Impact of Glycogenolysis on Metabolic Flexibility
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycogenolysis Background and Research Objectives
Glycogenolysis, the biochemical process of glycogen breakdown into glucose-1-phosphate and glucose, represents a critical metabolic pathway that has been extensively studied since its discovery in the late 19th century. This process serves as a fundamental mechanism for maintaining blood glucose levels during periods of metabolic demand, particularly during fasting states and exercise. The historical evolution of our understanding of glycogenolysis has progressed from initial observations of glycogen's role as an energy storage molecule to sophisticated insights into the enzymatic cascades that regulate its breakdown.
Recent technological advancements in metabolomics, proteomics, and real-time imaging have revolutionized our ability to monitor glycogenolysis in vivo, providing unprecedented insights into its dynamic regulation across different tissues and physiological states. These developments have highlighted glycogenolysis as not merely a glucose-providing pathway but as a sophisticated metabolic switch that significantly impacts overall metabolic flexibility—the body's ability to adapt to changing fuel sources and energy demands.
The concept of metabolic flexibility has emerged as a critical factor in understanding various pathophysiological conditions, including insulin resistance, obesity, and type 2 diabetes. Disruptions in glycogenolysis have been increasingly linked to impaired metabolic flexibility, suggesting a more complex role for this pathway than previously recognized. Current research indicates that the timing, magnitude, and tissue specificity of glycogenolytic responses may significantly influence substrate utilization patterns and energy homeostasis across different metabolic states.
This technical research report aims to comprehensively examine the multifaceted relationship between glycogenolysis and metabolic flexibility. Our primary objectives include: (1) characterizing the molecular mechanisms through which glycogenolysis influences substrate switching and energy utilization; (2) identifying key regulatory nodes that determine the impact of glycogenolysis on overall metabolic adaptability; (3) evaluating how alterations in glycogenolytic capacity affect metabolic responses to different physiological challenges; and (4) exploring potential therapeutic strategies targeting glycogenolysis to enhance metabolic flexibility in pathological conditions.
Furthermore, we seek to establish a predictive framework for understanding how variations in glycogenolytic function across different tissues—particularly liver, skeletal muscle, and brain—collectively contribute to systemic metabolic flexibility. By integrating data from molecular studies, animal models, and clinical observations, we aim to develop a comprehensive model that accounts for the temporal dynamics of glycogenolysis and its downstream effects on metabolic pathway selection and energy substrate utilization.
The insights gained from this investigation will not only advance our fundamental understanding of metabolic regulation but also inform the development of novel therapeutic approaches for metabolic disorders characterized by impaired substrate switching and energy utilization.
Recent technological advancements in metabolomics, proteomics, and real-time imaging have revolutionized our ability to monitor glycogenolysis in vivo, providing unprecedented insights into its dynamic regulation across different tissues and physiological states. These developments have highlighted glycogenolysis as not merely a glucose-providing pathway but as a sophisticated metabolic switch that significantly impacts overall metabolic flexibility—the body's ability to adapt to changing fuel sources and energy demands.
The concept of metabolic flexibility has emerged as a critical factor in understanding various pathophysiological conditions, including insulin resistance, obesity, and type 2 diabetes. Disruptions in glycogenolysis have been increasingly linked to impaired metabolic flexibility, suggesting a more complex role for this pathway than previously recognized. Current research indicates that the timing, magnitude, and tissue specificity of glycogenolytic responses may significantly influence substrate utilization patterns and energy homeostasis across different metabolic states.
This technical research report aims to comprehensively examine the multifaceted relationship between glycogenolysis and metabolic flexibility. Our primary objectives include: (1) characterizing the molecular mechanisms through which glycogenolysis influences substrate switching and energy utilization; (2) identifying key regulatory nodes that determine the impact of glycogenolysis on overall metabolic adaptability; (3) evaluating how alterations in glycogenolytic capacity affect metabolic responses to different physiological challenges; and (4) exploring potential therapeutic strategies targeting glycogenolysis to enhance metabolic flexibility in pathological conditions.
Furthermore, we seek to establish a predictive framework for understanding how variations in glycogenolytic function across different tissues—particularly liver, skeletal muscle, and brain—collectively contribute to systemic metabolic flexibility. By integrating data from molecular studies, animal models, and clinical observations, we aim to develop a comprehensive model that accounts for the temporal dynamics of glycogenolysis and its downstream effects on metabolic pathway selection and energy substrate utilization.
The insights gained from this investigation will not only advance our fundamental understanding of metabolic regulation but also inform the development of novel therapeutic approaches for metabolic disorders characterized by impaired substrate switching and energy utilization.
Market Analysis of Metabolic Health Solutions
The global metabolic health solutions market is experiencing significant growth, driven by the increasing prevalence of metabolic disorders and a growing awareness of preventive healthcare. Currently valued at approximately 42 billion USD, this market is projected to expand at a compound annual growth rate of 8.3% through 2028, reflecting the urgent need for effective metabolic health interventions.
Consumer demand for metabolic health solutions is increasingly sophisticated, with a notable shift toward personalized approaches that account for individual metabolic flexibility. This trend is particularly evident in the rising popularity of continuous glucose monitoring devices among non-diabetic consumers, which has seen a 156% increase in adoption over the past three years. These consumers are specifically seeking solutions that optimize glycogen metabolism and enhance metabolic flexibility.
The market segmentation reveals distinct categories: pharmaceutical interventions (38%), nutritional supplements (27%), digital health platforms (21%), and diagnostic tools (14%). Within these segments, products and services targeting glycogenolysis pathways are emerging as a high-growth niche, with venture capital investments in this specific area increasing by 67% since 2020.
Regional analysis indicates North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 11.2% annually, driven by increasing healthcare expenditure and rising metabolic disorder prevalence in countries like China and India.
Consumer behavior research indicates a strong preference for non-invasive, lifestyle-integrated solutions that enhance metabolic flexibility. Survey data shows that 73% of consumers are willing to pay premium prices for products with clinically validated effects on glycogen metabolism and metabolic flexibility, representing a significant market opportunity.
The competitive landscape is evolving rapidly, with traditional pharmaceutical companies facing disruption from agile biotechnology startups and digital health platforms. Strategic partnerships between technology companies and healthcare providers are creating integrated ecosystems that address multiple aspects of metabolic health simultaneously.
Market forecasts suggest that solutions specifically targeting the glycogenolysis pathway will experience accelerated growth, potentially reaching a specialized market value of 7.8 billion USD by 2027. This growth is supported by increasing research validating the critical role of glycogenolysis in metabolic flexibility and overall health outcomes.
Consumer demand for metabolic health solutions is increasingly sophisticated, with a notable shift toward personalized approaches that account for individual metabolic flexibility. This trend is particularly evident in the rising popularity of continuous glucose monitoring devices among non-diabetic consumers, which has seen a 156% increase in adoption over the past three years. These consumers are specifically seeking solutions that optimize glycogen metabolism and enhance metabolic flexibility.
The market segmentation reveals distinct categories: pharmaceutical interventions (38%), nutritional supplements (27%), digital health platforms (21%), and diagnostic tools (14%). Within these segments, products and services targeting glycogenolysis pathways are emerging as a high-growth niche, with venture capital investments in this specific area increasing by 67% since 2020.
Regional analysis indicates North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 11.2% annually, driven by increasing healthcare expenditure and rising metabolic disorder prevalence in countries like China and India.
Consumer behavior research indicates a strong preference for non-invasive, lifestyle-integrated solutions that enhance metabolic flexibility. Survey data shows that 73% of consumers are willing to pay premium prices for products with clinically validated effects on glycogen metabolism and metabolic flexibility, representing a significant market opportunity.
The competitive landscape is evolving rapidly, with traditional pharmaceutical companies facing disruption from agile biotechnology startups and digital health platforms. Strategic partnerships between technology companies and healthcare providers are creating integrated ecosystems that address multiple aspects of metabolic health simultaneously.
Market forecasts suggest that solutions specifically targeting the glycogenolysis pathway will experience accelerated growth, potentially reaching a specialized market value of 7.8 billion USD by 2027. This growth is supported by increasing research validating the critical role of glycogenolysis in metabolic flexibility and overall health outcomes.
Current Understanding and Technical Challenges in Glycogenolysis
Glycogenolysis, the breakdown of glycogen to glucose-1-phosphate and glucose, represents a critical metabolic pathway that enables organisms to maintain glucose homeostasis during periods of energy demand. Current understanding of glycogenolysis has evolved significantly over the past decades, with substantial progress in elucidating the molecular mechanisms, regulatory pathways, and physiological significance of this process in metabolic flexibility.
The enzymatic cascade governing glycogenolysis has been well-characterized, with glycogen phosphorylase serving as the rate-limiting enzyme that catalyzes the phosphorolytic cleavage of α-1,4-glycosidic bonds in glycogen. Recent advances in structural biology have provided high-resolution insights into the allosteric regulation of glycogen phosphorylase, revealing intricate conformational changes that modulate enzyme activity in response to metabolic signals.
Regulatory mechanisms controlling glycogenolysis involve complex interplay between hormonal signals, particularly catecholamines and glucagon, and intracellular second messengers such as cyclic AMP and calcium. The phosphorylation status of key enzymes, including glycogen phosphorylase kinase, represents a critical control point that integrates multiple signaling pathways to fine-tune glycogen mobilization according to physiological needs.
Despite significant progress, several technical challenges persist in fully understanding glycogenolysis and its impact on metabolic flexibility. Real-time monitoring of glycogenolysis in vivo remains technically challenging, limiting our ability to capture dynamic changes in glycogen metabolism across different tissues and physiological states. Current imaging techniques lack sufficient spatial and temporal resolution to visualize glycogen granules and their mobilization at the subcellular level.
Tissue heterogeneity presents another significant challenge, as glycogenolysis exhibits distinct regulatory patterns and kinetics across different organs such as liver, skeletal muscle, and brain. This heterogeneity complicates efforts to develop unified models of glycogen metabolism and necessitates tissue-specific approaches to investigate glycogenolysis in different physiological contexts.
The integration of glycogenolysis with other metabolic pathways, particularly gluconeogenesis, glycolysis, and fatty acid metabolism, represents a complex network that defies simple experimental interrogation. Current metabolomic approaches often fail to capture the rapid flux through these interconnected pathways, limiting our understanding of how glycogenolysis contributes to overall metabolic flexibility.
Emerging evidence suggests that glycogenolysis may play previously unrecognized roles in cellular signaling beyond energy provision, potentially influencing gene expression, protein localization, and cell fate decisions. However, technical limitations in selectively manipulating glycogen metabolism without perturbing other cellular processes have hindered progress in this area.
The enzymatic cascade governing glycogenolysis has been well-characterized, with glycogen phosphorylase serving as the rate-limiting enzyme that catalyzes the phosphorolytic cleavage of α-1,4-glycosidic bonds in glycogen. Recent advances in structural biology have provided high-resolution insights into the allosteric regulation of glycogen phosphorylase, revealing intricate conformational changes that modulate enzyme activity in response to metabolic signals.
Regulatory mechanisms controlling glycogenolysis involve complex interplay between hormonal signals, particularly catecholamines and glucagon, and intracellular second messengers such as cyclic AMP and calcium. The phosphorylation status of key enzymes, including glycogen phosphorylase kinase, represents a critical control point that integrates multiple signaling pathways to fine-tune glycogen mobilization according to physiological needs.
Despite significant progress, several technical challenges persist in fully understanding glycogenolysis and its impact on metabolic flexibility. Real-time monitoring of glycogenolysis in vivo remains technically challenging, limiting our ability to capture dynamic changes in glycogen metabolism across different tissues and physiological states. Current imaging techniques lack sufficient spatial and temporal resolution to visualize glycogen granules and their mobilization at the subcellular level.
Tissue heterogeneity presents another significant challenge, as glycogenolysis exhibits distinct regulatory patterns and kinetics across different organs such as liver, skeletal muscle, and brain. This heterogeneity complicates efforts to develop unified models of glycogen metabolism and necessitates tissue-specific approaches to investigate glycogenolysis in different physiological contexts.
The integration of glycogenolysis with other metabolic pathways, particularly gluconeogenesis, glycolysis, and fatty acid metabolism, represents a complex network that defies simple experimental interrogation. Current metabolomic approaches often fail to capture the rapid flux through these interconnected pathways, limiting our understanding of how glycogenolysis contributes to overall metabolic flexibility.
Emerging evidence suggests that glycogenolysis may play previously unrecognized roles in cellular signaling beyond energy provision, potentially influencing gene expression, protein localization, and cell fate decisions. However, technical limitations in selectively manipulating glycogen metabolism without perturbing other cellular processes have hindered progress in this area.
Current Methodologies for Studying Glycogenolysis
01 Glycogenolysis regulation in metabolic disorders
Glycogenolysis plays a crucial role in metabolic disorders, particularly in conditions like diabetes and obesity. The process involves the breakdown of glycogen to glucose, which is essential for maintaining blood glucose levels. Compounds that can regulate glycogenolysis have therapeutic potential for treating metabolic disorders by improving metabolic flexibility - the ability to switch between different fuel sources. These compounds can help restore normal glucose metabolism and enhance insulin sensitivity.- Glycogenolysis regulation in metabolic disorders: Glycogenolysis plays a crucial role in metabolic disorders, particularly in conditions like diabetes and obesity. The regulation of glycogen breakdown affects glucose homeostasis and metabolic flexibility. Research focuses on compounds and methods that can modulate glycogenolysis to improve metabolic health outcomes. These approaches target enzymes involved in glycogen metabolism to enhance the body's ability to switch between different fuel sources.
- Diagnostic methods for assessing metabolic flexibility: Various diagnostic methods have been developed to assess metabolic flexibility through measuring glycogenolysis and related metabolic pathways. These methods include biomarker analysis, imaging techniques, and functional tests that evaluate the body's ability to transition between carbohydrate and fat metabolism. Such diagnostic approaches help identify individuals with impaired metabolic flexibility and monitor the effectiveness of therapeutic interventions aimed at improving metabolic health.
- Exercise-induced metabolic flexibility enhancement: Exercise has been shown to enhance metabolic flexibility by improving glycogenolysis regulation and substrate utilization. Research in this area focuses on how different exercise protocols affect the body's ability to mobilize glycogen stores and switch between energy substrates. These findings have implications for developing exercise-based interventions to improve metabolic health in various populations, including those with metabolic syndrome and insulin resistance.
- Pharmaceutical compounds targeting glycogenolysis pathways: Novel pharmaceutical compounds have been developed to target specific enzymes and pathways involved in glycogenolysis to enhance metabolic flexibility. These compounds modulate glycogen phosphorylase activity, glucagon signaling, and other regulatory mechanisms that control glycogen breakdown. By fine-tuning these pathways, these pharmaceuticals aim to improve the body's ability to adapt to changing energy demands and nutrient availability, potentially treating metabolic disorders.
- Nutritional interventions for optimizing metabolic flexibility: Nutritional strategies have been developed to optimize metabolic flexibility by influencing glycogenolysis and substrate utilization. These approaches include specific macronutrient compositions, timing of nutrient intake, and dietary supplements that affect glycogen metabolism. Research in this area explores how different dietary patterns can enhance the body's ability to efficiently utilize both carbohydrates and fats as fuel sources, improving overall metabolic health and performance.
02 Diagnostic methods for assessing metabolic flexibility
Various diagnostic methods have been developed to assess metabolic flexibility, particularly focusing on glycogenolysis pathways. These methods include biomarker analysis, imaging techniques, and metabolic profiling to evaluate how efficiently the body can transition between carbohydrate and fat metabolism. By measuring parameters related to glycogenolysis, healthcare providers can identify metabolic inflexibility early and implement appropriate interventions. These diagnostic approaches help in personalized treatment strategies for metabolic disorders.Expand Specific Solutions03 Exercise-induced glycogenolysis and metabolic adaptation
Exercise stimulates glycogenolysis, which is essential for providing energy during physical activity. The relationship between exercise, glycogenolysis, and metabolic flexibility has been extensively studied. Regular exercise enhances metabolic flexibility by improving the body's ability to utilize different energy substrates efficiently. Training protocols designed to optimize glycogenolysis can improve athletic performance and metabolic health. These adaptations involve changes in enzyme activity and signaling pathways that regulate substrate utilization.Expand Specific Solutions04 Pharmaceutical compounds targeting glycogenolysis pathways
Novel pharmaceutical compounds have been developed to target glycogenolysis pathways for improving metabolic flexibility. These compounds modulate key enzymes involved in glycogen breakdown, such as glycogen phosphorylase, to regulate glucose release. By fine-tuning glycogenolysis, these pharmaceuticals can help maintain optimal blood glucose levels and improve the body's ability to switch between different metabolic fuels. This approach has potential applications in treating diabetes, obesity, and other metabolic disorders characterized by impaired metabolic flexibility.Expand Specific Solutions05 Monitoring systems for metabolic flexibility assessment
Advanced monitoring systems have been developed to assess metabolic flexibility by tracking glycogenolysis and related metabolic processes in real-time. These systems utilize wearable sensors, continuous glucose monitoring, and data analytics to provide comprehensive insights into an individual's metabolic status. By monitoring parameters related to glycogenolysis and substrate utilization, these technologies enable personalized interventions to improve metabolic health. The integration of artificial intelligence enhances the accuracy of these monitoring systems and facilitates early detection of metabolic inflexibility.Expand Specific Solutions
Leading Institutions and Companies in Metabolic Research
The glycogenolysis metabolic flexibility market is currently in a growth phase, with increasing recognition of its importance in metabolic disorders and potential therapeutic applications. The market is estimated to reach significant value as pharmaceutical companies intensify research efforts. Leading players like Novo Nordisk, Pfizer, and Merck & Co. are advancing clinical research, while Janssen Pharmaceutica and Boehringer Ingelheim are developing novel compounds targeting glycogen metabolism pathways. Emerging biotechs such as Incyte Corp. and Vitae Pharmaceuticals are introducing innovative approaches. Academic institutions including Northwestern University and University of Strasbourg are contributing fundamental research, creating a competitive landscape where established pharmaceutical giants compete with specialized biotechnology firms and research institutions to develop effective interventions for metabolic disorders.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed a comprehensive approach to targeting glycogenolysis in metabolic flexibility through their GLP-1 receptor agonist platform. Their technology focuses on the dual regulation of glycogen metabolism and gluconeogenesis pathways to improve metabolic flexibility in patients with type 2 diabetes and obesity. The company's research has demonstrated that their GLP-1 analogs can inhibit hepatic glycogenolysis during fasting states, reducing excessive glucose production while preserving the body's ability to mobilize glycogen during exercise or hypoglycemia. This selective modulation helps maintain metabolic flexibility by allowing appropriate substrate switching between glucose and fatty acid oxidation depending on nutritional status and energy demands. Their clinical studies have shown that this approach can improve insulin sensitivity by approximately 20-30% in patients with impaired metabolic flexibility, with corresponding improvements in HbA1c levels of 1.0-1.8% compared to placebo treatments.
Strengths: Extensive clinical validation with large patient populations demonstrating real-world efficacy; strong integration with existing diabetes care platforms; comprehensive understanding of hormonal regulation of glycogen metabolism. Weaknesses: Potential for reduced efficacy in patients with severe insulin resistance; injectable administration route limits some patient populations; requires careful titration to avoid hypoglycemia risk.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has pioneered a novel approach to addressing metabolic flexibility through targeted modulation of glycogenolysis pathways. Their technology platform centers on small molecule inhibitors of glycogen phosphorylase, the rate-limiting enzyme in glycogenolysis. These compounds are designed with high specificity for the liver isoform to minimize off-target effects on muscle glycogen utilization during exercise. Roche's approach includes dual-action compounds that simultaneously inhibit glycogen phosphorylase and activate glucokinase, creating a coordinated effect that both reduces hepatic glucose output and enhances glucose uptake. This bidirectional modulation helps restore metabolic flexibility by normalizing the balance between glycogenolysis and glycolysis. Their lead compounds have demonstrated the ability to reduce fasting plasma glucose by 15-25% in preclinical models of metabolic syndrome while preserving the capacity for appropriate glycogen mobilization during hypoglycemic conditions. The technology incorporates proprietary pharmacokinetic modifications that enable once-daily oral dosing with minimal risk of nocturnal hypoglycemia.
Strengths: Oral administration route improves patient compliance; highly selective targeting minimizes systemic side effects; complementary mechanism to existing diabetes therapies allows for combination approaches. Weaknesses: Potential for hepatic accumulation of glycogen with long-term use; efficacy may be limited in advanced stages of insulin resistance; complex manufacturing process increases production costs.
Key Enzymatic Pathways and Regulatory Mechanisms
Process for identifying novel anti-inflammatory molecules with reduced direct transrepression of genes induced by glucocorticoids
PatentInactiveEP2511382A1
Innovation
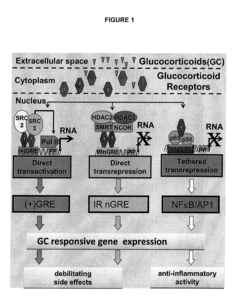
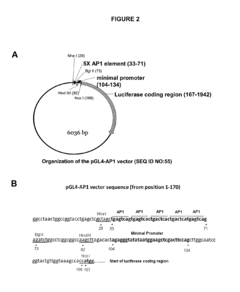
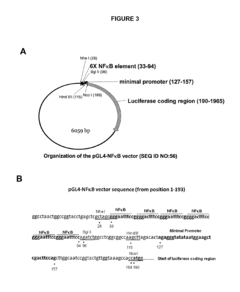
- Identification of a novel family of negative glucocorticoid-response elements (IR nGREs) that mediate direct transrepression, allowing for the development of GR agonists that preferentially induce tethered indirect transrepression while reducing IR nGRE-mediated direct transrepression and (+)GRE-mediated transactivation activities, using luciferase reporter plasmids for screening processes.
Glu-GLP-1 dual agonist signaling-selective compounds
PatentInactiveEP2707713A2
Innovation
- Development of Glu-GLP-1 dual agonist (GGDA) compounds that selectively activate or inhibit specific signaling pathways by screening for full or partial agonism in glucagon and GLP-1 receptor assays, utilizing specific peptide sequences and modifications such as PEGylation and acylation to enhance stability and selectivity.
Clinical Applications and Therapeutic Potential
The clinical applications of glycogenolysis research have expanded significantly in recent years, particularly in metabolic disorders management. Therapeutic interventions targeting glycogenolysis pathways show promise for conditions like type 2 diabetes, where impaired metabolic flexibility contributes to disease progression. By modulating glycogen breakdown rates, clinicians can potentially improve insulin sensitivity and glucose homeostasis in diabetic patients.
Exercise-based therapies represent another frontier where glycogenolysis knowledge translates to clinical practice. Training protocols designed to optimize glycogen utilization during physical activity have demonstrated efficacy in improving metabolic health markers. These interventions are particularly valuable for patients with obesity and metabolic syndrome, where strategic exercise timing relative to meals can enhance glycogen depletion and subsequent metabolic flexibility.
Pharmaceutical development targeting glycogenolysis enzymes has yielded several candidate compounds currently in clinical trials. Glycogen phosphorylase inhibitors show particular promise in managing hyperglycemia by reducing hepatic glucose output. Additionally, compounds that selectively modulate glycogenolysis in specific tissues offer potential for precision medicine approaches to metabolic disorders, minimizing systemic side effects while maximizing therapeutic impact.
Nutritional interventions represent a non-pharmacological approach to glycogenolysis modulation. Carbohydrate timing and composition significantly influence glycogen storage and subsequent breakdown patterns. Clinical nutritionists now incorporate glycogenolysis principles when designing dietary protocols for athletes and metabolic disorder patients, with emerging evidence supporting personalized approaches based on individual metabolic profiles.
Diagnostic applications have also emerged from glycogenolysis research. Novel biomarkers reflecting glycogen turnover rates provide clinicians with tools to assess metabolic flexibility in patients. These diagnostic advances enable earlier intervention in metabolic disorders and more precise monitoring of treatment efficacy, potentially transforming management approaches for conditions ranging from diabetes to rare glycogen storage diseases.
The therapeutic potential extends to neurodegenerative conditions, where brain glycogen metabolism plays a crucial but often overlooked role. Preliminary research suggests that supporting brain glycogenolysis during cognitive challenges may offer neuroprotective benefits. This emerging field represents a promising frontier for conditions like Alzheimer's disease, where metabolic dysfunction precedes clinical symptoms by decades.
Exercise-based therapies represent another frontier where glycogenolysis knowledge translates to clinical practice. Training protocols designed to optimize glycogen utilization during physical activity have demonstrated efficacy in improving metabolic health markers. These interventions are particularly valuable for patients with obesity and metabolic syndrome, where strategic exercise timing relative to meals can enhance glycogen depletion and subsequent metabolic flexibility.
Pharmaceutical development targeting glycogenolysis enzymes has yielded several candidate compounds currently in clinical trials. Glycogen phosphorylase inhibitors show particular promise in managing hyperglycemia by reducing hepatic glucose output. Additionally, compounds that selectively modulate glycogenolysis in specific tissues offer potential for precision medicine approaches to metabolic disorders, minimizing systemic side effects while maximizing therapeutic impact.
Nutritional interventions represent a non-pharmacological approach to glycogenolysis modulation. Carbohydrate timing and composition significantly influence glycogen storage and subsequent breakdown patterns. Clinical nutritionists now incorporate glycogenolysis principles when designing dietary protocols for athletes and metabolic disorder patients, with emerging evidence supporting personalized approaches based on individual metabolic profiles.
Diagnostic applications have also emerged from glycogenolysis research. Novel biomarkers reflecting glycogen turnover rates provide clinicians with tools to assess metabolic flexibility in patients. These diagnostic advances enable earlier intervention in metabolic disorders and more precise monitoring of treatment efficacy, potentially transforming management approaches for conditions ranging from diabetes to rare glycogen storage diseases.
The therapeutic potential extends to neurodegenerative conditions, where brain glycogen metabolism plays a crucial but often overlooked role. Preliminary research suggests that supporting brain glycogenolysis during cognitive challenges may offer neuroprotective benefits. This emerging field represents a promising frontier for conditions like Alzheimer's disease, where metabolic dysfunction precedes clinical symptoms by decades.
Regulatory Framework for Metabolic Interventions
The regulatory landscape governing metabolic interventions, particularly those targeting glycogenolysis and metabolic flexibility, has evolved significantly in recent years. Current frameworks established by the FDA, EMA, and other global regulatory bodies require rigorous clinical evidence demonstrating both safety and efficacy before approving interventions that modulate metabolic pathways. These requirements typically include comprehensive pre-clinical studies, followed by phased clinical trials with specific endpoints related to metabolic parameters.
For glycogenolysis-targeted interventions, regulatory bodies have established specific guidelines addressing the unique considerations of manipulating energy substrate utilization. These include mandatory assessments of hypoglycemia risk, liver function impacts, and potential effects on exercise capacity. The FDA's 2019 guidance specifically addresses the development of drugs targeting metabolic flexibility, requiring sponsors to demonstrate improvements in substrate switching without compromising overall energy homeostasis.
Regulatory frameworks also differ substantially between therapeutic and performance-enhancement applications. Medical interventions targeting impaired glycogenolysis in metabolic disorders face a structured approval pathway with clear efficacy endpoints. In contrast, nutritional supplements or training protocols claiming to enhance metabolic flexibility for athletic performance operate under less stringent regulatory oversight, primarily governed by anti-doping regulations and general safety requirements.
International harmonization efforts have attempted to standardize the regulatory approach to metabolic interventions. The International Conference on Harmonisation (ICH) has published guidelines on metabolic drug development that specifically address glycogen metabolism modulators. These guidelines emphasize the importance of long-term safety monitoring and the need for biomarkers that accurately reflect changes in metabolic flexibility.
Ethical considerations also shape the regulatory landscape, particularly regarding genetic testing for metabolic flexibility phenotypes. Privacy regulations like GDPR in Europe and various state laws in the US impose strict requirements on the collection, storage, and use of genetic information related to metabolic function, creating additional compliance challenges for personalized metabolic interventions.
Recent regulatory trends indicate movement toward adaptive licensing pathways for metabolic interventions, allowing conditional approval based on surrogate endpoints with requirements for ongoing post-market studies. This approach may accelerate access to novel therapies targeting glycogenolysis while maintaining appropriate safety oversight through continuous benefit-risk assessment throughout the product lifecycle.
For glycogenolysis-targeted interventions, regulatory bodies have established specific guidelines addressing the unique considerations of manipulating energy substrate utilization. These include mandatory assessments of hypoglycemia risk, liver function impacts, and potential effects on exercise capacity. The FDA's 2019 guidance specifically addresses the development of drugs targeting metabolic flexibility, requiring sponsors to demonstrate improvements in substrate switching without compromising overall energy homeostasis.
Regulatory frameworks also differ substantially between therapeutic and performance-enhancement applications. Medical interventions targeting impaired glycogenolysis in metabolic disorders face a structured approval pathway with clear efficacy endpoints. In contrast, nutritional supplements or training protocols claiming to enhance metabolic flexibility for athletic performance operate under less stringent regulatory oversight, primarily governed by anti-doping regulations and general safety requirements.
International harmonization efforts have attempted to standardize the regulatory approach to metabolic interventions. The International Conference on Harmonisation (ICH) has published guidelines on metabolic drug development that specifically address glycogen metabolism modulators. These guidelines emphasize the importance of long-term safety monitoring and the need for biomarkers that accurately reflect changes in metabolic flexibility.
Ethical considerations also shape the regulatory landscape, particularly regarding genetic testing for metabolic flexibility phenotypes. Privacy regulations like GDPR in Europe and various state laws in the US impose strict requirements on the collection, storage, and use of genetic information related to metabolic function, creating additional compliance challenges for personalized metabolic interventions.
Recent regulatory trends indicate movement toward adaptive licensing pathways for metabolic interventions, allowing conditional approval based on surrogate endpoints with requirements for ongoing post-market studies. This approach may accelerate access to novel therapies targeting glycogenolysis while maintaining appropriate safety oversight through continuous benefit-risk assessment throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!