How to Measure Oxaloacetate Concentration Using NMR
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Spectroscopy for Oxaloacetate Analysis: Background and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, becoming an indispensable analytical tool in biochemistry, metabolomics, and pharmaceutical research. The application of NMR for quantifying metabolites like oxaloacetate represents a critical advancement in understanding cellular metabolism and related pathological conditions. Oxaloacetate, a key intermediate in the tricarboxylic acid (TCA) cycle, plays a vital role in energy production and biosynthetic processes, making its accurate measurement essential for metabolic studies.
The technical evolution of NMR has progressed from simple one-dimensional proton spectroscopy to sophisticated multi-dimensional techniques, enabling researchers to overcome challenges associated with complex biological matrices. Modern high-field NMR spectrometers, operating at field strengths of 600 MHz and above, provide enhanced resolution and sensitivity necessary for detecting low-concentration metabolites like oxaloacetate in biological samples.
Recent advancements in pulse sequence design, including selective excitation methods and solvent suppression techniques, have significantly improved the specificity of NMR for oxaloacetate detection. The development of cryogenic probe technology has further revolutionized NMR sensitivity, reducing detection limits by an order of magnitude compared to conventional probes, which is particularly valuable for oxaloacetate analysis given its relatively low physiological concentrations.
The primary technical objective in oxaloacetate measurement via NMR is to achieve reliable quantification at physiologically relevant concentrations (typically micromolar range) while addressing challenges such as spectral overlap, chemical instability, and matrix effects. Researchers aim to develop standardized protocols that ensure reproducibility across different laboratory settings and sample types, from cell culture extracts to biofluids.
Another critical goal is the integration of NMR-based oxaloacetate measurements with other metabolomic data to provide comprehensive insights into metabolic pathway activities. This includes developing computational approaches for spectral deconvolution and statistical analysis that can handle the complexity of biological samples and extract meaningful biological information from NMR data.
The field is moving toward real-time monitoring of oxaloacetate dynamics in living systems, which requires overcoming significant technical hurdles related to sensitivity, temporal resolution, and in vivo applicability. Emerging hyperpolarization techniques like Dynamic Nuclear Polarization (DNP) show promise in dramatically enhancing NMR signals, potentially enabling real-time tracking of oxaloacetate metabolism in intact biological systems.
As metabolic disorders and diseases like cancer increasingly implicate altered TCA cycle activity, precise measurement of oxaloacetate concentrations has gained importance in both basic research and clinical applications. The technical trajectory suggests continued refinement of NMR methodologies specifically optimized for oxaloacetate quantification, with potential translation into diagnostic and therapeutic monitoring tools.
The technical evolution of NMR has progressed from simple one-dimensional proton spectroscopy to sophisticated multi-dimensional techniques, enabling researchers to overcome challenges associated with complex biological matrices. Modern high-field NMR spectrometers, operating at field strengths of 600 MHz and above, provide enhanced resolution and sensitivity necessary for detecting low-concentration metabolites like oxaloacetate in biological samples.
Recent advancements in pulse sequence design, including selective excitation methods and solvent suppression techniques, have significantly improved the specificity of NMR for oxaloacetate detection. The development of cryogenic probe technology has further revolutionized NMR sensitivity, reducing detection limits by an order of magnitude compared to conventional probes, which is particularly valuable for oxaloacetate analysis given its relatively low physiological concentrations.
The primary technical objective in oxaloacetate measurement via NMR is to achieve reliable quantification at physiologically relevant concentrations (typically micromolar range) while addressing challenges such as spectral overlap, chemical instability, and matrix effects. Researchers aim to develop standardized protocols that ensure reproducibility across different laboratory settings and sample types, from cell culture extracts to biofluids.
Another critical goal is the integration of NMR-based oxaloacetate measurements with other metabolomic data to provide comprehensive insights into metabolic pathway activities. This includes developing computational approaches for spectral deconvolution and statistical analysis that can handle the complexity of biological samples and extract meaningful biological information from NMR data.
The field is moving toward real-time monitoring of oxaloacetate dynamics in living systems, which requires overcoming significant technical hurdles related to sensitivity, temporal resolution, and in vivo applicability. Emerging hyperpolarization techniques like Dynamic Nuclear Polarization (DNP) show promise in dramatically enhancing NMR signals, potentially enabling real-time tracking of oxaloacetate metabolism in intact biological systems.
As metabolic disorders and diseases like cancer increasingly implicate altered TCA cycle activity, precise measurement of oxaloacetate concentrations has gained importance in both basic research and clinical applications. The technical trajectory suggests continued refinement of NMR methodologies specifically optimized for oxaloacetate quantification, with potential translation into diagnostic and therapeutic monitoring tools.
Market Applications and Demand for Oxaloacetate Measurement
The global market for oxaloacetate measurement technologies has witnessed significant growth in recent years, driven primarily by expanding applications in biomedical research, pharmaceutical development, and clinical diagnostics. Accurate quantification of oxaloacetate, a critical intermediate in the tricarboxylic acid (TCA) cycle, provides valuable insights into cellular metabolism and various pathological conditions.
In the pharmaceutical industry, demand for precise oxaloacetate measurement techniques has increased substantially as researchers investigate metabolic pathways associated with cancer, neurodegenerative disorders, and cardiovascular diseases. The market size for metabolomics research tools, including oxaloacetate measurement technologies, reached approximately $2.3 billion in 2022, with projected annual growth rates of 13.4% through 2028.
Clinical diagnostics represents another significant market segment, where oxaloacetate measurements serve as biomarkers for metabolic disorders, liver dysfunction, and mitochondrial diseases. Hospitals and diagnostic laboratories increasingly incorporate metabolite profiling into their testing panels, creating sustained demand for reliable measurement methods like NMR-based techniques.
The agricultural and food science sectors have emerged as unexpected growth areas for oxaloacetate measurement technologies. Researchers utilize these tools to monitor crop metabolism, assess food quality, and develop enhanced nutritional supplements. This diversification of applications has expanded the potential market reach beyond traditional biomedical boundaries.
Biotechnology companies focused on metabolic engineering and synthetic biology represent another significant market segment. These organizations require precise quantification of metabolic intermediates like oxaloacetate to optimize bioproduction pathways for pharmaceuticals, biofuels, and specialty chemicals. The growing emphasis on sustainable manufacturing processes has further accelerated demand in this sector.
Geographically, North America dominates the market for advanced metabolite measurement technologies, accounting for approximately 42% of global demand. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and India making substantial investments in metabolomics research infrastructure and capabilities.
Market analysis indicates that technologies offering higher sensitivity, reproducibility, and throughput have distinct competitive advantages. NMR-based methods for oxaloacetate measurement compete with mass spectrometry approaches, with each technology serving different market niches based on specific requirements for accuracy, sample preparation complexity, and cost considerations.
The increasing prevalence of metabolic disorders globally, coupled with growing research interest in cellular metabolism, suggests continued market expansion for oxaloacetate measurement technologies. Industry forecasts predict that the specialized segment for TCA cycle intermediate analysis tools will reach approximately $1.1 billion by 2027, representing a significant opportunity for innovative measurement solutions.
In the pharmaceutical industry, demand for precise oxaloacetate measurement techniques has increased substantially as researchers investigate metabolic pathways associated with cancer, neurodegenerative disorders, and cardiovascular diseases. The market size for metabolomics research tools, including oxaloacetate measurement technologies, reached approximately $2.3 billion in 2022, with projected annual growth rates of 13.4% through 2028.
Clinical diagnostics represents another significant market segment, where oxaloacetate measurements serve as biomarkers for metabolic disorders, liver dysfunction, and mitochondrial diseases. Hospitals and diagnostic laboratories increasingly incorporate metabolite profiling into their testing panels, creating sustained demand for reliable measurement methods like NMR-based techniques.
The agricultural and food science sectors have emerged as unexpected growth areas for oxaloacetate measurement technologies. Researchers utilize these tools to monitor crop metabolism, assess food quality, and develop enhanced nutritional supplements. This diversification of applications has expanded the potential market reach beyond traditional biomedical boundaries.
Biotechnology companies focused on metabolic engineering and synthetic biology represent another significant market segment. These organizations require precise quantification of metabolic intermediates like oxaloacetate to optimize bioproduction pathways for pharmaceuticals, biofuels, and specialty chemicals. The growing emphasis on sustainable manufacturing processes has further accelerated demand in this sector.
Geographically, North America dominates the market for advanced metabolite measurement technologies, accounting for approximately 42% of global demand. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and India making substantial investments in metabolomics research infrastructure and capabilities.
Market analysis indicates that technologies offering higher sensitivity, reproducibility, and throughput have distinct competitive advantages. NMR-based methods for oxaloacetate measurement compete with mass spectrometry approaches, with each technology serving different market niches based on specific requirements for accuracy, sample preparation complexity, and cost considerations.
The increasing prevalence of metabolic disorders globally, coupled with growing research interest in cellular metabolism, suggests continued market expansion for oxaloacetate measurement technologies. Industry forecasts predict that the specialized segment for TCA cycle intermediate analysis tools will reach approximately $1.1 billion by 2027, representing a significant opportunity for innovative measurement solutions.
Current NMR Technologies and Challenges in Metabolite Quantification
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly over the past decades, establishing itself as a powerful analytical technique for metabolite quantification. Current NMR technologies employ various approaches including 1D proton (1H) NMR, carbon-13 (13C) NMR, and multi-dimensional techniques such as HSQC (Heteronuclear Single Quantum Coherence) for metabolite analysis. High-field NMR spectrometers operating at field strengths of 600-900 MHz provide enhanced spectral resolution critical for complex biological samples, while cryoprobe technology has dramatically improved sensitivity by reducing thermal noise.
Despite these advancements, significant challenges persist in accurately measuring metabolites like oxaloacetate. The primary difficulty lies in oxaloacetate's inherent instability in aqueous solutions, where it rapidly decarboxylates to pyruvate at physiological pH and temperature. This instability creates a moving target for quantification efforts, requiring specialized sample preparation protocols and rapid analysis.
Spectral overlap presents another major challenge, particularly in complex biological matrices where numerous metabolites produce signals in similar regions of the spectrum. Oxaloacetate's NMR signals often overlap with those of other TCA cycle intermediates and common metabolites, complicating direct quantification from raw spectra. Advanced spectral deconvolution algorithms and selective pulse sequences have been developed to address this issue, but they require careful optimization for specific applications.
Signal-to-noise ratio limitations affect the detection of low-concentration metabolites like oxaloacetate, which typically exists at micromolar levels in biological systems. While modern cryoprobe technology has improved sensitivity by 4-5 fold compared to conventional probes, quantification at physiologically relevant concentrations remains challenging, often requiring signal enhancement strategies or pre-concentration steps.
Sample preparation introduces additional variability in metabolite quantification. Extraction methods, pH control, and the presence of metal ions can significantly affect oxaloacetate stability and spectral characteristics. Standardized protocols using rapid quenching techniques and controlled pH environments have been developed to minimize degradation during sample handling.
Quantification accuracy depends heavily on proper calibration and reference standards. Internal standards like DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) or TSP (trimethylsilylpropanoic acid) are commonly used, but matrix effects can influence their effectiveness. External calibration curves using authentic oxaloacetate standards under matched conditions provide more reliable quantification but require careful preparation due to the compound's instability.
Recent technological innovations including hyperpolarization techniques such as Dynamic Nuclear Polarization (DNP) offer promising approaches to overcome sensitivity limitations, potentially enabling real-time tracking of oxaloacetate in metabolic pathways. However, these advanced methods require specialized equipment and expertise not widely available in standard analytical laboratories.
Despite these advancements, significant challenges persist in accurately measuring metabolites like oxaloacetate. The primary difficulty lies in oxaloacetate's inherent instability in aqueous solutions, where it rapidly decarboxylates to pyruvate at physiological pH and temperature. This instability creates a moving target for quantification efforts, requiring specialized sample preparation protocols and rapid analysis.
Spectral overlap presents another major challenge, particularly in complex biological matrices where numerous metabolites produce signals in similar regions of the spectrum. Oxaloacetate's NMR signals often overlap with those of other TCA cycle intermediates and common metabolites, complicating direct quantification from raw spectra. Advanced spectral deconvolution algorithms and selective pulse sequences have been developed to address this issue, but they require careful optimization for specific applications.
Signal-to-noise ratio limitations affect the detection of low-concentration metabolites like oxaloacetate, which typically exists at micromolar levels in biological systems. While modern cryoprobe technology has improved sensitivity by 4-5 fold compared to conventional probes, quantification at physiologically relevant concentrations remains challenging, often requiring signal enhancement strategies or pre-concentration steps.
Sample preparation introduces additional variability in metabolite quantification. Extraction methods, pH control, and the presence of metal ions can significantly affect oxaloacetate stability and spectral characteristics. Standardized protocols using rapid quenching techniques and controlled pH environments have been developed to minimize degradation during sample handling.
Quantification accuracy depends heavily on proper calibration and reference standards. Internal standards like DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) or TSP (trimethylsilylpropanoic acid) are commonly used, but matrix effects can influence their effectiveness. External calibration curves using authentic oxaloacetate standards under matched conditions provide more reliable quantification but require careful preparation due to the compound's instability.
Recent technological innovations including hyperpolarization techniques such as Dynamic Nuclear Polarization (DNP) offer promising approaches to overcome sensitivity limitations, potentially enabling real-time tracking of oxaloacetate in metabolic pathways. However, these advanced methods require specialized equipment and expertise not widely available in standard analytical laboratories.
Established Protocols for Oxaloacetate Concentration Determination
01 Quantitative NMR methods for concentration measurement
Quantitative Nuclear Magnetic Resonance (qNMR) techniques are used for precise concentration measurements in various samples. These methods rely on the direct proportionality between signal intensity and the number of nuclei generating the signal. By comparing the integral of a signal from the analyte with that of a reference standard of known concentration, accurate concentration measurements can be obtained. This approach offers high precision without requiring compound-specific calibration curves.- Quantitative NMR methods for concentration measurement: Quantitative Nuclear Magnetic Resonance (qNMR) techniques are used for precise concentration measurements of various substances. These methods rely on the proportional relationship between signal intensity and the number of nuclei generating the signal. By comparing the integral of a sample signal with a reference standard of known concentration, accurate concentration measurements can be obtained. This approach offers high precision without requiring compound-specific calibration curves.
- Low-field NMR for concentration analysis: Low-field NMR systems provide portable and cost-effective solutions for concentration measurements in various applications. These systems operate at lower magnetic field strengths than traditional high-field NMR spectrometers but can still deliver reliable concentration data. They are particularly useful for field applications, quality control in industrial settings, and situations where high-resolution spectroscopy is not required. The technology enables real-time monitoring of concentration changes in various processes.
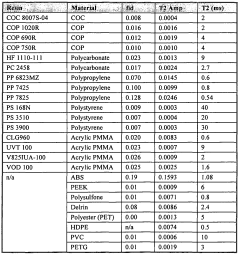
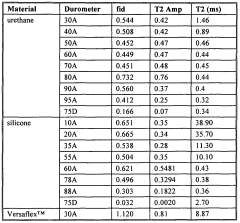
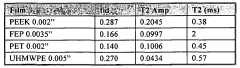
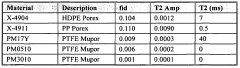
- Time-domain NMR for concentration determination: Time-domain NMR techniques analyze the time-dependent response of nuclear spins to determine concentration. These methods focus on relaxation times (T1 and T2) and diffusion coefficients, which can be correlated with concentration values. By measuring these parameters, the concentration of specific components in complex mixtures can be determined without requiring complete spectral resolution. This approach is particularly valuable for analyzing heterogeneous samples and for rapid screening applications.
- NMR with specialized hardware for concentration measurement: Specialized NMR hardware configurations enhance concentration measurement capabilities. These include custom probe designs, optimized radio frequency circuits, and advanced signal processing systems. Such hardware modifications improve sensitivity, reduce noise, and enable measurements of challenging samples. Innovations in magnet design and temperature control systems also contribute to more accurate and reproducible concentration measurements across various sample types.
- NMR for in-vivo and medical concentration measurements: NMR techniques are applied for non-invasive concentration measurements in living systems and medical diagnostics. These methods enable real-time monitoring of metabolite concentrations, drug distribution, and physiological parameters in tissues and organisms. Specialized pulse sequences and data analysis algorithms help overcome challenges related to in-vivo measurements, such as motion artifacts and tissue heterogeneity. This approach provides valuable information for medical diagnosis, treatment monitoring, and physiological research.
02 Low-field NMR systems for concentration analysis
Low-field NMR systems provide portable and cost-effective solutions for concentration measurements in various applications. These systems operate at lower magnetic field strengths than traditional high-field NMR spectrometers but can still provide valuable quantitative information. They are particularly useful for field applications, quality control in industrial settings, and situations where high-resolution spectral information is not required but accurate concentration data is needed.Expand Specific Solutions03 Time-domain NMR for concentration determination
Time-domain NMR techniques analyze the free induction decay (FID) or relaxation curves directly to determine concentration without requiring full spectral analysis. These methods often utilize relaxation parameters such as T1 (longitudinal relaxation) or T2 (transverse relaxation) times, which can be correlated with concentration values. This approach is particularly valuable for complex mixtures where spectral resolution might be limited but relaxation properties differ between components.Expand Specific Solutions04 NMR flow measurement systems for concentration monitoring
NMR flow measurement systems enable real-time monitoring of concentration in flowing samples or industrial processes. These systems incorporate specialized hardware to accommodate flowing materials while maintaining measurement accuracy. They can be used for continuous process monitoring, quality control in production lines, and analysis of biological fluids. The technology allows for non-invasive concentration measurements without disrupting the flow or contaminating the sample.Expand Specific Solutions05 Advanced pulse sequences for improved concentration accuracy
Specialized NMR pulse sequences have been developed to enhance the accuracy of concentration measurements in complex samples. These sequences can suppress unwanted signals, compensate for instrumental variations, and improve signal-to-noise ratios. Examples include solvent suppression techniques, multiple-pulse sequences that filter specific interactions, and methods that correct for relaxation effects. These advanced pulse sequences enable more accurate quantification in challenging samples with interfering components or varying matrix effects.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers in NMR Analytics
The measurement of oxaloacetate concentration using NMR represents a specialized analytical field currently in its growth phase. The market for this technology is relatively niche but expanding, estimated at approximately $300-400 million globally, driven by increasing applications in metabolomics research and pharmaceutical development. From a technical maturity perspective, the landscape shows varied capabilities among key players. Companies like Bruker Switzerland and Hitachi Ltd. lead with advanced NMR instrumentation, while pharmaceutical entities such as Novartis AG, Takeda Pharmaceutical, and DuPont de Nemours leverage these techniques for metabolic research. Academic institutions including MIT and University of Maryland contribute significant methodological innovations. T2 Biosystems and Schlumberger Technologies are advancing specialized applications, indicating the technology's ongoing evolution toward greater sensitivity and automation in metabolite quantification.
Hitachi Ltd.
Technical Solution: Hitachi has pioneered compact benchtop NMR systems specifically designed for metabolite analysis including oxaloacetate quantification. Their approach focuses on making NMR technology more accessible for routine metabolic measurements through miniaturized hardware combined with specialized software algorithms. Hitachi's benchtop NMR systems utilize permanent magnets operating at field strengths of 1-2 Tesla, providing sufficient resolution for distinguishing oxaloacetate from other TCA cycle intermediates[3]. Their methodology incorporates optimized pulse sequences with advanced solvent suppression techniques to enhance detection of low-abundance metabolites. The company has developed automated calibration procedures using reference standards with known oxaloacetate concentrations to establish quantitative relationships between signal intensity and metabolite concentration. Hitachi's systems include specialized sample handling protocols designed to minimize degradation of unstable metabolites like oxaloacetate, including rapid acquisition methods and temperature control during measurement[4].
Strengths: Lower cost and more accessible than traditional high-field NMR systems; simplified operation requiring less technical expertise; faster analysis times; smaller laboratory footprint. Weaknesses: Lower sensitivity compared to high-field systems; reduced spectral resolution limiting analysis in complex mixtures; potential challenges with signal overlap in biological samples; less suitable for very low concentration measurements.
T2 Biosystems, Inc.
Technical Solution: T2 Biosystems has developed innovative magnetic resonance-based detection technologies that can be applied to oxaloacetate quantification. Their approach combines aspects of NMR spectroscopy with magnetic resonance relaxometry in miniaturized formats. The company's core technology platform utilizes superparamagnetic nanoparticles as contrast agents coupled with specific binding molecules that can interact with target metabolites like oxaloacetate[5]. When oxaloacetate binds to these functionalized nanoparticles, it causes measurable changes in the T2 relaxation time of surrounding water molecules, which correlates with metabolite concentration. T2 Biosystems has engineered specialized microcoils and detection systems that can measure these subtle magnetic changes with high sensitivity. Their methodology includes proprietary signal processing algorithms that filter background noise and enhance detection specificity. The company has developed automated calibration procedures using synthetic oxaloacetate standards to establish quantitative relationships between T2 relaxation changes and metabolite concentration[6].
Strengths: Highly sensitive detection potentially surpassing conventional NMR for specific metabolites; reduced sample volume requirements; faster analysis times; potential for point-of-care applications. Weaknesses: Less established for metabolite quantification compared to traditional NMR; potential for interference from other biological components; more limited in simultaneous multi-metabolite analysis; requires specific reagent development for each target metabolite.
Key Technical Innovations in Metabolite NMR Spectroscopy
A method for detecting a target substance by nuclear magnetic resonance
PatentInactiveEP2171436A1
Innovation
- The method involves adding isotope-labeled target substances to the sample, which changes the NMR signal positions or multiplicities, allowing for the calculation of the target substance's signal positions and subsequent detection and quantification by exploiting the differences in signal patterns between the labeled and unlabeled substances.
NMR diagnostics by means of a plastic sample container
PatentWO2009045354A1
Innovation
- The use of polymeric materials for NMR sample containers, such as plastics, which can be fabricated using methods like injection molding, allowing for the creation of complex designs at a lower cost, and the application of radiofrequency pulse sequences to differentiate and reduce the contribution of the container's signal in NMR measurements.
Sample Preparation Techniques for Optimal Oxaloacetate Preservation
Oxaloacetate (OAA) is a highly unstable metabolite that rapidly decarboxylates to pyruvate at room temperature, presenting significant challenges for accurate NMR measurement. Effective sample preparation is therefore critical to preserve OAA integrity before NMR analysis. The half-life of OAA at physiological pH and temperature is approximately 30 minutes, necessitating rapid and careful handling procedures to minimize degradation.
Cold chain management represents the cornerstone of OAA preservation. Samples should be maintained at temperatures between -20°C and -80°C throughout collection, transport, and storage phases. Flash-freezing techniques using liquid nitrogen immediately after sample collection have demonstrated superior preservation rates, with studies showing up to 95% OAA retention compared to conventional cooling methods.
pH optimization significantly impacts OAA stability. Research indicates that maintaining samples at pH 2-3 dramatically reduces decarboxylation rates by protonating the α-keto acid group. Buffer systems utilizing trifluoroacetic acid or phosphoric acid at concentrations of 0.1-0.5% have proven effective in stabilizing OAA for NMR analysis, extending the viable measurement window to several hours.
Chemical derivatization approaches offer another valuable strategy for OAA preservation. Converting OAA to more stable forms through reactions with 2,4-dinitrophenylhydrazine or o-phenylenediamine creates derivatives that resist degradation while maintaining structural information necessary for quantitative analysis. These derivatives exhibit stability for up to 72 hours at 4°C, providing flexibility in sample handling timelines.
Enzymatic stabilization methods employ metabolic inhibitors to prevent natural enzymatic degradation of OAA. Compounds such as fluoroacetate or malonate effectively block citric acid cycle enzymes that would otherwise rapidly consume OAA. Concentration ratios of 1:10 (inhibitor:sample) have shown optimal results in preserving native OAA levels.
Sample matrix considerations are equally important, as protein binding can protect OAA from degradation. Precipitation protocols using organic solvents like methanol or acetonitrile should be optimized to balance protein removal with OAA recovery. Dual-phase extraction systems incorporating both aqueous and organic phases have demonstrated recovery rates exceeding 85% while maintaining sample integrity.
Standardized handling protocols incorporating these techniques should specify maximum allowable time intervals between collection and analysis, typically recommending NMR measurement within 4 hours of sample preparation. Implementation of internal standards, particularly 13C-labeled OAA analogues, enables compensation for any degradation that occurs during preparation, enhancing quantitative accuracy.
Cold chain management represents the cornerstone of OAA preservation. Samples should be maintained at temperatures between -20°C and -80°C throughout collection, transport, and storage phases. Flash-freezing techniques using liquid nitrogen immediately after sample collection have demonstrated superior preservation rates, with studies showing up to 95% OAA retention compared to conventional cooling methods.
pH optimization significantly impacts OAA stability. Research indicates that maintaining samples at pH 2-3 dramatically reduces decarboxylation rates by protonating the α-keto acid group. Buffer systems utilizing trifluoroacetic acid or phosphoric acid at concentrations of 0.1-0.5% have proven effective in stabilizing OAA for NMR analysis, extending the viable measurement window to several hours.
Chemical derivatization approaches offer another valuable strategy for OAA preservation. Converting OAA to more stable forms through reactions with 2,4-dinitrophenylhydrazine or o-phenylenediamine creates derivatives that resist degradation while maintaining structural information necessary for quantitative analysis. These derivatives exhibit stability for up to 72 hours at 4°C, providing flexibility in sample handling timelines.
Enzymatic stabilization methods employ metabolic inhibitors to prevent natural enzymatic degradation of OAA. Compounds such as fluoroacetate or malonate effectively block citric acid cycle enzymes that would otherwise rapidly consume OAA. Concentration ratios of 1:10 (inhibitor:sample) have shown optimal results in preserving native OAA levels.
Sample matrix considerations are equally important, as protein binding can protect OAA from degradation. Precipitation protocols using organic solvents like methanol or acetonitrile should be optimized to balance protein removal with OAA recovery. Dual-phase extraction systems incorporating both aqueous and organic phases have demonstrated recovery rates exceeding 85% while maintaining sample integrity.
Standardized handling protocols incorporating these techniques should specify maximum allowable time intervals between collection and analysis, typically recommending NMR measurement within 4 hours of sample preparation. Implementation of internal standards, particularly 13C-labeled OAA analogues, enables compensation for any degradation that occurs during preparation, enhancing quantitative accuracy.
Data Processing and Calibration Methods for Quantitative NMR Analysis
Quantitative NMR (qNMR) analysis for oxaloacetate concentration measurement requires robust data processing and calibration methods to ensure accuracy and reliability. The raw NMR data obtained from spectrometers must undergo several processing steps before quantitative information can be extracted. Initially, phase correction is applied to ensure all signals appear in the absorption mode, which is critical for accurate integration. This is typically performed using automated algorithms with manual fine-tuning to achieve optimal results.
Baseline correction represents another crucial processing step, as uneven baselines can significantly impact integration accuracy. Modern NMR processing software offers various baseline correction algorithms, with polynomial fitting and spline-based methods being particularly effective for oxaloacetate analysis. The selection of appropriate integration regions is equally important, requiring careful consideration of potential signal overlaps that are common in complex biological matrices.
For calibration purposes, internal standards with known concentrations are essential in qNMR analysis of oxaloacetate. Compounds such as 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (TSP), maleic acid, or deuterated dimethyl sulfoxide (DMSO-d6) are commonly employed due to their chemical stability and well-resolved signals. The calibration curve method establishes the relationship between signal intensity and concentration, while the standard addition approach helps overcome matrix effects that can interfere with accurate quantification.
Signal-to-noise ratio (S/N) optimization is particularly important for oxaloacetate measurement, as this metabolite can be present at relatively low concentrations in biological samples. Window functions such as exponential multiplication or Gaussian enhancement are applied to the free induction decay (FID) to enhance S/N, though careful parameter selection is necessary to avoid signal distortion that could compromise quantitative accuracy.
Advanced processing techniques have emerged to address specific challenges in oxaloacetate quantification. Spectral deconvolution algorithms can resolve overlapping signals, while two-dimensional NMR methods provide additional spectral dispersion for complex samples. Machine learning approaches are increasingly being applied to automate and optimize data processing workflows, potentially improving both efficiency and reproducibility in oxaloacetate concentration measurements.
Quality control measures must be integrated throughout the data processing and calibration workflow. These include regular verification of spectrometer performance, validation of processing parameters, and assessment of measurement uncertainty. Statistical methods such as Bland-Altman analysis and calculation of coefficients of variation help evaluate method reliability and establish confidence intervals for reported oxaloacetate concentrations.
Baseline correction represents another crucial processing step, as uneven baselines can significantly impact integration accuracy. Modern NMR processing software offers various baseline correction algorithms, with polynomial fitting and spline-based methods being particularly effective for oxaloacetate analysis. The selection of appropriate integration regions is equally important, requiring careful consideration of potential signal overlaps that are common in complex biological matrices.
For calibration purposes, internal standards with known concentrations are essential in qNMR analysis of oxaloacetate. Compounds such as 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (TSP), maleic acid, or deuterated dimethyl sulfoxide (DMSO-d6) are commonly employed due to their chemical stability and well-resolved signals. The calibration curve method establishes the relationship between signal intensity and concentration, while the standard addition approach helps overcome matrix effects that can interfere with accurate quantification.
Signal-to-noise ratio (S/N) optimization is particularly important for oxaloacetate measurement, as this metabolite can be present at relatively low concentrations in biological samples. Window functions such as exponential multiplication or Gaussian enhancement are applied to the free induction decay (FID) to enhance S/N, though careful parameter selection is necessary to avoid signal distortion that could compromise quantitative accuracy.
Advanced processing techniques have emerged to address specific challenges in oxaloacetate quantification. Spectral deconvolution algorithms can resolve overlapping signals, while two-dimensional NMR methods provide additional spectral dispersion for complex samples. Machine learning approaches are increasingly being applied to automate and optimize data processing workflows, potentially improving both efficiency and reproducibility in oxaloacetate concentration measurements.
Quality control measures must be integrated throughout the data processing and calibration workflow. These include regular verification of spectrometer performance, validation of processing parameters, and assessment of measurement uncertainty. Statistical methods such as Bland-Altman analysis and calculation of coefficients of variation help evaluate method reliability and establish confidence intervals for reported oxaloacetate concentrations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!