-species mechanisms remain less developed. Companies like Lonza and Ajinomoto are advancing commercial applications, while research institutes such as VIB and Max Planck Society drive fundamental understanding of species-specific glycogenolysis pathways.
Ghent University
Technical Solution: Ghent University has pioneered research on species-specific glycogenolysis through their Comparative Glycobiology Research Group. Their technical approach employs isotope-labeled glucose tracking to monitor glycogen breakdown kinetics across diverse organisms from bacteria to mammals. Their studies have documented significant differences in the regulatory mechanisms of glycogenolysis between species, particularly in the phosphorylation cascades that activate glycogen phosphorylase. Their research has revealed that while mammals primarily use epinephrine and glucagon for glycogenolysis regulation, fish utilize different catecholamine profiles with varying receptor affinities[2]. Additionally, they've characterized unique glycogen structures in certain invertebrates that affect the rate and efficiency of glycogenolysis, demonstrating how evolutionary adaptations in glycogen architecture influence metabolic response to energy demands[4]. Their comparative genomics platform has identified novel regulatory elements in glycogenolysis pathways across evolutionary distant species.
Strengths: Exceptional breadth of species comparison from prokaryotes to complex mammals provides comprehensive evolutionary perspective on glycogenolysis adaptation. Weaknesses: Some of their analytical methods require specialized equipment limiting widespread application of their techniques in resource-limited research settings.
Vlaams Instituut voor Biotechnologie - Flanders Institute
Technical Solution: The Flanders Institute has developed a multi-omics platform specifically designed to investigate glycogenolysis across species barriers. Their technical approach combines proteomics, metabolomics, and advanced imaging to characterize species-specific differences in glycogen breakdown pathways. Their research has identified significant variations in the phosphorylase kinase complex structure and activation mechanisms between mammals, birds, and reptiles. Using CRISPR-engineered cellular models, they've demonstrated how species-specific mutations in glycogen phosphorylase affect enzyme activity and regulation[5]. Their comparative studies have revealed that aquatic species possess unique adaptations in their glycogenolysis pathways that enable rapid energy mobilization under hypoxic conditions, with different allosteric regulators than those found in terrestrial animals[6]. The institute has also mapped species-specific differences in the subcellular localization of glycogenolysis enzymes, showing how compartmentalization varies across evolutionary lineages to optimize metabolic efficiency.
Strengths: Their integrated multi-omics approach provides unprecedented resolution of species-specific glycogenolysis regulation at molecular and cellular levels. Weaknesses: Research has focused primarily on model organisms, with limited data on exotic species that might reveal novel regulatory mechanisms.
Key Enzymatic and Regulatory Differences Between Species
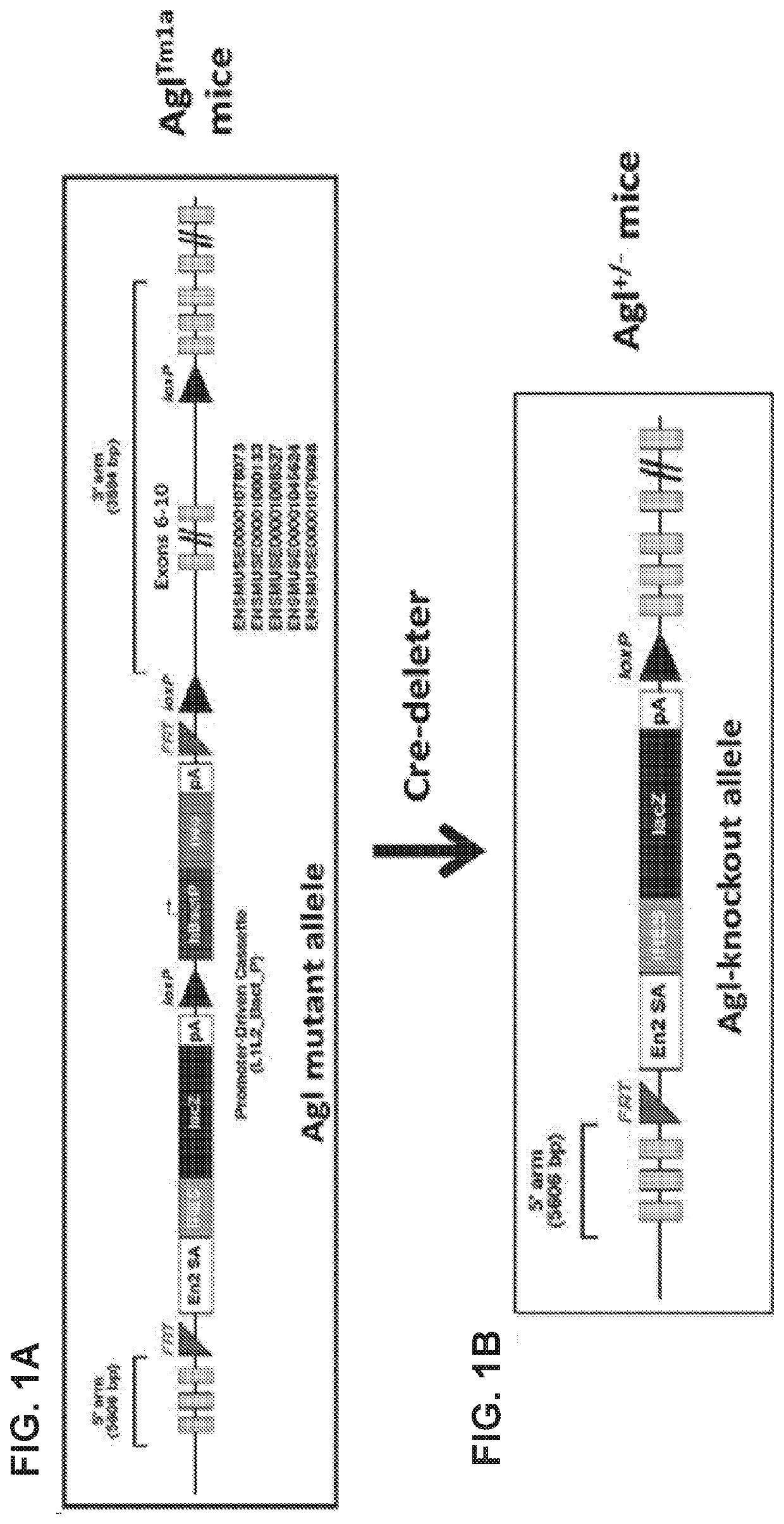
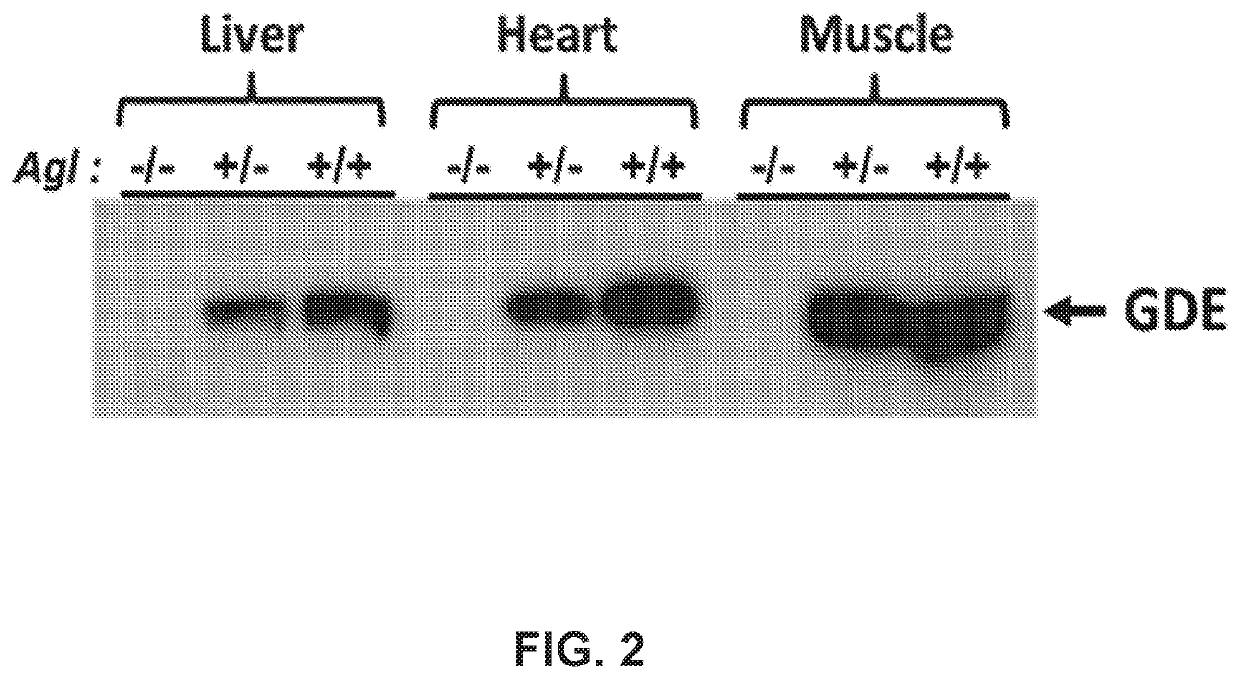
Compositions and Methods for the Treatment of Genetic Diseases
PatentPendingUS20220105204A1
Innovation
- The use of a microbial glycogen debranching enzyme encoded by a nucleic acid sequence optimized for mammalian expression, delivered via vectors with tissue-specific or immunotolerant dual promoters to prevent immune responses and achieve broader tissue correction.
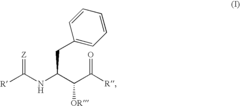
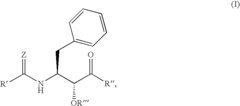
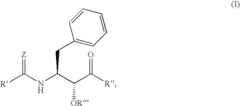
N-(indole-2-carbonyl) and H-thieno[2,3-b]pyrrole-5-carboxamide anti-diabetic agents
PatentInactiveUS6992101B2
Innovation
- Development of specific substituted N-(indole-2-carbonyl)amides and 6H-thieno[2,3-b]pyrrole-5-carboxamides and their prodrugs, which act as glycogen phosphorylase inhibitors, to treat diabetes, insulin resistance, diabetic complications, hypertension, and cardiovascular issues by regulating glycogenolysis and insulin levels.
Metabolic Disease Implications Across Different Species
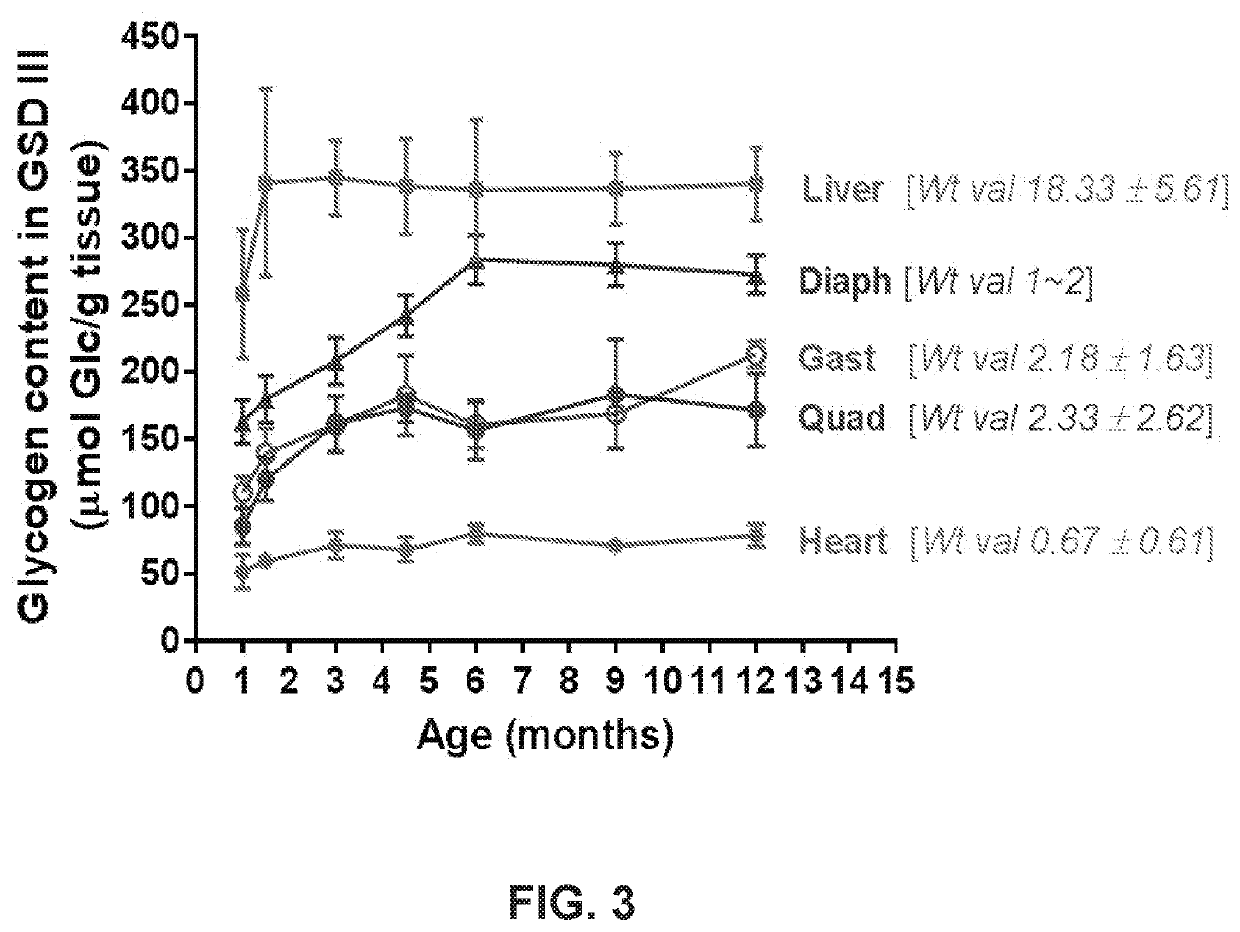
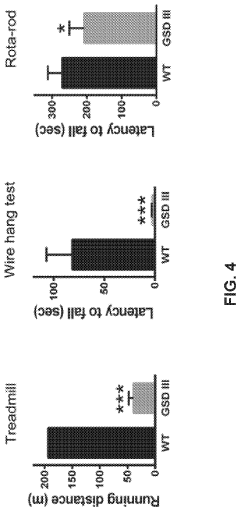
The interspecies variations in glycogenolysis mechanisms have profound implications for metabolic disease manifestations and therapeutic approaches. Mammals exhibit distinct patterns of glycogen metabolism disorders, with humans showing particular vulnerability to glycogen storage diseases (GSDs) that affect liver and muscle tissues. These conditions manifest differently across species due to evolutionary adaptations in enzyme expression and regulatory pathways.
In rodent models, particularly mice and rats, glycogenolysis disorders typically present with more severe hypoglycemic episodes compared to humans, reflecting their higher metabolic rates and greater reliance on glucose as an energy substrate. This distinction has complicated the translation of rodent-based research findings to human clinical applications, necessitating careful consideration when developing therapeutic interventions.
Canine species demonstrate unique glycogenolysis patterns that have made them valuable models for studying certain human GSDs. Dogs with naturally occurring phosphofructokinase deficiency exhibit hemolytic anemia and exercise intolerance that closely mirror human manifestations, providing insights into potential treatment modalities that may cross species boundaries.
Avian species, with their distinctive glucose metabolism adapted for flight, show remarkable resistance to diabetes-like conditions despite high blood glucose levels. Their glycogenolysis pathways incorporate specialized regulatory mechanisms that prevent glucotoxicity, offering potential insights for novel therapeutic approaches to human diabetes management.
Aquatic mammals like seals and whales have evolved specialized glycogenolysis regulation to accommodate prolonged diving and fasting periods. These adaptations include enhanced glycogen storage capacity and controlled release mechanisms that prevent harmful metabolic fluctuations. Understanding these evolutionary adaptations may inform new approaches to managing metabolic syndrome and insulin resistance in humans.
The differential expression of glycogen phosphorylase isoenzymes across species correlates with varying susceptibility to metabolic disorders. Primates and humans express patterns that render them particularly vulnerable to type 2 diabetes when combined with dietary factors, while certain hibernating mammals demonstrate remarkable metabolic flexibility without pathological consequences.
These interspecies differences highlight the importance of comparative metabolic research in developing targeted therapies. Recent advances in gene therapy approaches for GSDs have demonstrated variable efficacy across species, underscoring the need for species-specific considerations in treatment development. The emerging field of evolutionary medicine increasingly recognizes these variations as critical factors in translating basic research into effective clinical interventions for metabolic disorders.
In rodent models, particularly mice and rats, glycogenolysis disorders typically present with more severe hypoglycemic episodes compared to humans, reflecting their higher metabolic rates and greater reliance on glucose as an energy substrate. This distinction has complicated the translation of rodent-based research findings to human clinical applications, necessitating careful consideration when developing therapeutic interventions.
Canine species demonstrate unique glycogenolysis patterns that have made them valuable models for studying certain human GSDs. Dogs with naturally occurring phosphofructokinase deficiency exhibit hemolytic anemia and exercise intolerance that closely mirror human manifestations, providing insights into potential treatment modalities that may cross species boundaries.
Avian species, with their distinctive glucose metabolism adapted for flight, show remarkable resistance to diabetes-like conditions despite high blood glucose levels. Their glycogenolysis pathways incorporate specialized regulatory mechanisms that prevent glucotoxicity, offering potential insights for novel therapeutic approaches to human diabetes management.
Aquatic mammals like seals and whales have evolved specialized glycogenolysis regulation to accommodate prolonged diving and fasting periods. These adaptations include enhanced glycogen storage capacity and controlled release mechanisms that prevent harmful metabolic fluctuations. Understanding these evolutionary adaptations may inform new approaches to managing metabolic syndrome and insulin resistance in humans.
The differential expression of glycogen phosphorylase isoenzymes across species correlates with varying susceptibility to metabolic disorders. Primates and humans express patterns that render them particularly vulnerable to type 2 diabetes when combined with dietary factors, while certain hibernating mammals demonstrate remarkable metabolic flexibility without pathological consequences.
These interspecies differences highlight the importance of comparative metabolic research in developing targeted therapies. Recent advances in gene therapy approaches for GSDs have demonstrated variable efficacy across species, underscoring the need for species-specific considerations in treatment development. The emerging field of evolutionary medicine increasingly recognizes these variations as critical factors in translating basic research into effective clinical interventions for metabolic disorders.
Biotechnological Applications of Species-Specific Glycogen Metabolism
The interspecies variations in glycogen metabolism present unique opportunities for biotechnological applications. Species-specific glycogenolysis pathways can be leveraged to develop targeted enzymatic systems for industrial processes. For instance, the rapid glycogen breakdown mechanisms in certain fish species that enable burst swimming can inform the design of high-efficiency biocatalysts for rapid starch processing in food industries.
Mammalian liver glycogenolysis, particularly in humans and rodents, has been extensively studied for pharmaceutical applications. The differential regulation of glycogen phosphorylase between species provides templates for designing selective inhibitors that can target specific metabolic disorders without cross-species effects. This species specificity is crucial for developing drugs that modulate glycogen metabolism in humans while minimizing adverse effects observed in preclinical animal models.
Agricultural biotechnology benefits from understanding species variations in glycogen metabolism. Plant starch mobilization mechanisms, while distinct from animal glycogenolysis, share evolutionary relationships that can be exploited for crop improvement. Engineering crops with modified glycogen/starch metabolism pathways based on insights from diverse species can enhance yield stability under varying environmental conditions and improve nutritional profiles.
Microbial glycogen metabolism offers perhaps the most immediate biotechnological potential. The diverse glycogenolysis pathways in bacteria and fungi can be harnessed for bioremediation applications. Some microorganisms efficiently mobilize glycogen under nutrient-limited conditions, making them excellent candidates for environmental cleanup technologies where carbon source utilization efficiency is paramount.
Biofuel production represents another promising application area. The varied efficiency of glycogen breakdown across species can inform the development of optimized microbial strains for biofuel production. By incorporating glycogenolysis mechanisms from species that rapidly mobilize glycogen reserves, researchers can potentially enhance the conversion efficiency of biomass to biofuels.
Diagnostic technologies also benefit from species-specific glycogen metabolism knowledge. Distinctive glycogenolysis patterns can serve as biomarkers for identifying particular organisms in complex biological samples. This has applications in food safety testing, environmental monitoring, and clinical diagnostics where rapid species identification is crucial.
Enzyme engineering based on cross-species comparison of glycogenolytic enzymes enables the development of novel biocatalysts with enhanced stability, activity, or substrate specificity. These engineered enzymes can find applications in various industries, from textile processing to pharmaceutical manufacturing, where specific glycosidic bond hydrolysis is required under controlled conditions.
Mammalian liver glycogenolysis, particularly in humans and rodents, has been extensively studied for pharmaceutical applications. The differential regulation of glycogen phosphorylase between species provides templates for designing selective inhibitors that can target specific metabolic disorders without cross-species effects. This species specificity is crucial for developing drugs that modulate glycogen metabolism in humans while minimizing adverse effects observed in preclinical animal models.
Agricultural biotechnology benefits from understanding species variations in glycogen metabolism. Plant starch mobilization mechanisms, while distinct from animal glycogenolysis, share evolutionary relationships that can be exploited for crop improvement. Engineering crops with modified glycogen/starch metabolism pathways based on insights from diverse species can enhance yield stability under varying environmental conditions and improve nutritional profiles.
Microbial glycogen metabolism offers perhaps the most immediate biotechnological potential. The diverse glycogenolysis pathways in bacteria and fungi can be harnessed for bioremediation applications. Some microorganisms efficiently mobilize glycogen under nutrient-limited conditions, making them excellent candidates for environmental cleanup technologies where carbon source utilization efficiency is paramount.
Biofuel production represents another promising application area. The varied efficiency of glycogen breakdown across species can inform the development of optimized microbial strains for biofuel production. By incorporating glycogenolysis mechanisms from species that rapidly mobilize glycogen reserves, researchers can potentially enhance the conversion efficiency of biomass to biofuels.
Diagnostic technologies also benefit from species-specific glycogen metabolism knowledge. Distinctive glycogenolysis patterns can serve as biomarkers for identifying particular organisms in complex biological samples. This has applications in food safety testing, environmental monitoring, and clinical diagnostics where rapid species identification is crucial.
Enzyme engineering based on cross-species comparison of glycogenolytic enzymes enables the development of novel biocatalysts with enhanced stability, activity, or substrate specificity. These engineered enzymes can find applications in various industries, from textile processing to pharmaceutical manufacturing, where specific glycosidic bond hydrolysis is required under controlled conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!