How to Improve MRI Sensitivity with Ferrofluid Contrast Agents?
JUL 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MRI Ferrofluid Evolution
The evolution of ferrofluid contrast agents in MRI technology represents a significant advancement in medical imaging. Initially developed in the 1960s, ferrofluids have undergone substantial refinement to enhance their efficacy in MRI applications. The early stages of ferrofluid development focused on creating stable colloidal suspensions of magnetic nanoparticles, primarily iron oxides, in carrier liquids.
In the 1980s and 1990s, researchers began exploring the potential of ferrofluids as MRI contrast agents. The first generation of these agents utilized relatively large iron oxide particles, which limited their effectiveness due to rapid clearance from the bloodstream. However, this period saw crucial advancements in nanoparticle synthesis and surface modification techniques, laying the groundwork for more sophisticated contrast agents.
The turn of the millennium marked a significant leap forward with the introduction of superparamagnetic iron oxide nanoparticles (SPIONs). These particles, typically ranging from 10 to 100 nm in size, exhibited superior magnetic properties and improved biocompatibility. The development of SPIONs led to enhanced T2 and T2* contrast in MRI, particularly beneficial for liver and lymph node imaging.
From 2005 to 2015, research efforts concentrated on optimizing particle size, shape, and surface coatings to improve circulation time and targeting capabilities. This period saw the emergence of ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs), which demonstrated longer blood half-lives and the ability to penetrate smaller vasculature, expanding their potential applications.
Recent years have witnessed a shift towards multifunctional ferrofluid contrast agents. These advanced formulations combine imaging capabilities with therapeutic functions, paving the way for theranostic applications. Innovations in this area include the development of pH-responsive ferrofluids for targeted drug delivery and thermosensitive ferrofluids for hyperthermia treatment.
The latest frontier in ferrofluid evolution involves the integration of artificial intelligence and machine learning algorithms to optimize contrast agent design and image interpretation. This approach promises to enhance the sensitivity and specificity of MRI diagnostics further, potentially revolutionizing early disease detection and personalized medicine.
Throughout this evolution, continuous improvements in synthesis methods, surface functionalization, and biocompatibility have been crucial. These advancements have not only enhanced the imaging capabilities of ferrofluid contrast agents but also addressed safety concerns, making them increasingly viable for clinical use.
In the 1980s and 1990s, researchers began exploring the potential of ferrofluids as MRI contrast agents. The first generation of these agents utilized relatively large iron oxide particles, which limited their effectiveness due to rapid clearance from the bloodstream. However, this period saw crucial advancements in nanoparticle synthesis and surface modification techniques, laying the groundwork for more sophisticated contrast agents.
The turn of the millennium marked a significant leap forward with the introduction of superparamagnetic iron oxide nanoparticles (SPIONs). These particles, typically ranging from 10 to 100 nm in size, exhibited superior magnetic properties and improved biocompatibility. The development of SPIONs led to enhanced T2 and T2* contrast in MRI, particularly beneficial for liver and lymph node imaging.
From 2005 to 2015, research efforts concentrated on optimizing particle size, shape, and surface coatings to improve circulation time and targeting capabilities. This period saw the emergence of ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs), which demonstrated longer blood half-lives and the ability to penetrate smaller vasculature, expanding their potential applications.
Recent years have witnessed a shift towards multifunctional ferrofluid contrast agents. These advanced formulations combine imaging capabilities with therapeutic functions, paving the way for theranostic applications. Innovations in this area include the development of pH-responsive ferrofluids for targeted drug delivery and thermosensitive ferrofluids for hyperthermia treatment.
The latest frontier in ferrofluid evolution involves the integration of artificial intelligence and machine learning algorithms to optimize contrast agent design and image interpretation. This approach promises to enhance the sensitivity and specificity of MRI diagnostics further, potentially revolutionizing early disease detection and personalized medicine.
Throughout this evolution, continuous improvements in synthesis methods, surface functionalization, and biocompatibility have been crucial. These advancements have not only enhanced the imaging capabilities of ferrofluid contrast agents but also addressed safety concerns, making them increasingly viable for clinical use.
Clinical Demand Analysis
The clinical demand for improved MRI sensitivity using ferrofluid contrast agents is driven by the need for more accurate and detailed imaging in various medical fields. Magnetic Resonance Imaging (MRI) has become an indispensable tool in modern medicine, offering non-invasive visualization of internal body structures. However, there is a constant push for higher resolution and better contrast to detect smaller lesions, characterize tissue properties more precisely, and enable earlier disease detection.
In oncology, enhanced MRI sensitivity could lead to the detection of smaller tumors and metastases, potentially improving cancer staging and treatment planning. This is particularly crucial in brain cancer imaging, where precise delineation of tumor boundaries can significantly impact surgical outcomes and radiation therapy planning. The ability to detect microscopic tumor spread could revolutionize cancer management strategies.
Cardiovascular imaging stands to benefit greatly from improved MRI sensitivity. Enhanced visualization of blood flow patterns, vessel wall abnormalities, and myocardial perfusion could lead to earlier detection of cardiovascular diseases and more accurate assessment of treatment efficacy. This is especially important in diagnosing and monitoring conditions such as atherosclerosis, coronary artery disease, and heart valve disorders.
Neurological applications of high-sensitivity MRI are vast and growing. Improved imaging could aid in the early detection of neurodegenerative diseases such as Alzheimer's and Parkinson's, potentially before clinical symptoms manifest. It could also enhance the understanding of brain function and connectivity, benefiting both clinical practice and neuroscience research.
In musculoskeletal imaging, increased MRI sensitivity could improve the detection of subtle cartilage defects, ligament tears, and early signs of arthritis. This would enable earlier intervention and more personalized treatment strategies in orthopedics and rheumatology.
The demand for pediatric imaging with minimal radiation exposure makes MRI an attractive option. Enhanced sensitivity could reduce the need for sedation or anesthesia in young patients by shortening scan times while maintaining or improving image quality.
Molecular imaging is an emerging field that could be revolutionized by more sensitive MRI techniques. The ability to detect and quantify specific molecular markers in vivo could provide invaluable insights into disease processes, drug efficacy, and personalized medicine approaches.
As healthcare moves towards preventive and personalized medicine, the demand for high-sensitivity imaging techniques is expected to grow. Improved MRI sensitivity could enable population screening for various diseases, allowing for earlier interventions and potentially reducing healthcare costs in the long term.
In oncology, enhanced MRI sensitivity could lead to the detection of smaller tumors and metastases, potentially improving cancer staging and treatment planning. This is particularly crucial in brain cancer imaging, where precise delineation of tumor boundaries can significantly impact surgical outcomes and radiation therapy planning. The ability to detect microscopic tumor spread could revolutionize cancer management strategies.
Cardiovascular imaging stands to benefit greatly from improved MRI sensitivity. Enhanced visualization of blood flow patterns, vessel wall abnormalities, and myocardial perfusion could lead to earlier detection of cardiovascular diseases and more accurate assessment of treatment efficacy. This is especially important in diagnosing and monitoring conditions such as atherosclerosis, coronary artery disease, and heart valve disorders.
Neurological applications of high-sensitivity MRI are vast and growing. Improved imaging could aid in the early detection of neurodegenerative diseases such as Alzheimer's and Parkinson's, potentially before clinical symptoms manifest. It could also enhance the understanding of brain function and connectivity, benefiting both clinical practice and neuroscience research.
In musculoskeletal imaging, increased MRI sensitivity could improve the detection of subtle cartilage defects, ligament tears, and early signs of arthritis. This would enable earlier intervention and more personalized treatment strategies in orthopedics and rheumatology.
The demand for pediatric imaging with minimal radiation exposure makes MRI an attractive option. Enhanced sensitivity could reduce the need for sedation or anesthesia in young patients by shortening scan times while maintaining or improving image quality.
Molecular imaging is an emerging field that could be revolutionized by more sensitive MRI techniques. The ability to detect and quantify specific molecular markers in vivo could provide invaluable insights into disease processes, drug efficacy, and personalized medicine approaches.
As healthcare moves towards preventive and personalized medicine, the demand for high-sensitivity imaging techniques is expected to grow. Improved MRI sensitivity could enable population screening for various diseases, allowing for earlier interventions and potentially reducing healthcare costs in the long term.
Ferrofluid MRI Challenges
The use of ferrofluid contrast agents in Magnetic Resonance Imaging (MRI) presents several significant challenges that researchers and medical professionals must address to improve MRI sensitivity effectively. These challenges span across various domains, including material science, biocompatibility, and imaging technology.
One of the primary challenges lies in the development of ferrofluid contrast agents with optimal magnetic properties. The magnetic nanoparticles within the ferrofluid must possess sufficient magnetic susceptibility to enhance MRI contrast while maintaining stability in physiological conditions. Achieving the right balance between particle size, composition, and magnetic behavior is crucial for maximizing sensitivity without compromising image quality or patient safety.
Biocompatibility and toxicity concerns pose another significant hurdle. Ferrofluids introduced into the body must be non-toxic, non-immunogenic, and easily cleared from the system after imaging. Ensuring that these contrast agents do not accumulate in organs or tissues over time is essential to prevent potential long-term health effects. Additionally, the surface chemistry of the nanoparticles must be carefully engineered to prevent aggregation and maintain colloidal stability in biological environments.
The biodistribution and targeting efficiency of ferrofluid contrast agents present further challenges. Developing methods to direct these agents to specific tissues or organs of interest while minimizing non-specific uptake is crucial for improving MRI sensitivity. This requires innovative approaches in nanoparticle functionalization and the incorporation of targeting moieties that can selectively bind to desired biological markers.
From an imaging perspective, optimizing MRI pulse sequences and hardware to fully exploit the unique properties of ferrofluid contrast agents is a complex task. Conventional MRI protocols may not be ideally suited for these novel contrast agents, necessitating the development of specialized imaging techniques that can maximize sensitivity and contrast enhancement while minimizing artifacts.
The scalability and reproducibility of ferrofluid contrast agent production also pose significant challenges. Ensuring consistent quality, size distribution, and magnetic properties across different batches is crucial for reliable clinical applications. This requires the development of robust manufacturing processes and stringent quality control measures.
Regulatory hurdles and clinical translation represent another set of challenges. Obtaining regulatory approval for new contrast agents involves extensive safety and efficacy studies, which can be time-consuming and costly. Demonstrating clear advantages over existing contrast agents in terms of sensitivity, specificity, and safety is essential for successful clinical adoption.
Addressing these multifaceted challenges requires interdisciplinary collaboration between material scientists, chemists, biologists, and imaging experts. Overcoming these obstacles will pave the way for significant advancements in MRI technology, potentially revolutionizing diagnostic capabilities and improving patient outcomes.
One of the primary challenges lies in the development of ferrofluid contrast agents with optimal magnetic properties. The magnetic nanoparticles within the ferrofluid must possess sufficient magnetic susceptibility to enhance MRI contrast while maintaining stability in physiological conditions. Achieving the right balance between particle size, composition, and magnetic behavior is crucial for maximizing sensitivity without compromising image quality or patient safety.
Biocompatibility and toxicity concerns pose another significant hurdle. Ferrofluids introduced into the body must be non-toxic, non-immunogenic, and easily cleared from the system after imaging. Ensuring that these contrast agents do not accumulate in organs or tissues over time is essential to prevent potential long-term health effects. Additionally, the surface chemistry of the nanoparticles must be carefully engineered to prevent aggregation and maintain colloidal stability in biological environments.
The biodistribution and targeting efficiency of ferrofluid contrast agents present further challenges. Developing methods to direct these agents to specific tissues or organs of interest while minimizing non-specific uptake is crucial for improving MRI sensitivity. This requires innovative approaches in nanoparticle functionalization and the incorporation of targeting moieties that can selectively bind to desired biological markers.
From an imaging perspective, optimizing MRI pulse sequences and hardware to fully exploit the unique properties of ferrofluid contrast agents is a complex task. Conventional MRI protocols may not be ideally suited for these novel contrast agents, necessitating the development of specialized imaging techniques that can maximize sensitivity and contrast enhancement while minimizing artifacts.
The scalability and reproducibility of ferrofluid contrast agent production also pose significant challenges. Ensuring consistent quality, size distribution, and magnetic properties across different batches is crucial for reliable clinical applications. This requires the development of robust manufacturing processes and stringent quality control measures.
Regulatory hurdles and clinical translation represent another set of challenges. Obtaining regulatory approval for new contrast agents involves extensive safety and efficacy studies, which can be time-consuming and costly. Demonstrating clear advantages over existing contrast agents in terms of sensitivity, specificity, and safety is essential for successful clinical adoption.
Addressing these multifaceted challenges requires interdisciplinary collaboration between material scientists, chemists, biologists, and imaging experts. Overcoming these obstacles will pave the way for significant advancements in MRI technology, potentially revolutionizing diagnostic capabilities and improving patient outcomes.
Current Ferrofluid Tech
01 Magnetic nanoparticles as contrast agents
Ferrofluid contrast agents utilize magnetic nanoparticles to enhance imaging sensitivity in various medical imaging techniques. These nanoparticles can be engineered to have specific magnetic properties, allowing for improved contrast and detection of biological structures or processes. The sensitivity of these contrast agents can be optimized by controlling particle size, composition, and surface modifications.- Magnetic nanoparticles as contrast agents: Ferrofluid contrast agents utilize magnetic nanoparticles to enhance imaging sensitivity in medical diagnostics. These nanoparticles can be engineered to target specific tissues or organs, improving contrast and resolution in various imaging modalities such as MRI. The size, composition, and surface properties of the nanoparticles can be optimized to increase sensitivity and specificity of detection.
- Optical properties of ferrofluids for imaging: Ferrofluids exhibit unique optical properties that can be exploited for high-sensitivity imaging applications. When subjected to magnetic fields, these fluids can change their refractive index or light scattering properties, allowing for dynamic contrast enhancement. This principle is applied in various optical imaging techniques to improve sensitivity and resolution.
- Multimodal imaging with ferrofluid contrast agents: Ferrofluid contrast agents can be designed for use in multiple imaging modalities simultaneously, such as MRI and optical imaging. This multimodal approach enhances overall sensitivity by combining the strengths of different imaging techniques. The agents can be tailored to provide complementary information, improving diagnostic accuracy and sensitivity across various imaging platforms.
- Sensitivity enhancement through magnetic field manipulation: The sensitivity of ferrofluid contrast agents can be significantly improved by manipulating external magnetic fields. By applying specific field strengths or patterns, the magnetic properties of the ferrofluid can be optimized for maximum contrast. This approach allows for dynamic adjustment of sensitivity during imaging procedures, enhancing the detection of subtle anatomical or functional changes.
- Biocompatibility and targeted delivery for improved sensitivity: Enhancing the biocompatibility and targeted delivery of ferrofluid contrast agents can significantly improve their sensitivity in vivo. Surface modifications and functionalization of the nanoparticles can reduce non-specific interactions, increase circulation time, and promote accumulation in target tissues. This approach leads to higher local concentrations of the contrast agent, resulting in improved signal-to-noise ratios and overall sensitivity in diagnostic imaging.
02 Ferrofluid-based optical devices
Ferrofluids are used in optical devices to enhance sensitivity and control light transmission. These devices utilize the magnetic properties of ferrofluids to create tunable optical elements, such as lenses or filters. The sensitivity of these devices can be adjusted by manipulating the ferrofluid's response to external magnetic fields, allowing for precise control of optical properties.Expand Specific Solutions03 Ferrofluid-enhanced MRI contrast
Ferrofluid contrast agents are employed to improve the sensitivity of Magnetic Resonance Imaging (MRI). These agents can be designed to target specific tissues or organs, enhancing local contrast and allowing for better visualization of anatomical structures or pathological conditions. The sensitivity of MRI can be further improved by optimizing the magnetic properties and biodistribution of the ferrofluid contrast agents.Expand Specific Solutions04 Ferrofluid-based sensors and actuators
Ferrofluids are utilized in the development of highly sensitive sensors and actuators. These devices leverage the unique properties of ferrofluids to detect small changes in magnetic fields or to generate precise mechanical movements. The sensitivity of these sensors and actuators can be enhanced by optimizing the ferrofluid composition and the design of the surrounding magnetic structures.Expand Specific Solutions05 Biocompatibility and targeting of ferrofluid contrast agents
Improving the biocompatibility and targeting capabilities of ferrofluid contrast agents is crucial for enhancing their sensitivity in biological applications. This involves developing surface modifications that reduce toxicity, improve stability in physiological conditions, and enable specific binding to target molecules or cells. These advancements allow for more sensitive and selective imaging or therapeutic applications using ferrofluid-based contrast agents.Expand Specific Solutions
Key MRI Industry Players
The development of ferrofluid contrast agents for improving MRI sensitivity is in an early stage, with significant potential for growth. The market size is expanding as researchers explore novel applications in medical imaging. While the technology is still evolving, several key players are driving innovation. Companies like Bayer Schering Pharma AG and EPIX Pharmaceuticals are leveraging their expertise in contrast agents to develop advanced ferrofluid-based solutions. Academic institutions such as Northwestern University and Case Western Reserve University are conducting cutting-edge research to enhance MRI sensitivity. Collaborations between industry and academia are accelerating progress, with potential for breakthrough advancements in diagnostic imaging capabilities.
Schering AG
Technical Solution: Schering AG has developed innovative ferrofluid contrast agents for MRI, focusing on superparamagnetic iron oxide nanoparticles (SPIONs). Their approach involves coating SPIONs with biocompatible materials to enhance stability and reduce toxicity. The company has optimized particle size distribution to achieve maximum T2 relaxivity, resulting in improved negative contrast enhancement. Their ferrofluid agents demonstrate high magnetic susceptibility, allowing for lower dosages while maintaining excellent image quality. Schering AG has also explored functionalization of these nanoparticles for targeted imaging, potentially enabling early detection of specific pathologies[1][3].
Strengths: High relaxivity, reduced toxicity, and potential for targeted imaging. Weaknesses: Limited to T2-weighted imaging, potential for long-term retention in the body.
Washington University in St. Louis
Technical Solution: Researchers at Washington University in St. Louis have developed innovative approaches to improve MRI sensitivity using ferrofluid contrast agents. Their work focuses on the synthesis of novel iron oxide nanoparticles with enhanced magnetic properties and improved biocompatibility. They have explored the use of various coating materials and surface modifications to optimize the pharmacokinetics and biodistribution of the contrast agents. The team has also investigated the potential of using ferrofluid contrast agents in conjunction with advanced MRI techniques such as magnetic particle imaging (MPI) and magnetic resonance fingerprinting (MRF) to further enhance sensitivity and specificity. Additionally, they have conducted extensive in vivo studies to evaluate the safety and efficacy of their ferrofluid contrast agents in various disease models[7][8].
Strengths: Innovative nanoparticle designs, integration with advanced MRI techniques, and comprehensive in vivo validation. Weaknesses: Potential challenges in scaling up production and regulatory approval for clinical use.
Ferrofluid Patent Review
Compositions and methods for magnetic resonance imaging contrast agents
PatentActiveUS20090053141A1
Innovation
- Development of monodisperse, protein polymer-based contrast agents with genetically engineered protein backbones capable of chelating multiple paramagnetic ions, such as gadolinium(III), to enhance MRI signal intensity and safety by being biodegradable and non-toxic.
Magnetic ferroferric oxide nanoparticle, and preparation method therefor and use thereof
PatentPendingEP4467161A1
Innovation
- Development of magnetic ferroferric oxide nanoparticles with a hydrophilic macromolecule, specifically coprecipitated to achieve a particle size between 2-5 nm, enhancing r1 values and reducing the r2/r1 ratio, improving water solubility and stability, and incorporating hydrophilic macromolecules like polyglutamic acid or polyaspartic acid for surface modification.
Safety and Regulations
The safety and regulatory aspects of using ferrofluid contrast agents to improve MRI sensitivity are of paramount importance in the development and implementation of this technology. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established stringent guidelines for the approval and use of contrast agents in medical imaging.
One of the primary safety concerns with ferrofluid contrast agents is their potential toxicity and long-term effects on the human body. Extensive preclinical and clinical studies are required to assess the pharmacokinetics, biodistribution, and elimination of these agents. Regulatory agencies demand comprehensive data on acute and chronic toxicity, as well as potential genotoxicity and carcinogenicity.
The size and composition of ferrofluid nanoparticles are critical factors in their safety profile. Particles must be designed to avoid accumulation in organs and tissues, and to facilitate efficient clearance from the body. Coating materials used to stabilize the nanoparticles must also be thoroughly evaluated for biocompatibility and potential immunogenicity.
Regulatory requirements extend to the manufacturing process of ferrofluid contrast agents. Good Manufacturing Practices (GMP) must be strictly adhered to, ensuring consistency, purity, and quality of the final product. Rigorous quality control measures are necessary to prevent contamination and ensure batch-to-batch reproducibility.
Clinical trials for ferrofluid contrast agents typically follow a phased approach, starting with small-scale safety studies and progressing to larger efficacy trials. These studies must demonstrate not only the improved sensitivity of MRI imaging but also an acceptable safety profile compared to existing contrast agents.
Post-market surveillance is another crucial aspect of the regulatory framework. Manufacturers are required to implement robust pharmacovigilance systems to monitor and report any adverse events associated with the use of ferrofluid contrast agents in clinical practice.
Environmental considerations also play a role in the regulatory landscape. The production, use, and disposal of ferrofluid contrast agents must comply with environmental protection regulations to minimize potential ecological impacts.
As the technology advances, regulatory agencies may need to adapt their guidelines to address the unique characteristics of ferrofluid contrast agents. This may involve developing new testing protocols or modifying existing ones to adequately assess the safety and efficacy of these novel agents.
One of the primary safety concerns with ferrofluid contrast agents is their potential toxicity and long-term effects on the human body. Extensive preclinical and clinical studies are required to assess the pharmacokinetics, biodistribution, and elimination of these agents. Regulatory agencies demand comprehensive data on acute and chronic toxicity, as well as potential genotoxicity and carcinogenicity.
The size and composition of ferrofluid nanoparticles are critical factors in their safety profile. Particles must be designed to avoid accumulation in organs and tissues, and to facilitate efficient clearance from the body. Coating materials used to stabilize the nanoparticles must also be thoroughly evaluated for biocompatibility and potential immunogenicity.
Regulatory requirements extend to the manufacturing process of ferrofluid contrast agents. Good Manufacturing Practices (GMP) must be strictly adhered to, ensuring consistency, purity, and quality of the final product. Rigorous quality control measures are necessary to prevent contamination and ensure batch-to-batch reproducibility.
Clinical trials for ferrofluid contrast agents typically follow a phased approach, starting with small-scale safety studies and progressing to larger efficacy trials. These studies must demonstrate not only the improved sensitivity of MRI imaging but also an acceptable safety profile compared to existing contrast agents.
Post-market surveillance is another crucial aspect of the regulatory framework. Manufacturers are required to implement robust pharmacovigilance systems to monitor and report any adverse events associated with the use of ferrofluid contrast agents in clinical practice.
Environmental considerations also play a role in the regulatory landscape. The production, use, and disposal of ferrofluid contrast agents must comply with environmental protection regulations to minimize potential ecological impacts.
As the technology advances, regulatory agencies may need to adapt their guidelines to address the unique characteristics of ferrofluid contrast agents. This may involve developing new testing protocols or modifying existing ones to adequately assess the safety and efficacy of these novel agents.
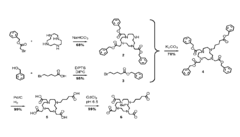
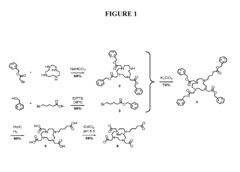
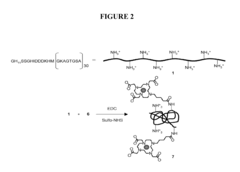
Nanoparticle Synthesis
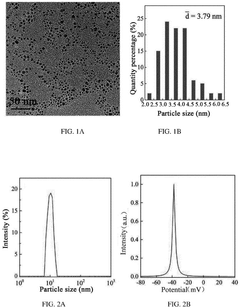
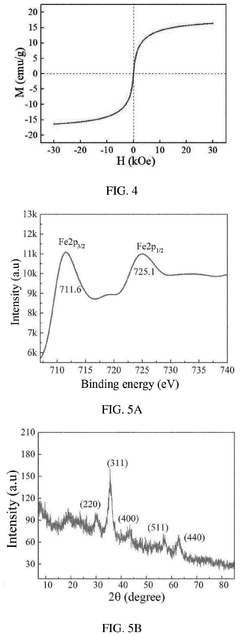
Nanoparticle synthesis plays a crucial role in developing ferrofluid contrast agents for improving MRI sensitivity. The process involves carefully controlled chemical reactions to produce magnetic nanoparticles with specific size, shape, and surface properties. These nanoparticles typically consist of iron oxide cores, such as magnetite (Fe3O4) or maghemite (γ-Fe2O3), coated with biocompatible materials.
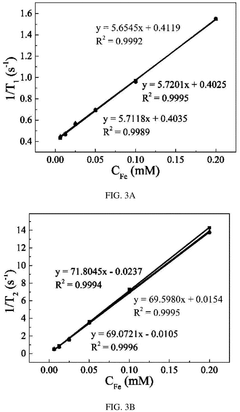
One common method for synthesizing magnetic nanoparticles is co-precipitation. This technique involves mixing ferric and ferrous salts in an alkaline solution under inert atmosphere. The reaction conditions, including pH, temperature, and ionic strength, are carefully controlled to achieve the desired particle size and magnetic properties. Co-precipitation offers advantages such as simplicity, scalability, and cost-effectiveness.
Thermal decomposition is another widely used method for producing high-quality magnetic nanoparticles. This process involves the decomposition of organometallic precursors in high-boiling organic solvents in the presence of surfactants. By adjusting reaction parameters like temperature, time, and precursor concentration, researchers can precisely control the size and shape of the nanoparticles.
Hydrothermal synthesis is a versatile technique that utilizes high-temperature and high-pressure conditions to produce magnetic nanoparticles. This method allows for the synthesis of nanoparticles with various morphologies, including spheres, cubes, and rods. The hydrothermal approach offers advantages in terms of particle crystallinity and uniformity.
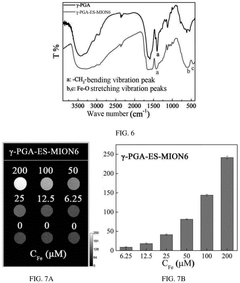
Surface modification of the synthesized nanoparticles is a critical step in developing effective ferrofluid contrast agents. Various coating materials, such as dextran, polyethylene glycol (PEG), and silica, are employed to enhance biocompatibility, stability, and functionality. These coatings also provide anchor points for attaching targeting ligands or therapeutic agents, enabling multifunctional applications.
Recent advancements in nanoparticle synthesis have focused on developing novel core-shell structures and hybrid nanoparticles. These innovations aim to combine the magnetic properties of iron oxide with other functional materials, such as gold or quantum dots, to create multimodal imaging agents with enhanced sensitivity and specificity.
Continuous efforts are being made to optimize synthesis protocols for large-scale production while maintaining precise control over nanoparticle properties. This includes the development of microfluidic and flow synthesis techniques, which offer improved reproducibility and scalability compared to traditional batch methods.
One common method for synthesizing magnetic nanoparticles is co-precipitation. This technique involves mixing ferric and ferrous salts in an alkaline solution under inert atmosphere. The reaction conditions, including pH, temperature, and ionic strength, are carefully controlled to achieve the desired particle size and magnetic properties. Co-precipitation offers advantages such as simplicity, scalability, and cost-effectiveness.
Thermal decomposition is another widely used method for producing high-quality magnetic nanoparticles. This process involves the decomposition of organometallic precursors in high-boiling organic solvents in the presence of surfactants. By adjusting reaction parameters like temperature, time, and precursor concentration, researchers can precisely control the size and shape of the nanoparticles.
Hydrothermal synthesis is a versatile technique that utilizes high-temperature and high-pressure conditions to produce magnetic nanoparticles. This method allows for the synthesis of nanoparticles with various morphologies, including spheres, cubes, and rods. The hydrothermal approach offers advantages in terms of particle crystallinity and uniformity.
Surface modification of the synthesized nanoparticles is a critical step in developing effective ferrofluid contrast agents. Various coating materials, such as dextran, polyethylene glycol (PEG), and silica, are employed to enhance biocompatibility, stability, and functionality. These coatings also provide anchor points for attaching targeting ligands or therapeutic agents, enabling multifunctional applications.
Recent advancements in nanoparticle synthesis have focused on developing novel core-shell structures and hybrid nanoparticles. These innovations aim to combine the magnetic properties of iron oxide with other functional materials, such as gold or quantum dots, to create multimodal imaging agents with enhanced sensitivity and specificity.
Continuous efforts are being made to optimize synthesis protocols for large-scale production while maintaining precise control over nanoparticle properties. This includes the development of microfluidic and flow synthesis techniques, which offer improved reproducibility and scalability compared to traditional batch methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!