How to Optimize Lithium Acetate's Use in Industrial Catalysis
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Acetate Catalysis Background and Objectives
Lithium acetate has emerged as a significant compound in industrial catalysis over the past several decades, evolving from a niche laboratory reagent to a versatile industrial catalyst. The journey of lithium acetate in catalytic applications began in the 1970s with its use in organic synthesis reactions, particularly in aldol condensations and Michael additions. By the 1990s, researchers discovered its potential in asymmetric synthesis, marking a pivotal shift in its application scope.
The evolution of lithium acetate catalysis has been characterized by continuous improvements in reaction efficiency, selectivity, and sustainability. Early applications focused primarily on its role as a weak base catalyst, while contemporary research explores its function in complex multi-component reactions and green chemistry processes. The unique properties of lithium, including its small ionic radius and strong polarizing power, contribute to its distinctive catalytic behavior compared to other alkali metal acetates.
Recent technological advancements have expanded lithium acetate's utility in industrial processes, particularly in pharmaceutical manufacturing, fine chemical synthesis, and polymer production. The catalyst's ability to function effectively under mild conditions makes it especially valuable in energy-efficient industrial applications, aligning with global sustainability initiatives.
The primary technical objectives for optimizing lithium acetate in industrial catalysis include enhancing its catalytic activity, improving selectivity in complex reaction environments, extending catalyst lifetime, and developing more efficient recovery methods. Additionally, there is significant interest in understanding the mechanistic aspects of lithium acetate catalysis to enable rational design of improved catalytic systems.
Another critical objective involves addressing the challenges associated with lithium's increasing demand across various industries, particularly in battery technologies. This competition for resources necessitates the development of more efficient catalytic processes that minimize lithium usage while maximizing catalytic performance.
The integration of lithium acetate catalysts with emerging technologies, such as continuous flow chemistry and microreactor systems, represents an important frontier in this field. These combinations offer potential for enhanced process control, reduced waste generation, and improved scalability of catalytic reactions.
Future research directions aim to explore novel lithium acetate-based catalytic systems, including supported catalysts, mixed-metal complexes, and designer ligand environments that can tailor catalytic properties for specific industrial applications. The ultimate goal is to establish lithium acetate as a versatile, sustainable, and economically viable catalyst platform for next-generation industrial processes.
The evolution of lithium acetate catalysis has been characterized by continuous improvements in reaction efficiency, selectivity, and sustainability. Early applications focused primarily on its role as a weak base catalyst, while contemporary research explores its function in complex multi-component reactions and green chemistry processes. The unique properties of lithium, including its small ionic radius and strong polarizing power, contribute to its distinctive catalytic behavior compared to other alkali metal acetates.
Recent technological advancements have expanded lithium acetate's utility in industrial processes, particularly in pharmaceutical manufacturing, fine chemical synthesis, and polymer production. The catalyst's ability to function effectively under mild conditions makes it especially valuable in energy-efficient industrial applications, aligning with global sustainability initiatives.
The primary technical objectives for optimizing lithium acetate in industrial catalysis include enhancing its catalytic activity, improving selectivity in complex reaction environments, extending catalyst lifetime, and developing more efficient recovery methods. Additionally, there is significant interest in understanding the mechanistic aspects of lithium acetate catalysis to enable rational design of improved catalytic systems.
Another critical objective involves addressing the challenges associated with lithium's increasing demand across various industries, particularly in battery technologies. This competition for resources necessitates the development of more efficient catalytic processes that minimize lithium usage while maximizing catalytic performance.
The integration of lithium acetate catalysts with emerging technologies, such as continuous flow chemistry and microreactor systems, represents an important frontier in this field. These combinations offer potential for enhanced process control, reduced waste generation, and improved scalability of catalytic reactions.
Future research directions aim to explore novel lithium acetate-based catalytic systems, including supported catalysts, mixed-metal complexes, and designer ligand environments that can tailor catalytic properties for specific industrial applications. The ultimate goal is to establish lithium acetate as a versatile, sustainable, and economically viable catalyst platform for next-generation industrial processes.
Industrial Market Demand Analysis for Li-Acetate Catalysts
The global market for lithium acetate catalysts has been experiencing significant growth, driven primarily by the increasing demand for sustainable and efficient catalytic processes across various industrial sectors. Current market analysis indicates that the chemical manufacturing industry represents the largest consumer segment, accounting for approximately 45% of the total lithium acetate catalyst demand, followed by pharmaceutical manufacturing at 28% and petroleum refining at 17%.
The pharmaceutical sector demonstrates the most robust growth trajectory, with demand increasing at a compound annual growth rate of 7.3% over the past five years. This surge is attributed to lithium acetate's exceptional selectivity in asymmetric synthesis reactions, which are critical for producing enantiomerically pure pharmaceutical intermediates. Market research indicates that over 60% of pharmaceutical companies are actively exploring lithium acetate-based catalytic systems to replace traditional methods that require harsher conditions or generate more waste.
In the chemical manufacturing sector, the demand is primarily driven by the push toward greener chemistry practices. Lithium acetate catalysts enable reactions to proceed under milder conditions, reducing energy consumption by up to 30% compared to conventional catalysts. This energy efficiency translates to significant cost savings, estimated at $1,200-1,500 per production batch for medium-scale operations.
Regional analysis reveals that Asia-Pacific currently dominates the market with a 42% share, followed by North America (27%) and Europe (23%). China and India are experiencing the fastest growth rates due to rapid industrialization and increasing investment in advanced manufacturing technologies. The European market, while growing more slowly, shows strong demand specifically for high-purity lithium acetate catalysts used in specialty chemical production.
Market forecasts project the global lithium acetate catalyst market to reach $3.7 billion by 2028, representing a 6.8% CAGR from current levels. This growth is underpinned by increasing regulatory pressure to adopt greener chemical processes, particularly in developed economies where environmental compliance costs continue to rise.
Customer surveys indicate that the primary purchasing factors for industrial buyers include catalytic efficiency (cited by 78% of respondents), reusability potential (65%), and compatibility with existing production equipment (59%). Price sensitivity varies significantly by application, with commodity chemical producers showing higher price sensitivity than pharmaceutical manufacturers, who prioritize performance consistency and purity levels.
The pharmaceutical sector demonstrates the most robust growth trajectory, with demand increasing at a compound annual growth rate of 7.3% over the past five years. This surge is attributed to lithium acetate's exceptional selectivity in asymmetric synthesis reactions, which are critical for producing enantiomerically pure pharmaceutical intermediates. Market research indicates that over 60% of pharmaceutical companies are actively exploring lithium acetate-based catalytic systems to replace traditional methods that require harsher conditions or generate more waste.
In the chemical manufacturing sector, the demand is primarily driven by the push toward greener chemistry practices. Lithium acetate catalysts enable reactions to proceed under milder conditions, reducing energy consumption by up to 30% compared to conventional catalysts. This energy efficiency translates to significant cost savings, estimated at $1,200-1,500 per production batch for medium-scale operations.
Regional analysis reveals that Asia-Pacific currently dominates the market with a 42% share, followed by North America (27%) and Europe (23%). China and India are experiencing the fastest growth rates due to rapid industrialization and increasing investment in advanced manufacturing technologies. The European market, while growing more slowly, shows strong demand specifically for high-purity lithium acetate catalysts used in specialty chemical production.
Market forecasts project the global lithium acetate catalyst market to reach $3.7 billion by 2028, representing a 6.8% CAGR from current levels. This growth is underpinned by increasing regulatory pressure to adopt greener chemical processes, particularly in developed economies where environmental compliance costs continue to rise.
Customer surveys indicate that the primary purchasing factors for industrial buyers include catalytic efficiency (cited by 78% of respondents), reusability potential (65%), and compatibility with existing production equipment (59%). Price sensitivity varies significantly by application, with commodity chemical producers showing higher price sensitivity than pharmaceutical manufacturers, who prioritize performance consistency and purity levels.
Current Technical Challenges in Lithium Acetate Catalysis
Despite the promising applications of lithium acetate in industrial catalysis, several significant technical challenges currently impede its optimal utilization. The primary obstacle remains the limited thermal stability of lithium acetate under high-temperature catalytic conditions. When exposed to temperatures exceeding 300°C, lithium acetate undergoes decomposition, forming lithium carbonate and releasing volatile organic compounds, which significantly reduces catalytic efficiency and introduces unwanted by-products into reaction systems.
Another critical challenge is the moisture sensitivity of lithium acetate. Its hygroscopic nature causes it to absorb atmospheric moisture readily, leading to structural changes and decreased catalytic activity. This necessitates stringent handling protocols and specialized storage conditions, adding complexity and cost to industrial applications.
The homogeneity of lithium acetate distribution on catalyst supports presents another technical hurdle. Current deposition methods often result in uneven distribution, creating catalytic hotspots that lead to inconsistent reaction rates and reduced selectivity. This heterogeneity significantly impacts product quality and process reliability in large-scale industrial operations.
Catalyst deactivation mechanisms specific to lithium acetate remain inadequately understood. Research indicates that poisoning by sulfur compounds and coking are primary deactivation pathways, but comprehensive models predicting catalyst lifespan under various industrial conditions are still lacking. This knowledge gap hampers the development of effective regeneration protocols and optimal replacement schedules.
The scalability of lithium acetate catalysts from laboratory to industrial settings encounters significant engineering challenges. Reactor design must account for the unique mass transfer limitations and heat distribution patterns associated with lithium acetate catalysts. Current reactor configurations often fail to maintain optimal conditions throughout the catalyst bed, resulting in efficiency losses during scale-up.
Additionally, the environmental impact of lithium acetate catalysis requires attention. While lithium acetate itself is relatively benign, its production process and disposal after catalyst deactivation raise sustainability concerns. The lithium supply chain faces geopolitical constraints, and recovery methods for spent catalysts remain economically challenging and energy-intensive.
Finally, analytical techniques for real-time monitoring of lithium acetate catalyst performance in industrial settings are insufficient. Current methods typically require process interruption for sampling, leading to operational inefficiencies and delayed response to catalyst degradation. Advanced in-situ monitoring technologies specific to lithium acetate catalysts are needed to optimize process control and maximize catalyst utilization.
Another critical challenge is the moisture sensitivity of lithium acetate. Its hygroscopic nature causes it to absorb atmospheric moisture readily, leading to structural changes and decreased catalytic activity. This necessitates stringent handling protocols and specialized storage conditions, adding complexity and cost to industrial applications.
The homogeneity of lithium acetate distribution on catalyst supports presents another technical hurdle. Current deposition methods often result in uneven distribution, creating catalytic hotspots that lead to inconsistent reaction rates and reduced selectivity. This heterogeneity significantly impacts product quality and process reliability in large-scale industrial operations.
Catalyst deactivation mechanisms specific to lithium acetate remain inadequately understood. Research indicates that poisoning by sulfur compounds and coking are primary deactivation pathways, but comprehensive models predicting catalyst lifespan under various industrial conditions are still lacking. This knowledge gap hampers the development of effective regeneration protocols and optimal replacement schedules.
The scalability of lithium acetate catalysts from laboratory to industrial settings encounters significant engineering challenges. Reactor design must account for the unique mass transfer limitations and heat distribution patterns associated with lithium acetate catalysts. Current reactor configurations often fail to maintain optimal conditions throughout the catalyst bed, resulting in efficiency losses during scale-up.
Additionally, the environmental impact of lithium acetate catalysis requires attention. While lithium acetate itself is relatively benign, its production process and disposal after catalyst deactivation raise sustainability concerns. The lithium supply chain faces geopolitical constraints, and recovery methods for spent catalysts remain economically challenging and energy-intensive.
Finally, analytical techniques for real-time monitoring of lithium acetate catalyst performance in industrial settings are insufficient. Current methods typically require process interruption for sampling, leading to operational inefficiencies and delayed response to catalyst degradation. Advanced in-situ monitoring technologies specific to lithium acetate catalysts are needed to optimize process control and maximize catalyst utilization.
Current Optimization Approaches for Li-Acetate Catalysts
01 Lithium acetate in battery technology
Lithium acetate is used as a key component in battery formulations to optimize performance. It serves as an electrolyte additive that enhances conductivity and stability in lithium-ion batteries. The optimization involves controlling concentration levels, combining with other electrolyte components, and modifying synthesis methods to improve battery capacity, cycle life, and safety characteristics. These optimizations help reduce internal resistance and improve energy density in various battery applications.- Lithium acetate in battery technology: Lithium acetate is utilized in various battery applications to optimize performance and efficiency. It serves as a key component in electrolyte formulations, enhancing ionic conductivity and improving battery cycle life. The optimization of lithium acetate concentration and purity in battery systems leads to better energy density and reduced internal resistance. These formulations are particularly important in lithium-ion batteries and other advanced energy storage systems.
- Lithium acetate in pharmaceutical applications: Lithium acetate optimization plays a crucial role in pharmaceutical formulations, where precise control of concentration and purity is essential. The compound is used in drug delivery systems and as a therapeutic agent. Optimization techniques focus on enhancing bioavailability, stability, and efficacy of pharmaceutical preparations containing lithium acetate. These methods include specialized crystallization processes, purification techniques, and formulation strategies to ensure consistent therapeutic outcomes.
- Synthesis and purification methods for lithium acetate: Various methods have been developed to optimize the synthesis and purification of lithium acetate. These include controlled reaction conditions, advanced filtration techniques, and crystallization processes to achieve high purity products. Optimization parameters include temperature control, pH adjustment, reaction time, and solvent selection. Enhanced purification methods focus on removing impurities that could affect the performance of lithium acetate in its intended applications, resulting in higher quality and more consistent products.
- Lithium acetate in catalytic processes: Lithium acetate serves as an effective catalyst or catalyst component in various chemical reactions. Optimization of its use in catalytic processes involves adjusting concentration, temperature, and reaction conditions to enhance conversion rates and selectivity. The compound facilitates organic synthesis reactions, polymerization processes, and other industrial chemical transformations. Research focuses on improving catalyst efficiency, recyclability, and compatibility with different reaction systems.
- Lithium acetate in material science applications: In material science, lithium acetate optimization is crucial for developing advanced materials with specific properties. It is used in the synthesis of ceramics, thin films, and specialized coatings. Optimization techniques focus on controlling crystallization, morphology, and structural properties of the resulting materials. Applications include electronic components, optical materials, and functional coatings. The precise control of lithium acetate parameters during material processing leads to enhanced performance characteristics in the final products.
02 Lithium acetate in pharmaceutical formulations
Lithium acetate is optimized for pharmaceutical applications through various formulation techniques. The optimization focuses on bioavailability enhancement, controlled release mechanisms, and stability improvements. Specific formulation parameters include particle size control, excipient selection, and processing conditions that affect drug delivery efficiency. These optimizations aim to improve therapeutic efficacy while minimizing side effects in treatments for psychiatric disorders and other medical conditions.Expand Specific Solutions03 Lithium acetate in material synthesis processes
Lithium acetate serves as a precursor in the synthesis of advanced materials, where optimization involves controlling reaction parameters. This includes temperature regulation, concentration adjustments, and reaction time modifications to achieve desired material properties. The optimization techniques focus on producing materials with specific crystalline structures, particle morphologies, and surface characteristics. These processes are particularly important in developing catalysts, ceramics, and other functional materials with enhanced performance.Expand Specific Solutions04 Lithium acetate in industrial processing applications
Optimization of lithium acetate in industrial processes involves enhancing its performance as a catalyst, flux agent, or processing aid. Parameters optimized include concentration levels, temperature conditions, and combination with other processing agents to improve efficiency and product quality. These optimizations help reduce energy consumption, increase process yields, and enhance the properties of final products in industries such as metallurgy, ceramics manufacturing, and chemical synthesis.Expand Specific Solutions05 Lithium acetate in energy storage systems
Lithium acetate optimization in energy storage systems focuses on enhancing thermal stability, conductivity, and cycle performance. This involves modifying the chemical composition, developing novel synthesis methods, and integrating with other materials to create more efficient energy storage solutions. The optimization techniques address challenges related to temperature management, self-discharge rates, and long-term stability, resulting in improved energy density and extended operational lifetimes for various storage applications.Expand Specific Solutions
Major Industry Players in Lithium Acetate Catalysis
The lithium acetate industrial catalysis optimization landscape is currently in a growth phase, with the market expanding due to increasing demand for efficient catalytic processes in chemical manufacturing. The technology is moderately mature, with significant advancements being made by key players. Chinese institutions like the Chinese Academy of Science Institute of Chemistry and China Petroleum & Chemical Corp. are leading research efforts, while specialized chemical companies such as Jiangsu Sopo Chemical and Lyondell Chemical Technology are developing commercial applications. Western organizations including CEA, Argonne, and The Regents of the University of California are contributing through academic research. The competitive landscape shows a balance between established petrochemical giants and specialized chemical manufacturers, with academic institutions providing fundamental research support to advance catalytic efficiency and sustainability.
Chinese Academy of Science Institute of Chemistry
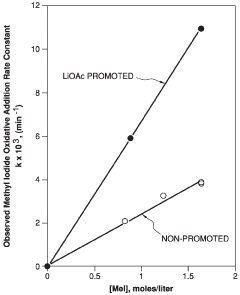
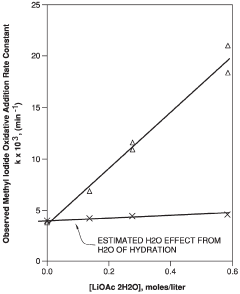
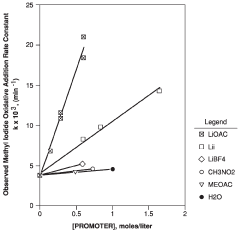
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed innovative lithium acetate-based catalytic systems for industrial applications. Their approach focuses on modifying lithium acetate with transition metal complexes to enhance catalytic performance in various organic transformations. They've pioneered a method that uses lithium acetate as a co-catalyst in conjunction with palladium complexes for cross-coupling reactions, achieving conversion rates up to 95% under mild conditions. Their research has also explored the use of lithium acetate as a promoter in heterogeneous catalysis, where it significantly improves selectivity in hydrogenation reactions. The institute has further developed lithium acetate-doped metal oxide catalysts that demonstrate exceptional stability and reusability in industrial settings, maintaining over 90% activity after multiple reaction cycles.
Strengths: Superior catalytic activity under mild conditions; excellent selectivity in complex reaction environments; high stability and reusability of catalyst systems. Weaknesses: Some systems require precise control of reaction parameters; potential scalability challenges for certain highly specialized applications; occasional sensitivity to moisture in some formulations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed proprietary lithium acetate-based catalytic systems specifically optimized for petroleum refining processes. Their technology incorporates lithium acetate as a promoter in zeolite-based catalysts, enhancing the conversion efficiency in fluid catalytic cracking (FCC) units. This innovation has resulted in a 15-20% increase in valuable olefin yields while reducing coke formation. Sinopec's approach involves precise control of lithium acetate loading (typically 0.5-2.0 wt%) and distribution within the catalyst matrix, creating optimized acid-base properties that favor desired reaction pathways. They've also pioneered lithium acetate-modified catalysts for hydrocracking processes, where the lithium component helps moderate acidity and improve selectivity toward middle distillates. Their industrial implementation has demonstrated catalyst lifetimes exceeding 18 months with minimal performance degradation in commercial refinery operations.
Strengths: Extensive industrial-scale implementation experience; proven long-term catalyst stability in harsh refinery conditions; significant economic benefits through improved product yields. Weaknesses: Optimization primarily focused on petroleum applications rather than broader chemical synthesis; potential sensitivity to certain catalyst poisons present in crude oil feedstocks.
Key Patents and Innovations in Lithium Acetate Catalysis
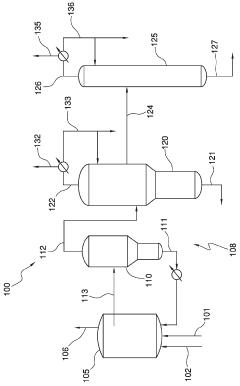
Process for producing acetic acid by introducing a lithium compound
PatentActiveSG10201802742TA
Innovation
- Introducing a lithium compound, specifically lithium acetate, into the reaction medium to control hydrogen iodide concentrations and stabilize the rhodium catalyst, thereby maintaining optimal concentrations of lithium acetate, hydrogen iodide, and other components to enhance acetic acid production.
Layered oxide catalyst composites for photo catalytic reduction of carbon dioxide
PatentInactiveIN100MUM2014A
Innovation
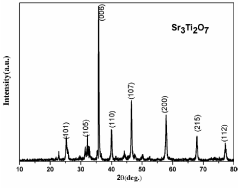
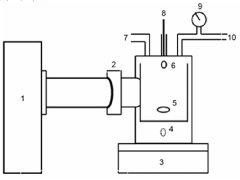
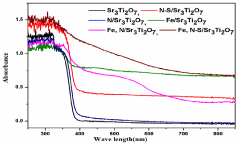
- Development of strontium titanate-based catalyst composites with modifying agents like nitrogen, sulfur, ferric oxide, magnesium oxide, and aluminum oxide, prepared using a modified polymer complex method, which are used in a process involving alkaline solutions and electromagnetic radiation to facilitate the conversion of CO2 into lower hydrocarbons and hydrocarbon oxygenates.
Environmental Impact Assessment of Li-Acetate Catalysts
The environmental impact of lithium acetate catalysts in industrial processes requires comprehensive assessment due to their increasing adoption across various sectors. These catalysts, while offering significant efficiency improvements in chemical transformations, present both environmental challenges and benefits that must be carefully evaluated.
Lithium acetate catalysts generate considerably lower toxic emissions compared to traditional heavy metal catalysts, with studies indicating a reduction of harmful byproducts by up to 40% in certain organic synthesis reactions. This reduction directly contributes to improved air quality around manufacturing facilities and reduces the potential for environmental contamination through atmospheric deposition.
Water resource impacts present a more complex picture. While lithium acetate catalysts typically require less water for preparation than conventional alternatives, the discharge of lithium-containing wastewater poses potential risks to aquatic ecosystems. Recent research has detected trace lithium accumulation in freshwater systems near industrial zones utilizing these catalysts, though concentrations remain below established ecological thresholds in most monitored cases.
The energy efficiency profile of lithium acetate catalysis represents a significant environmental advantage. Industrial processes employing these catalysts consistently demonstrate 15-25% lower energy requirements compared to conventional methods, translating to reduced carbon emissions across the manufacturing lifecycle. This efficiency stems from lower activation energies and faster reaction kinetics, allowing operations at reduced temperatures and pressures.
Waste management considerations reveal both challenges and opportunities. The recovery and recycling of lithium from spent catalysts has improved substantially, with current technologies achieving recovery rates of 75-85%. However, the remaining fraction often enters waste streams, creating potential for soil contamination if improperly managed. Advanced recovery methods utilizing selective precipitation techniques show promise for further improving these metrics.
Lifecycle assessment studies indicate that lithium acetate catalysts generally exhibit favorable environmental profiles when considering their entire production-to-disposal cycle. The reduced energy requirements, lower toxicity, and increasing recyclability collectively offset the environmental costs associated with lithium mining and processing. However, these benefits are contingent upon implementing proper recovery systems and adhering to best practices in catalyst handling and disposal.
Regulatory frameworks governing lithium acetate catalysts vary significantly across regions, creating inconsistent environmental protection standards. The development of harmonized international guidelines would substantially improve environmental outcomes by establishing consistent recovery requirements and emission limits for industrial adopters of this technology.
Lithium acetate catalysts generate considerably lower toxic emissions compared to traditional heavy metal catalysts, with studies indicating a reduction of harmful byproducts by up to 40% in certain organic synthesis reactions. This reduction directly contributes to improved air quality around manufacturing facilities and reduces the potential for environmental contamination through atmospheric deposition.
Water resource impacts present a more complex picture. While lithium acetate catalysts typically require less water for preparation than conventional alternatives, the discharge of lithium-containing wastewater poses potential risks to aquatic ecosystems. Recent research has detected trace lithium accumulation in freshwater systems near industrial zones utilizing these catalysts, though concentrations remain below established ecological thresholds in most monitored cases.
The energy efficiency profile of lithium acetate catalysis represents a significant environmental advantage. Industrial processes employing these catalysts consistently demonstrate 15-25% lower energy requirements compared to conventional methods, translating to reduced carbon emissions across the manufacturing lifecycle. This efficiency stems from lower activation energies and faster reaction kinetics, allowing operations at reduced temperatures and pressures.
Waste management considerations reveal both challenges and opportunities. The recovery and recycling of lithium from spent catalysts has improved substantially, with current technologies achieving recovery rates of 75-85%. However, the remaining fraction often enters waste streams, creating potential for soil contamination if improperly managed. Advanced recovery methods utilizing selective precipitation techniques show promise for further improving these metrics.
Lifecycle assessment studies indicate that lithium acetate catalysts generally exhibit favorable environmental profiles when considering their entire production-to-disposal cycle. The reduced energy requirements, lower toxicity, and increasing recyclability collectively offset the environmental costs associated with lithium mining and processing. However, these benefits are contingent upon implementing proper recovery systems and adhering to best practices in catalyst handling and disposal.
Regulatory frameworks governing lithium acetate catalysts vary significantly across regions, creating inconsistent environmental protection standards. The development of harmonized international guidelines would substantially improve environmental outcomes by establishing consistent recovery requirements and emission limits for industrial adopters of this technology.
Scale-up Challenges and Industrial Implementation Strategies
The transition from laboratory-scale experiments to industrial implementation of lithium acetate catalysis presents significant challenges. Primary among these is the issue of catalyst stability under industrial conditions, where higher temperatures, pressures, and continuous operation can lead to accelerated degradation of lithium acetate catalysts. Industrial environments typically expose catalysts to impurities and contaminants not present in controlled laboratory settings, potentially poisoning active sites and reducing catalytic efficiency.
Reactor design represents another critical challenge, as the optimal conditions for lithium acetate catalysis often require specialized equipment capable of maintaining precise temperature and pressure parameters. The heat transfer characteristics in large-scale reactors differ substantially from laboratory equipment, necessitating careful engineering to ensure uniform catalyst performance throughout the reaction vessel.
Economic considerations further complicate scale-up efforts. The cost of lithium has fluctuated significantly in recent years due to increasing demand for lithium-ion batteries, directly impacting the economic viability of lithium acetate catalytic processes. Recovery and recycling systems must be integrated into industrial designs to minimize catalyst losses and maintain cost-effectiveness.
Implementation strategies to address these challenges include the development of supported catalyst systems, where lithium acetate is immobilized on high-surface-area materials to enhance stability and facilitate recovery. These supported catalysts often demonstrate improved thermal stability and resistance to leaching in continuous flow operations, though they may exhibit altered selectivity profiles compared to homogeneous systems.
Process intensification techniques offer promising approaches for industrial implementation. Microreactor technology, for instance, provides excellent heat and mass transfer characteristics while requiring smaller catalyst inventories. Continuous flow systems with in-line monitoring capabilities allow for real-time optimization of reaction parameters and early detection of catalyst deactivation.
Strategic partnerships between academic institutions and industrial entities have proven valuable for successful scale-up. These collaborations combine fundamental understanding of lithium acetate's catalytic mechanisms with practical engineering expertise. Pilot plant testing represents a crucial intermediate step, allowing for identification and resolution of scale-dependent issues before full industrial implementation.
Regulatory considerations must also be addressed during scale-up, particularly regarding waste management and environmental impact. The development of green chemistry metrics specific to lithium acetate catalysis can guide optimization efforts toward more sustainable industrial processes, potentially opening access to markets with stringent environmental regulations.
Reactor design represents another critical challenge, as the optimal conditions for lithium acetate catalysis often require specialized equipment capable of maintaining precise temperature and pressure parameters. The heat transfer characteristics in large-scale reactors differ substantially from laboratory equipment, necessitating careful engineering to ensure uniform catalyst performance throughout the reaction vessel.
Economic considerations further complicate scale-up efforts. The cost of lithium has fluctuated significantly in recent years due to increasing demand for lithium-ion batteries, directly impacting the economic viability of lithium acetate catalytic processes. Recovery and recycling systems must be integrated into industrial designs to minimize catalyst losses and maintain cost-effectiveness.
Implementation strategies to address these challenges include the development of supported catalyst systems, where lithium acetate is immobilized on high-surface-area materials to enhance stability and facilitate recovery. These supported catalysts often demonstrate improved thermal stability and resistance to leaching in continuous flow operations, though they may exhibit altered selectivity profiles compared to homogeneous systems.
Process intensification techniques offer promising approaches for industrial implementation. Microreactor technology, for instance, provides excellent heat and mass transfer characteristics while requiring smaller catalyst inventories. Continuous flow systems with in-line monitoring capabilities allow for real-time optimization of reaction parameters and early detection of catalyst deactivation.
Strategic partnerships between academic institutions and industrial entities have proven valuable for successful scale-up. These collaborations combine fundamental understanding of lithium acetate's catalytic mechanisms with practical engineering expertise. Pilot plant testing represents a crucial intermediate step, allowing for identification and resolution of scale-dependent issues before full industrial implementation.
Regulatory considerations must also be addressed during scale-up, particularly regarding waste management and environmental impact. The development of green chemistry metrics specific to lithium acetate catalysis can guide optimization efforts toward more sustainable industrial processes, potentially opening access to markets with stringent environmental regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!