How to Minimize Carryover in GC-MS for Multi-Analytes
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Carryover Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, becoming an indispensable analytical technique in various fields including environmental analysis, forensic science, food safety, and pharmaceutical research. The integration of gas chromatography's separation capabilities with mass spectrometry's identification power has created a powerful tool for multi-analyte detection and quantification.
Carryover, the persistent presence of analytes from previous injections in subsequent analyses, represents one of the most challenging issues in GC-MS methodology. This phenomenon has become increasingly problematic as detection limits have decreased to parts-per-billion or even parts-per-trillion levels, where even minimal contamination can significantly impact results.
The evolution of GC-MS technology has seen remarkable improvements in sensitivity and selectivity, but these advancements have paradoxically made carryover issues more apparent and critical. Historical approaches to mitigate carryover have included system baking, solvent washes, and hardware modifications, with varying degrees of success depending on analyte properties and matrix complexity.
Current trends in GC-MS development focus on automated sample preparation, intelligent software algorithms for carryover detection, and novel materials for GC components that minimize analyte adsorption. The industry is moving toward integrated solutions that address carryover at multiple points in the analytical workflow rather than isolated interventions.
The primary objective of this technical research is to comprehensively evaluate existing strategies for minimizing carryover in GC-MS systems when analyzing multiple analytes simultaneously. We aim to identify the most effective approaches for different classes of compounds, particularly focusing on challenging analytes such as polar compounds, high-boiling point substances, and those prone to adsorption on system surfaces.
Secondary objectives include quantifying the impact of various instrument parameters on carryover, assessing the effectiveness of different washing solvents and procedures, and exploring emerging technologies that show promise in reducing carryover. Additionally, we seek to develop a systematic framework for method development that incorporates carryover minimization as a fundamental consideration rather than a troubleshooting afterthought.
The ultimate goal is to establish best practices that can be implemented across different GC-MS platforms and applications, providing analysts with practical, evidence-based strategies to enhance data quality and reliability. This research acknowledges the complex interplay between analyte properties, matrix effects, and instrument design in carryover phenomena, aiming to deliver holistic solutions that address these multifaceted challenges.
Carryover, the persistent presence of analytes from previous injections in subsequent analyses, represents one of the most challenging issues in GC-MS methodology. This phenomenon has become increasingly problematic as detection limits have decreased to parts-per-billion or even parts-per-trillion levels, where even minimal contamination can significantly impact results.
The evolution of GC-MS technology has seen remarkable improvements in sensitivity and selectivity, but these advancements have paradoxically made carryover issues more apparent and critical. Historical approaches to mitigate carryover have included system baking, solvent washes, and hardware modifications, with varying degrees of success depending on analyte properties and matrix complexity.
Current trends in GC-MS development focus on automated sample preparation, intelligent software algorithms for carryover detection, and novel materials for GC components that minimize analyte adsorption. The industry is moving toward integrated solutions that address carryover at multiple points in the analytical workflow rather than isolated interventions.
The primary objective of this technical research is to comprehensively evaluate existing strategies for minimizing carryover in GC-MS systems when analyzing multiple analytes simultaneously. We aim to identify the most effective approaches for different classes of compounds, particularly focusing on challenging analytes such as polar compounds, high-boiling point substances, and those prone to adsorption on system surfaces.
Secondary objectives include quantifying the impact of various instrument parameters on carryover, assessing the effectiveness of different washing solvents and procedures, and exploring emerging technologies that show promise in reducing carryover. Additionally, we seek to develop a systematic framework for method development that incorporates carryover minimization as a fundamental consideration rather than a troubleshooting afterthought.
The ultimate goal is to establish best practices that can be implemented across different GC-MS platforms and applications, providing analysts with practical, evidence-based strategies to enhance data quality and reliability. This research acknowledges the complex interplay between analyte properties, matrix effects, and instrument design in carryover phenomena, aiming to deliver holistic solutions that address these multifaceted challenges.
Market Demand for High-Precision Analytical Methods
The analytical chemistry market has witnessed a significant surge in demand for high-precision analytical methods, particularly in Gas Chromatography-Mass Spectrometry (GC-MS) applications. This growth is primarily driven by increasingly stringent regulatory requirements across pharmaceutical, environmental, food safety, and forensic sectors. The global analytical instrumentation market, valued at approximately $85 billion in 2022, is projected to grow at a compound annual growth rate of 6.2% through 2028, with GC-MS systems representing a substantial segment of this expansion.
Industries requiring multi-analyte detection capabilities are particularly invested in advanced GC-MS technologies that minimize carryover effects. The pharmaceutical sector, facing rigorous FDA and EMA regulations, demands analytical methods capable of detecting trace contaminants at parts-per-billion levels without false positives from previous sample residues. This market segment alone contributes nearly 35% to the overall demand for high-precision analytical methods.
Environmental testing laboratories represent another significant market driver, with over 20,000 facilities worldwide requiring regular analysis of complex environmental samples containing multiple analytes at varying concentration levels. The ability to analyze consecutive samples without cross-contamination is critical for accurate environmental monitoring and regulatory compliance reporting.
The food and beverage industry has emerged as a rapidly growing segment, with increasing concerns about food safety and authenticity driving demand for sensitive multi-residue analytical methods. Market research indicates that approximately 70% of food testing laboratories cite carryover as a major challenge affecting their analytical workflow efficiency and result reliability.
Contract Research Organizations (CROs) and forensic laboratories constitute a specialized market segment with particularly high demands for analytical precision. These facilities often analyze diverse sample types in sequence, making carryover minimization essential for maintaining data integrity and legal defensibility of results.
The clinical diagnostics sector presents an emerging opportunity, with growing applications of GC-MS in metabolomics and clinical toxicology. This segment is expected to grow at 8.5% annually, outpacing the broader market, as personalized medicine approaches drive the need for more sensitive and reliable analytical methods.
Market feedback indicates that laboratories are willing to invest 15-20% premium for analytical systems and methodologies that demonstrably reduce carryover issues, highlighting the significant economic value placed on solving this technical challenge. This premium pricing potential represents a compelling commercial opportunity for technology developers who can effectively address carryover minimization in multi-analyte GC-MS applications.
Industries requiring multi-analyte detection capabilities are particularly invested in advanced GC-MS technologies that minimize carryover effects. The pharmaceutical sector, facing rigorous FDA and EMA regulations, demands analytical methods capable of detecting trace contaminants at parts-per-billion levels without false positives from previous sample residues. This market segment alone contributes nearly 35% to the overall demand for high-precision analytical methods.
Environmental testing laboratories represent another significant market driver, with over 20,000 facilities worldwide requiring regular analysis of complex environmental samples containing multiple analytes at varying concentration levels. The ability to analyze consecutive samples without cross-contamination is critical for accurate environmental monitoring and regulatory compliance reporting.
The food and beverage industry has emerged as a rapidly growing segment, with increasing concerns about food safety and authenticity driving demand for sensitive multi-residue analytical methods. Market research indicates that approximately 70% of food testing laboratories cite carryover as a major challenge affecting their analytical workflow efficiency and result reliability.
Contract Research Organizations (CROs) and forensic laboratories constitute a specialized market segment with particularly high demands for analytical precision. These facilities often analyze diverse sample types in sequence, making carryover minimization essential for maintaining data integrity and legal defensibility of results.
The clinical diagnostics sector presents an emerging opportunity, with growing applications of GC-MS in metabolomics and clinical toxicology. This segment is expected to grow at 8.5% annually, outpacing the broader market, as personalized medicine approaches drive the need for more sensitive and reliable analytical methods.
Market feedback indicates that laboratories are willing to invest 15-20% premium for analytical systems and methodologies that demonstrably reduce carryover issues, highlighting the significant economic value placed on solving this technical challenge. This premium pricing potential represents a compelling commercial opportunity for technology developers who can effectively address carryover minimization in multi-analyte GC-MS applications.
Current Challenges in GC-MS Carryover Reduction
Despite significant advancements in GC-MS technology, carryover remains a persistent challenge that compromises analytical accuracy, especially when analyzing multiple analytes with diverse chemical properties. Carryover occurs when residues from previous injections contaminate subsequent samples, leading to false positives, quantification errors, and reduced instrument reliability. This issue becomes particularly problematic in multi-analyte methods where compounds with varying volatility, polarity, and thermal stability are analyzed simultaneously.
The primary technical obstacles in minimizing carryover stem from several interconnected factors. First, the interaction between analytes and the GC system components, particularly the inlet liner, column, and detector surfaces, creates adsorption sites where compounds can accumulate. High-boiling point compounds and those with active functional groups (such as amines, carboxylic acids, and phenols) are especially prone to adsorption and subsequent slow release during later analyses.
Temperature programming presents another significant challenge. While higher temperatures can reduce carryover by promoting complete volatilization of residual compounds, they may also accelerate column degradation and increase background noise. Conversely, insufficient temperatures fail to effectively clear high-boiling residues, creating a difficult optimization problem that varies with each analyte mixture.
Sample matrix complexity further exacerbates carryover issues. Non-volatile matrix components can accumulate in the GC system, creating new active sites that enhance analyte adsorption. This matrix effect is particularly challenging in environmental, biological, and food samples where complex backgrounds are unavoidable.
Modern high-sensitivity MS detectors have paradoxically intensified carryover concerns. As detection limits have decreased to parts-per-trillion levels, even minute carryover becomes analytically significant. This heightened sensitivity has transformed what were once acceptable levels of system contamination into problematic interferences.
Autosampler design and operation contribute additional complications. Needle washing procedures, while necessary, often fail to completely remove all analyte residues, especially for compounds with strong affinity for the needle material. The compromise between thorough cleaning and analytical throughput creates practical limitations in high-volume testing environments.
The diversity of analytes in multi-residue methods creates perhaps the most fundamental challenge. Optimizing conditions to minimize carryover for one class of compounds often increases it for others, making universal solutions elusive. This is particularly evident in applications such as pesticide residue analysis, forensic toxicology, and metabolomics, where hundreds of chemically diverse compounds may be targeted in a single analytical run.
The primary technical obstacles in minimizing carryover stem from several interconnected factors. First, the interaction between analytes and the GC system components, particularly the inlet liner, column, and detector surfaces, creates adsorption sites where compounds can accumulate. High-boiling point compounds and those with active functional groups (such as amines, carboxylic acids, and phenols) are especially prone to adsorption and subsequent slow release during later analyses.
Temperature programming presents another significant challenge. While higher temperatures can reduce carryover by promoting complete volatilization of residual compounds, they may also accelerate column degradation and increase background noise. Conversely, insufficient temperatures fail to effectively clear high-boiling residues, creating a difficult optimization problem that varies with each analyte mixture.
Sample matrix complexity further exacerbates carryover issues. Non-volatile matrix components can accumulate in the GC system, creating new active sites that enhance analyte adsorption. This matrix effect is particularly challenging in environmental, biological, and food samples where complex backgrounds are unavoidable.
Modern high-sensitivity MS detectors have paradoxically intensified carryover concerns. As detection limits have decreased to parts-per-trillion levels, even minute carryover becomes analytically significant. This heightened sensitivity has transformed what were once acceptable levels of system contamination into problematic interferences.
Autosampler design and operation contribute additional complications. Needle washing procedures, while necessary, often fail to completely remove all analyte residues, especially for compounds with strong affinity for the needle material. The compromise between thorough cleaning and analytical throughput creates practical limitations in high-volume testing environments.
The diversity of analytes in multi-residue methods creates perhaps the most fundamental challenge. Optimizing conditions to minimize carryover for one class of compounds often increases it for others, making universal solutions elusive. This is particularly evident in applications such as pesticide residue analysis, forensic toxicology, and metabolomics, where hundreds of chemically diverse compounds may be targeted in a single analytical run.
Established Carryover Reduction Methodologies
01 Cleaning methods to reduce carryover in GC-MS systems
Various cleaning methods can be employed to reduce carryover in GC-MS systems. These include using solvent washes, thermal cleaning procedures, and specialized cleaning agents to remove residual analytes from the injection port, column, and detector components. Implementing regular cleaning protocols helps maintain system performance and ensures accurate analytical results by preventing contamination from previous samples.- Cleaning methods to reduce carryover in GC-MS systems: Various cleaning methods can be employed to reduce carryover in GC-MS systems. These include using solvent washes, thermal cleaning procedures, and specialized cleaning agents to remove residual analytes from the injection port, column, and detector components. Implementing regular cleaning protocols helps maintain system performance and ensures accurate analytical results by preventing contamination from previous samples.
- Hardware modifications to minimize carryover effects: Specific hardware modifications can be implemented to minimize carryover in GC-MS systems. These include redesigned injection ports, improved liner designs, inert flow path components, and specialized sample introduction systems. These modifications aim to reduce sample contact with active surfaces, minimize dead volumes, and improve sample transfer efficiency, thereby reducing the potential for analyte carryover between injections.
- Optimized method parameters to prevent carryover: Optimizing method parameters is crucial for preventing carryover in GC-MS analysis. This includes adjusting injection techniques, temperature programming, carrier gas flow rates, and detector settings. Higher injection port and transfer line temperatures can help volatilize residual compounds, while optimized temperature ramps can ensure complete elution of high-boiling compounds, reducing their potential to carry over to subsequent analyses.
- Specialized sample preparation techniques: Specialized sample preparation techniques can significantly reduce carryover issues in GC-MS analysis. These include dilution protocols, filtration methods, solid-phase extraction, and derivatization approaches that improve volatility and reduce adsorption. Proper sample handling and preparation not only improve analytical sensitivity but also minimize the risk of introducing contaminants that could contribute to carryover problems.
- Software solutions and data processing techniques: Software solutions and advanced data processing techniques can help address carryover issues in GC-MS analysis. These include blank subtraction algorithms, carryover correction models, and automated system suitability testing. Intelligent software can detect potential carryover, implement corrective actions such as additional cleaning cycles, and apply mathematical corrections to compensate for residual signals from previous injections, improving data accuracy and reliability.
02 Hardware modifications to minimize carryover effects
Specific hardware modifications can be implemented to minimize carryover in GC-MS systems. These include redesigned injection ports, improved liner designs, inert flow path components, and specialized connection systems that reduce dead volume. Such hardware improvements help prevent sample residue accumulation in critical system components and facilitate more complete sample transfer, thereby reducing carryover between consecutive analyses.Expand Specific Solutions03 Optimized sample introduction techniques
Optimized sample introduction techniques can significantly reduce carryover in GC-MS analysis. These include programmed temperature vaporization (PTV) injection, cold on-column injection, and specialized autosampler techniques that minimize contact between the sample and potential carryover sites. Proper sample preparation methods and the use of internal standards can also help compensate for any residual carryover effects.Expand Specific Solutions04 Software solutions for carryover compensation
Software solutions can be employed to address carryover issues in GC-MS systems. These include algorithms for carryover detection and correction, automated system suitability testing, and data processing methods that can mathematically compensate for carryover effects. Such software approaches help improve data quality and reliability without requiring extensive hardware modifications or time-consuming cleaning procedures.Expand Specific Solutions05 System design improvements for reduced carryover
Comprehensive system design improvements focus on reducing carryover in GC-MS instruments. These include integrated flow path designs with minimal dead volume, temperature-controlled transfer lines, improved detector designs, and materials with reduced adsorption properties. Modern GC-MS systems incorporate these design elements to minimize sample interaction with system components and reduce the potential for carryover between analyses.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The GC-MS carryover minimization market is currently in a growth phase, with increasing demand for high-precision analytical solutions across pharmaceutical, environmental, and food safety sectors. The competitive landscape is dominated by established analytical instrumentation leaders including Agilent Technologies, Thermo Finnigan (Thermo Fisher Scientific), and Shimadzu Corporation, who offer comprehensive solutions addressing carryover challenges. These companies have developed advanced technologies such as improved inlet systems, specialized column technologies, and automated washing procedures. The market is characterized by continuous innovation in sample introduction systems, with mid-tier players like LECO Corporation and Bruker Scientific focusing on specialized applications. Technical maturity varies across solution types, with newer automated sample preparation systems showing rapid advancement while fundamental column chemistry continues steady improvement through incremental innovations.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed a multi-faceted approach to minimize carryover in GC-MS systems, particularly for challenging multi-analyte applications. Their AOC-6000 Plus Multifunctional Autosampler incorporates advanced sample handling techniques including programmable pre- and post-injection solvent rinses with up to four different solvents to match the polarity range of target analytes. The system employs needle washing in both inner and outer needle surfaces, significantly reducing sample transfer contamination. Shimadzu's ClickTek technology enables tool-free column and liner changes, minimizing potential contamination during maintenance procedures. Their proprietary inert sample flow path utilizes ultra-inert materials throughout the system, from injection port to detector, reducing active sites where analytes might adhere. For particularly challenging applications, Shimadzu has developed specialized low-carryover injection ports with optimized temperature profiles and gas flows that minimize condensation zones. Their GCMS-TQ series incorporates a high-efficiency ion source design that reduces contamination and features automated source cleaning functions to maintain performance without disassembly.
Strengths: Comprehensive autosampler washing protocols with multiple solvent capability; tool-free maintenance reduces contamination risks during routine operations; specialized injection port designs for challenging applications. Weaknesses: Multiple solvent washing systems increase operational costs and waste generation; some advanced features require premium system configurations; may require more frequent preventive maintenance compared to simpler systems.
LECO Corp.
Technical Solution: LECO Corporation has developed specialized technologies to address carryover challenges in GC-MS systems, particularly for their comprehensive two-dimensional gas chromatography (GCxGC) platforms. Their approach focuses on thermal management throughout the analytical system to prevent analyte condensation. LECO's Thermal Modulation technology incorporates rapid heating and cooling cycles that efficiently transfer analytes between columns without cold spots where compounds might accumulate. Their Pegasus BT 4D system features a specially designed transfer line and ion source that maintains consistent high temperatures across all surfaces, minimizing areas where less volatile compounds might condense and later desorb. For multi-analyte applications, LECO has developed ChromaTOF software with Statistical Compare functionality that can identify potential carryover peaks by comparing patterns across sequential runs. Their QuadJet thermal modulator design eliminates cryogenic liquid requirements while maintaining efficient analyte transfer, reducing cold spots that contribute to carryover. LECO's folded flight path time-of-flight technology provides high sensitivity without the ion trapping effects that can exacerbate carryover in other mass analyzer designs.
Strengths: Specialized thermal management throughout the system effectively prevents condensation zones; advanced software tools help identify and quantify potential carryover; high-temperature operation capability for analyzing less volatile compounds. Weaknesses: Comprehensive GCxGC systems represent significant capital investment; thermal modulation technology requires specialized training; higher operating temperatures may accelerate column degradation in some applications.
Critical Technologies for Multi-Analyte Carryover Prevention
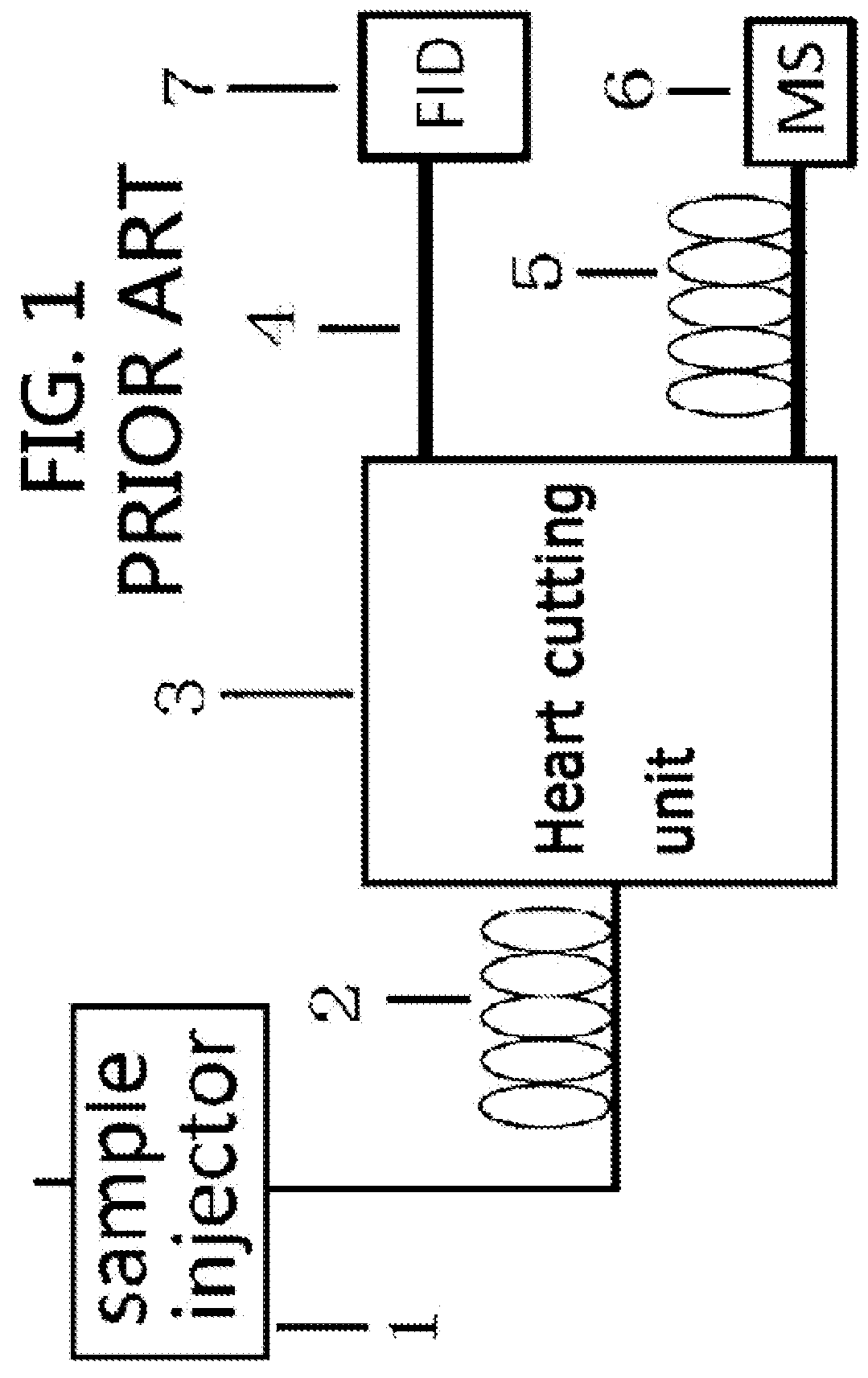
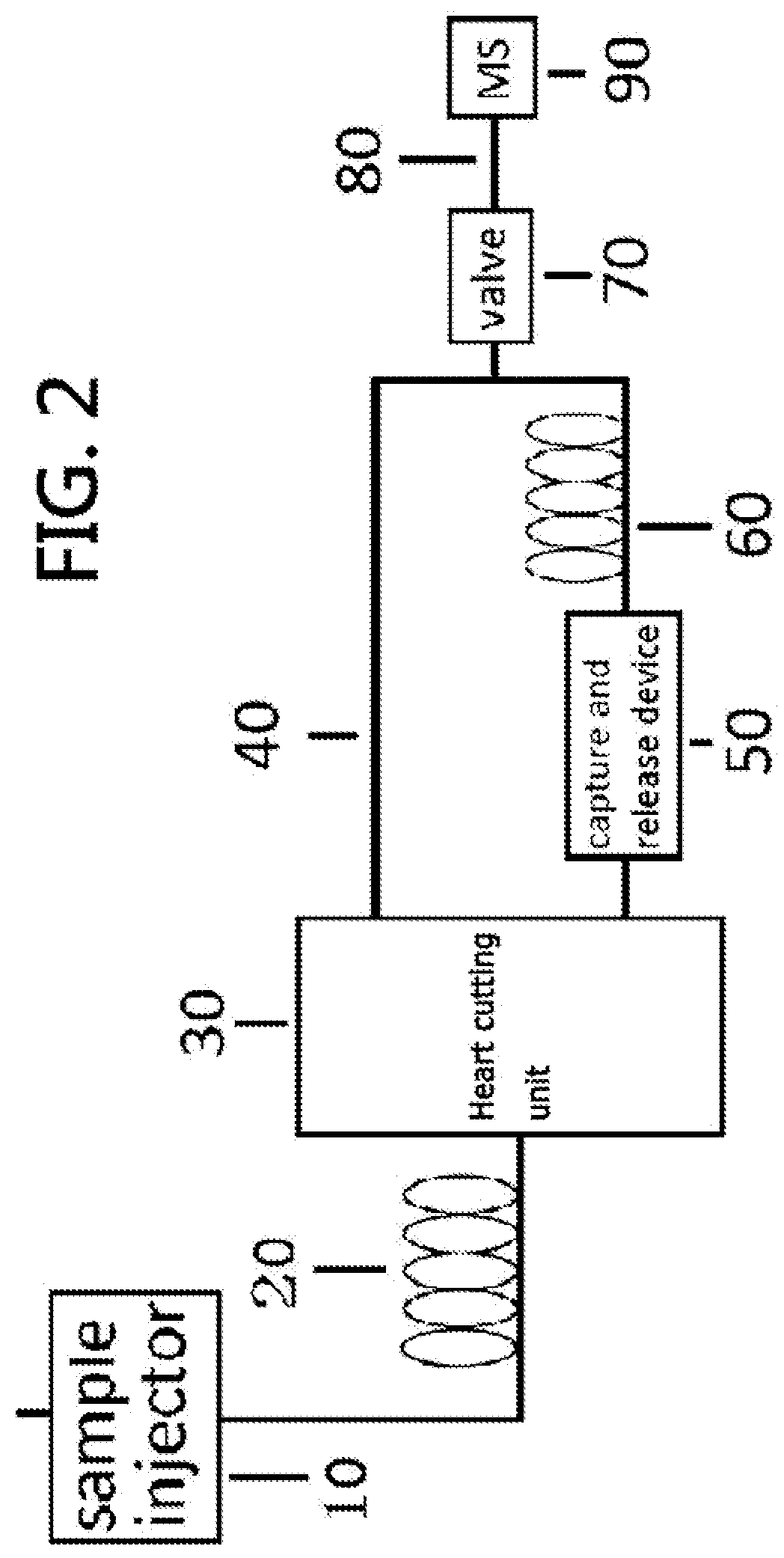
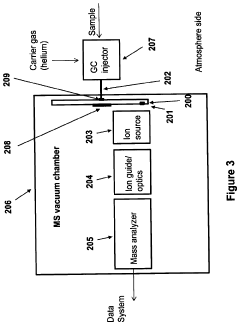
Gas chromatography-mass spectrometry method and gas chromatography-mass spectrometry apparatus therefor having a capture and release device
PatentActiveUS9228984B2
Innovation
- A capture and release device with a switching valve is integrated into the GC-MS system, allowing for the capture and release of eluted compounds using cooling and heating units, enabling simultaneous analysis of both simple and complex compounds by rotating the switching valve to connect different capillary columns to the mass spectrometer.
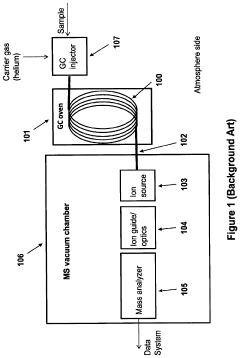
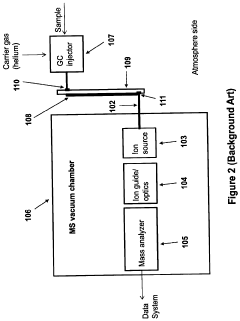
Portable MEMS GC-ms system
PatentActiveUS20200378930A1
Innovation
- The integration of a MEMS GC column with an integrated heater inside the MS vacuum system, leveraging the high thermal isolation properties of a vacuum to minimize heating power losses, and incorporating non-active cooling methods like a periodically activated cold finger for efficient temperature control.
Validation Protocols for Carryover Assessment
Establishing robust validation protocols for carryover assessment is essential for ensuring the reliability and accuracy of GC-MS analyses involving multiple analytes. These protocols must be systematically designed to quantify, monitor, and minimize carryover effects that can compromise analytical results. The foundation of effective validation begins with determining the carryover threshold specific to the analytical method and target compounds, typically set at 0.1-0.5% of the preceding sample's concentration.
A comprehensive validation protocol should include a series of blank injections following high-concentration standard samples, with the number of blanks determined by the persistence of carryover observed in preliminary studies. The concentration gradient approach is particularly valuable, wherein standards of increasing concentration are analyzed, followed by blanks to establish the concentration at which carryover becomes significant. This establishes the upper limit of quantification for the method.
Statistical evaluation forms a critical component of validation protocols, requiring replicate analyses to calculate the mean, standard deviation, and relative standard deviation of carryover measurements. Acceptance criteria must be clearly defined before validation begins, typically specifying that carryover should not exceed a predetermined percentage of the lower limit of quantification for each analyte.
Matrix-specific validation is essential as different sample matrices can significantly influence carryover behavior. The protocol should include assessment of carryover in all relevant matrices encountered in routine analysis, as complex biological or environmental samples may exhibit different carryover characteristics compared to simple standard solutions.
Long-term monitoring protocols should be incorporated to evaluate carryover trends over time, which can indicate system degradation or contamination buildup. This includes regular system suitability tests with carryover assessment as part of routine quality control procedures. Documentation requirements must specify detailed recording of all carryover evaluation parameters, including injection sequences, concentrations, peak areas in blanks, and calculated carryover percentages.
Corrective action procedures must be clearly outlined in the validation protocol, detailing specific steps to be taken when carryover exceeds acceptable limits. These may include system cleaning, component replacement, or method modification. Finally, the protocol should include periodic revalidation schedules to ensure continued compliance with carryover specifications, particularly after major maintenance events or changes to the analytical system.
A comprehensive validation protocol should include a series of blank injections following high-concentration standard samples, with the number of blanks determined by the persistence of carryover observed in preliminary studies. The concentration gradient approach is particularly valuable, wherein standards of increasing concentration are analyzed, followed by blanks to establish the concentration at which carryover becomes significant. This establishes the upper limit of quantification for the method.
Statistical evaluation forms a critical component of validation protocols, requiring replicate analyses to calculate the mean, standard deviation, and relative standard deviation of carryover measurements. Acceptance criteria must be clearly defined before validation begins, typically specifying that carryover should not exceed a predetermined percentage of the lower limit of quantification for each analyte.
Matrix-specific validation is essential as different sample matrices can significantly influence carryover behavior. The protocol should include assessment of carryover in all relevant matrices encountered in routine analysis, as complex biological or environmental samples may exhibit different carryover characteristics compared to simple standard solutions.
Long-term monitoring protocols should be incorporated to evaluate carryover trends over time, which can indicate system degradation or contamination buildup. This includes regular system suitability tests with carryover assessment as part of routine quality control procedures. Documentation requirements must specify detailed recording of all carryover evaluation parameters, including injection sequences, concentrations, peak areas in blanks, and calculated carryover percentages.
Corrective action procedures must be clearly outlined in the validation protocol, detailing specific steps to be taken when carryover exceeds acceptable limits. These may include system cleaning, component replacement, or method modification. Finally, the protocol should include periodic revalidation schedules to ensure continued compliance with carryover specifications, particularly after major maintenance events or changes to the analytical system.
Environmental and Sustainability Considerations
The environmental impact of analytical chemistry practices has become increasingly important in modern laboratory operations. When addressing carryover issues in GC-MS multi-analyte analysis, environmental and sustainability considerations must be integrated into solution strategies. Traditional approaches to minimize carryover often involve extensive solvent washing procedures and frequent replacement of consumables, which can generate significant chemical waste and increase the environmental footprint of analytical processes.
Solvent consumption represents one of the most substantial environmental concerns in GC-MS analysis. High-purity solvents used for cleaning and sample preparation are often derived from non-renewable resources and may contain hazardous compounds. Implementing solvent recycling systems and adopting greener alternative solvents can substantially reduce environmental impact while maintaining analytical performance. For instance, replacing chlorinated solvents with bio-based alternatives has shown promising results in reducing carryover without compromising separation efficiency.
Energy consumption is another critical environmental factor in GC-MS operations. The high temperatures required for inlet cleaning and column conditioning contribute significantly to laboratory energy usage. Optimizing temperature programs and implementing intelligent standby modes can reduce energy consumption by up to 30% while simultaneously minimizing carryover through more efficient thermal cleaning cycles.
Consumable waste management presents additional sustainability challenges. Inlet liners, septa, and columns require regular replacement to control carryover, generating substantial laboratory waste. Manufacturers are now developing more durable components with extended lifespans and improved inertness, reducing both waste generation and the frequency of system maintenance. Some innovative materials incorporate recyclable or biodegradable elements without sacrificing analytical performance.
Water usage in laboratory operations also merits consideration. Advanced cooling systems and more efficient vacuum pumps can significantly reduce water consumption while maintaining optimal instrument performance. Closed-loop cooling systems represent a particularly effective approach for laboratories in water-stressed regions.
The implementation of green analytical chemistry principles in carryover reduction strategies aligns with broader sustainability goals and increasingly stringent environmental regulations. Organizations like the American Chemical Society's Green Chemistry Institute have developed specific guidelines for sustainable analytical practices, providing a framework for laboratories to evaluate and improve their environmental performance while addressing technical challenges like carryover in multi-analyte GC-MS applications.
Solvent consumption represents one of the most substantial environmental concerns in GC-MS analysis. High-purity solvents used for cleaning and sample preparation are often derived from non-renewable resources and may contain hazardous compounds. Implementing solvent recycling systems and adopting greener alternative solvents can substantially reduce environmental impact while maintaining analytical performance. For instance, replacing chlorinated solvents with bio-based alternatives has shown promising results in reducing carryover without compromising separation efficiency.
Energy consumption is another critical environmental factor in GC-MS operations. The high temperatures required for inlet cleaning and column conditioning contribute significantly to laboratory energy usage. Optimizing temperature programs and implementing intelligent standby modes can reduce energy consumption by up to 30% while simultaneously minimizing carryover through more efficient thermal cleaning cycles.
Consumable waste management presents additional sustainability challenges. Inlet liners, septa, and columns require regular replacement to control carryover, generating substantial laboratory waste. Manufacturers are now developing more durable components with extended lifespans and improved inertness, reducing both waste generation and the frequency of system maintenance. Some innovative materials incorporate recyclable or biodegradable elements without sacrificing analytical performance.
Water usage in laboratory operations also merits consideration. Advanced cooling systems and more efficient vacuum pumps can significantly reduce water consumption while maintaining optimal instrument performance. Closed-loop cooling systems represent a particularly effective approach for laboratories in water-stressed regions.
The implementation of green analytical chemistry principles in carryover reduction strategies aligns with broader sustainability goals and increasingly stringent environmental regulations. Organizations like the American Chemical Society's Green Chemistry Institute have developed specific guidelines for sustainable analytical practices, providing a framework for laboratories to evaluate and improve their environmental performance while addressing technical challenges like carryover in multi-analyte GC-MS applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!