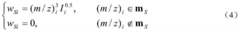How to Resolve GC-MS Peak Overlap in High-Resolution Work
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Peak Overlap Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the 1950s, becoming an indispensable analytical technique in various fields including environmental analysis, forensic science, pharmaceutical research, and metabolomics. The technique combines the separation capabilities of gas chromatography with the detection specificity of mass spectrometry, allowing for the identification and quantification of complex mixtures of volatile and semi-volatile compounds.
Peak overlap in GC-MS represents one of the most persistent challenges in high-resolution analytical work. This phenomenon occurs when two or more compounds elute from the chromatographic column at similar retention times, resulting in overlapping peaks that complicate accurate identification and quantification. The problem has become increasingly significant as analytical demands have shifted toward more complex matrices and lower detection limits.
The evolution of GC-MS technology has been marked by continuous improvements in resolution, sensitivity, and data processing capabilities. Early systems from the 1970s and 1980s offered limited resolution, making peak overlap a common and often insurmountable problem. The 1990s saw significant advancements in column technology and instrument design, while the 2000s brought sophisticated software solutions for deconvolution.
Despite these advances, peak overlap remains a critical challenge in high-resolution work, particularly when analyzing complex environmental samples, biological matrices, or when regulatory compliance requires unambiguous compound identification. The increasing demand for comprehensive analysis of complex mixtures has further highlighted the limitations of current methodologies.
The primary objective of addressing GC-MS peak overlap is to enhance the accuracy and reliability of analytical results in high-resolution applications. This includes improving compound identification confidence, enhancing quantitative precision, and reducing false positives and negatives that can result from inadequately resolved chromatographic peaks.
Secondary objectives include increasing laboratory throughput by minimizing the need for sample re-analysis or alternative analytical approaches, reducing the expertise barrier for complex sample analysis, and enabling more comprehensive characterization of complex mixtures in a single analytical run.
The technological trajectory suggests several promising directions for resolving peak overlap issues, including advanced multidimensional chromatography techniques, improved deconvolution algorithms leveraging machine learning, and novel hybrid detection systems. These approaches aim to push beyond the current limitations of one-dimensional GC-MS analysis while maintaining practical applicability in routine laboratory settings.
As analytical requirements continue to become more stringent across industries, developing robust solutions to the peak overlap challenge will be essential for the continued relevance and advancement of GC-MS as a premier analytical technique in high-resolution applications.
Peak overlap in GC-MS represents one of the most persistent challenges in high-resolution analytical work. This phenomenon occurs when two or more compounds elute from the chromatographic column at similar retention times, resulting in overlapping peaks that complicate accurate identification and quantification. The problem has become increasingly significant as analytical demands have shifted toward more complex matrices and lower detection limits.
The evolution of GC-MS technology has been marked by continuous improvements in resolution, sensitivity, and data processing capabilities. Early systems from the 1970s and 1980s offered limited resolution, making peak overlap a common and often insurmountable problem. The 1990s saw significant advancements in column technology and instrument design, while the 2000s brought sophisticated software solutions for deconvolution.
Despite these advances, peak overlap remains a critical challenge in high-resolution work, particularly when analyzing complex environmental samples, biological matrices, or when regulatory compliance requires unambiguous compound identification. The increasing demand for comprehensive analysis of complex mixtures has further highlighted the limitations of current methodologies.
The primary objective of addressing GC-MS peak overlap is to enhance the accuracy and reliability of analytical results in high-resolution applications. This includes improving compound identification confidence, enhancing quantitative precision, and reducing false positives and negatives that can result from inadequately resolved chromatographic peaks.
Secondary objectives include increasing laboratory throughput by minimizing the need for sample re-analysis or alternative analytical approaches, reducing the expertise barrier for complex sample analysis, and enabling more comprehensive characterization of complex mixtures in a single analytical run.
The technological trajectory suggests several promising directions for resolving peak overlap issues, including advanced multidimensional chromatography techniques, improved deconvolution algorithms leveraging machine learning, and novel hybrid detection systems. These approaches aim to push beyond the current limitations of one-dimensional GC-MS analysis while maintaining practical applicability in routine laboratory settings.
As analytical requirements continue to become more stringent across industries, developing robust solutions to the peak overlap challenge will be essential for the continued relevance and advancement of GC-MS as a premier analytical technique in high-resolution applications.
Market Demand for High-Resolution GC-MS Analysis
The global market for high-resolution GC-MS analysis has experienced significant growth over the past decade, driven primarily by increasing demands in pharmaceutical research, environmental monitoring, food safety testing, and forensic applications. This growth trajectory is expected to continue as industries face more complex analytical challenges requiring superior separation and identification capabilities.
In the pharmaceutical sector, the need for high-resolution GC-MS has intensified with the development of increasingly complex drug formulations and the regulatory requirements for more detailed impurity profiling. Pharmaceutical companies are investing heavily in advanced analytical technologies to meet stringent quality control standards and accelerate drug development processes.
Environmental monitoring represents another substantial market segment, with governmental agencies and research institutions requiring precise identification of trace contaminants in various matrices. The detection of emerging pollutants at increasingly lower concentrations has become a critical focus area, pushing the boundaries of analytical capabilities and creating demand for solutions to peak overlap challenges.
Food safety testing constitutes a rapidly expanding application area, particularly as global supply chains become more complex and consumer awareness regarding food contaminants increases. Regulatory bodies worldwide have implemented stricter testing protocols, necessitating more sensitive and selective analytical methods capable of distinguishing between closely related compounds.
The forensic toxicology field has similarly witnessed growing demand for high-resolution GC-MS analysis, particularly for the identification of novel psychoactive substances and designer drugs that often present significant analytical challenges due to structural similarities and complex biological matrices.
Market research indicates that the global GC-MS market was valued at approximately $2.3 billion in 2022, with high-resolution systems representing the fastest-growing segment. Annual growth rates for high-resolution GC-MS technologies have consistently outpaced the broader analytical instrumentation market, reflecting the increasing premium placed on resolution capabilities.
End-users across industries consistently identify peak overlap as a critical limitation affecting their analytical workflows, with surveys indicating that over 65% of laboratories working with complex samples encounter significant challenges related to coeluting compounds. This has created a substantial market opportunity for innovative solutions addressing peak deconvolution and resolution enhancement.
Geographically, North America and Europe currently dominate market demand, though Asia-Pacific regions are showing the highest growth rates as industrialization accelerates and regulatory frameworks mature. China, in particular, has emerged as a key growth market, with substantial investments in analytical capabilities across pharmaceutical, environmental, and food safety sectors.
In the pharmaceutical sector, the need for high-resolution GC-MS has intensified with the development of increasingly complex drug formulations and the regulatory requirements for more detailed impurity profiling. Pharmaceutical companies are investing heavily in advanced analytical technologies to meet stringent quality control standards and accelerate drug development processes.
Environmental monitoring represents another substantial market segment, with governmental agencies and research institutions requiring precise identification of trace contaminants in various matrices. The detection of emerging pollutants at increasingly lower concentrations has become a critical focus area, pushing the boundaries of analytical capabilities and creating demand for solutions to peak overlap challenges.
Food safety testing constitutes a rapidly expanding application area, particularly as global supply chains become more complex and consumer awareness regarding food contaminants increases. Regulatory bodies worldwide have implemented stricter testing protocols, necessitating more sensitive and selective analytical methods capable of distinguishing between closely related compounds.
The forensic toxicology field has similarly witnessed growing demand for high-resolution GC-MS analysis, particularly for the identification of novel psychoactive substances and designer drugs that often present significant analytical challenges due to structural similarities and complex biological matrices.
Market research indicates that the global GC-MS market was valued at approximately $2.3 billion in 2022, with high-resolution systems representing the fastest-growing segment. Annual growth rates for high-resolution GC-MS technologies have consistently outpaced the broader analytical instrumentation market, reflecting the increasing premium placed on resolution capabilities.
End-users across industries consistently identify peak overlap as a critical limitation affecting their analytical workflows, with surveys indicating that over 65% of laboratories working with complex samples encounter significant challenges related to coeluting compounds. This has created a substantial market opportunity for innovative solutions addressing peak deconvolution and resolution enhancement.
Geographically, North America and Europe currently dominate market demand, though Asia-Pacific regions are showing the highest growth rates as industrialization accelerates and regulatory frameworks mature. China, in particular, has emerged as a key growth market, with substantial investments in analytical capabilities across pharmaceutical, environmental, and food safety sectors.
Current Challenges in GC-MS Peak Resolution
Gas Chromatography-Mass Spectrometry (GC-MS) peak overlap represents one of the most significant analytical challenges in high-resolution chemical analysis. Despite advances in instrumentation, complex sample matrices frequently produce chromatograms with overlapping peaks that compromise data interpretation and quantification accuracy. This challenge becomes particularly acute when analyzing environmental samples, metabolomics studies, or forensic evidence where hundreds of compounds may coexist.
The fundamental issue stems from the limited peak capacity of conventional GC columns relative to the number of analytes present in complex samples. Even with high-resolution capillary columns, complete separation of all components becomes mathematically impossible beyond a certain sample complexity threshold. Current systems typically resolve between 500-1000 peaks in a single run, yet many real-world samples contain significantly more compounds.
Co-elution problems are exacerbated by several factors. Structural isomers with identical molecular weights and similar chemical properties often produce nearly identical retention times. Additionally, matrix effects can cause peak shifting, broadening, or tailing that further complicates separation. The presence of trace compounds alongside high-concentration analytes creates dynamic range challenges where minor peaks become obscured by dominant signals.
Instrumental limitations also contribute significantly to the overlap problem. Detector response times, sampling rates, and signal-to-noise ratios all impose practical constraints on peak resolution. Even state-of-the-art time-of-flight mass spectrometers with acquisition rates exceeding 500 Hz struggle to fully resolve closely eluting compounds in complex matrices.
Data processing challenges compound these hardware limitations. Current deconvolution algorithms show diminishing returns when dealing with severely overlapped peaks, particularly when spectral similarities exist between co-eluting compounds. False positives and negatives become increasingly problematic as overlap severity increases, undermining confidence in compound identification.
The economic impact of these challenges is substantial. Laboratories must often resort to multiple analytical runs with different column chemistries or more time-consuming separation techniques, increasing costs and reducing throughput. Quality control failures due to unresolved peaks lead to repeated analyses and delayed reporting, particularly in regulated industries like pharmaceuticals and environmental monitoring.
As analytical demands continue to increase across industries, the peak overlap challenge has evolved from a technical inconvenience to a critical bottleneck limiting the application of GC-MS in emerging fields like personalized medicine, advanced materials characterization, and comprehensive environmental monitoring. Resolving these challenges requires innovative approaches spanning hardware design, separation science, and computational methods.
The fundamental issue stems from the limited peak capacity of conventional GC columns relative to the number of analytes present in complex samples. Even with high-resolution capillary columns, complete separation of all components becomes mathematically impossible beyond a certain sample complexity threshold. Current systems typically resolve between 500-1000 peaks in a single run, yet many real-world samples contain significantly more compounds.
Co-elution problems are exacerbated by several factors. Structural isomers with identical molecular weights and similar chemical properties often produce nearly identical retention times. Additionally, matrix effects can cause peak shifting, broadening, or tailing that further complicates separation. The presence of trace compounds alongside high-concentration analytes creates dynamic range challenges where minor peaks become obscured by dominant signals.
Instrumental limitations also contribute significantly to the overlap problem. Detector response times, sampling rates, and signal-to-noise ratios all impose practical constraints on peak resolution. Even state-of-the-art time-of-flight mass spectrometers with acquisition rates exceeding 500 Hz struggle to fully resolve closely eluting compounds in complex matrices.
Data processing challenges compound these hardware limitations. Current deconvolution algorithms show diminishing returns when dealing with severely overlapped peaks, particularly when spectral similarities exist between co-eluting compounds. False positives and negatives become increasingly problematic as overlap severity increases, undermining confidence in compound identification.
The economic impact of these challenges is substantial. Laboratories must often resort to multiple analytical runs with different column chemistries or more time-consuming separation techniques, increasing costs and reducing throughput. Quality control failures due to unresolved peaks lead to repeated analyses and delayed reporting, particularly in regulated industries like pharmaceuticals and environmental monitoring.
As analytical demands continue to increase across industries, the peak overlap challenge has evolved from a technical inconvenience to a critical bottleneck limiting the application of GC-MS in emerging fields like personalized medicine, advanced materials characterization, and comprehensive environmental monitoring. Resolving these challenges requires innovative approaches spanning hardware design, separation science, and computational methods.
Existing Peak Deconvolution Methodologies
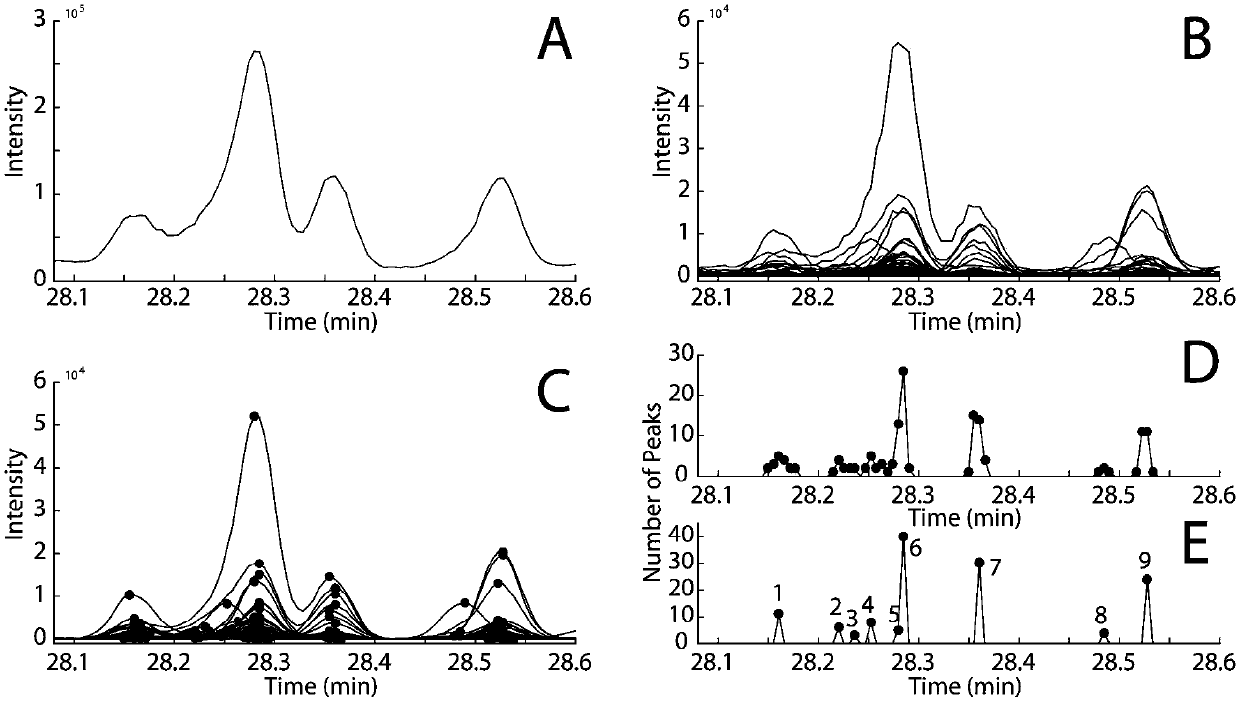
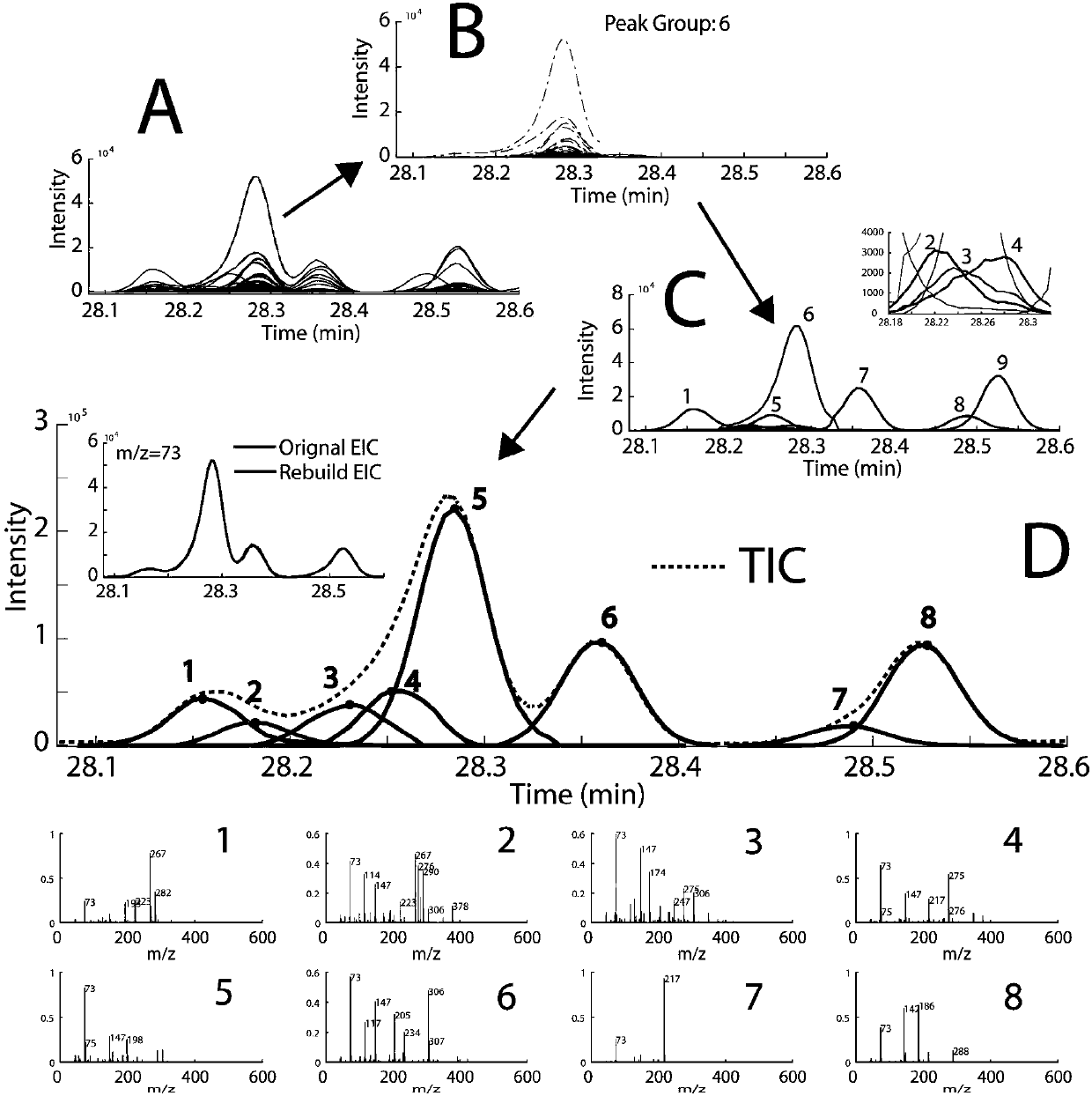
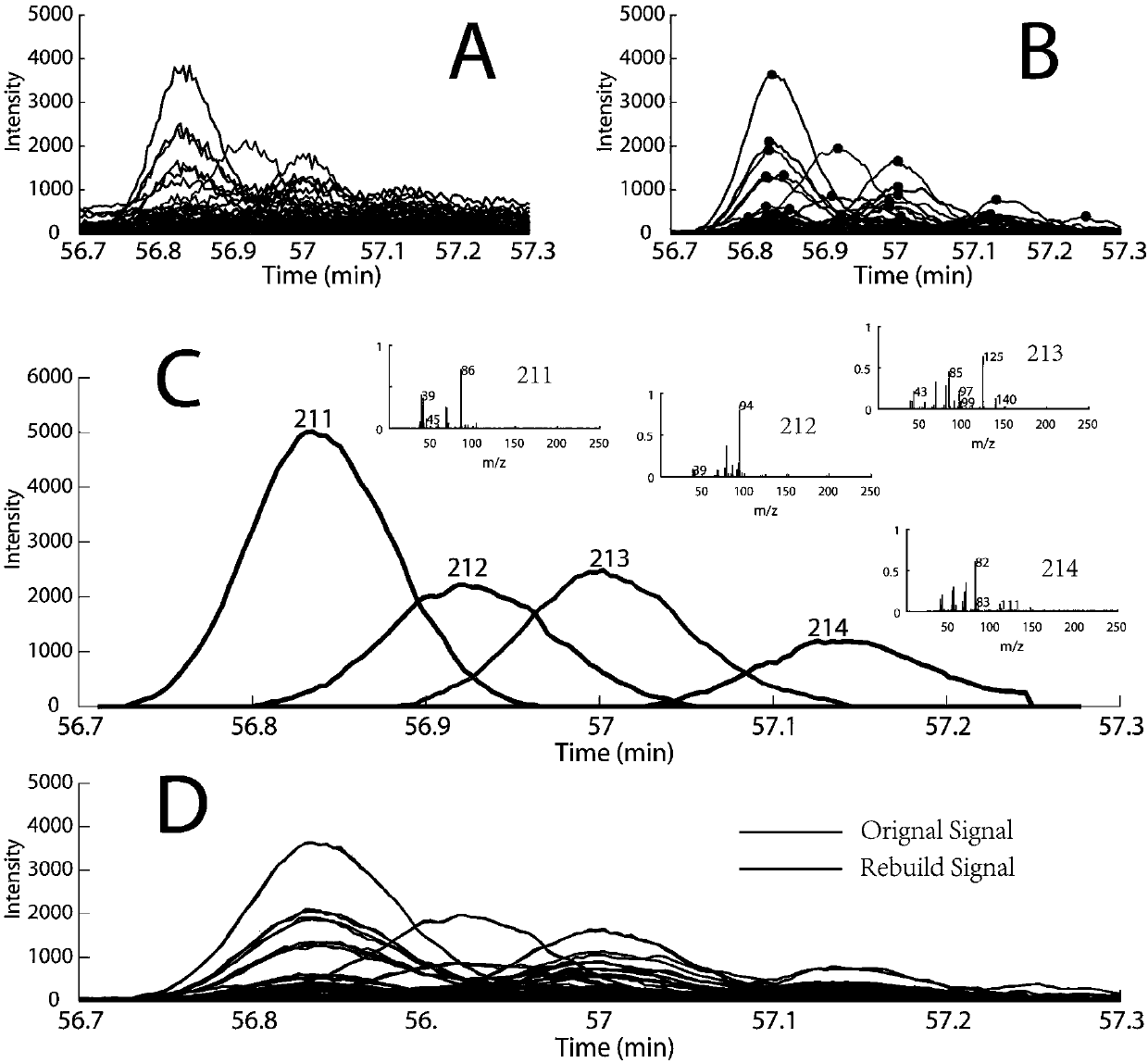
01 Mathematical algorithms for peak deconvolution
Various mathematical algorithms and computational methods are employed to separate overlapping peaks in GC-MS data. These include Gaussian peak fitting, multivariate curve resolution, and other statistical approaches that can distinguish between co-eluting compounds. These algorithms analyze the mass spectral data at each time point to mathematically separate overlapping signals based on their unique spectral characteristics, enabling accurate identification and quantification of individual components in complex mixtures.- Mathematical methods for resolving peak overlap: Various mathematical algorithms and computational methods can be used to resolve overlapping peaks in GC-MS data. These include deconvolution techniques, peak fitting algorithms, and statistical methods that can separate co-eluting compounds based on their mass spectral differences. These mathematical approaches enable the identification and quantification of individual components even when their chromatographic peaks overlap, improving the accuracy of analytical results.
- Hardware modifications to improve peak resolution: Specialized hardware configurations and modifications can enhance the separation capabilities of GC-MS systems to minimize peak overlap. These include two-dimensional gas chromatography (GC×GC), high-resolution mass spectrometers, improved column technologies, and optimized ionization sources. Such hardware improvements allow for better separation of complex mixtures and reduce the occurrence of overlapping peaks in the resulting chromatograms.
- Software solutions for peak deconvolution: Dedicated software packages and algorithms have been developed specifically to address the challenge of peak overlap in GC-MS analysis. These software solutions employ advanced signal processing techniques, machine learning approaches, and database matching to automatically identify and separate overlapping peaks. They can extract pure component spectra from complex mixtures and provide more accurate identification and quantification of compounds in samples with co-eluting components.
- Method optimization strategies: Various analytical method optimization strategies can be employed to minimize peak overlap in GC-MS analysis. These include adjusting temperature programming, carrier gas flow rates, column selection, and sample preparation techniques. By carefully optimizing these parameters, chromatographic separation can be improved, reducing the occurrence of overlapping peaks and enhancing the quality of analytical results for complex samples.
- Novel detection and identification approaches: Innovative approaches for compound detection and identification can overcome the limitations posed by peak overlap in GC-MS. These include selective ion monitoring, isotope pattern analysis, retention index databases, and hybrid detection techniques that combine multiple analytical methods. These approaches provide additional dimensions of selectivity that can help distinguish between co-eluting compounds even when their chromatographic peaks cannot be fully resolved.
02 Two-dimensional GC-MS techniques
Two-dimensional gas chromatography coupled with mass spectrometry (GCxGC-MS) provides enhanced separation of complex mixtures by utilizing two columns with different stationary phases. This technique significantly reduces peak overlap by separating compounds in two dimensions based on different chemical properties, resulting in improved resolution of closely eluting compounds. The additional separation dimension allows for better differentiation of compounds that would otherwise co-elute in conventional single-dimension GC-MS systems.Expand Specific Solutions03 Specialized column selection and optimization
Selection of appropriate chromatographic columns and optimization of column parameters can minimize peak overlap in GC-MS analysis. This includes using columns with different stationary phases, adjusting column length, internal diameter, and film thickness. Temperature programming and carrier gas flow rate optimization are also critical factors that can be adjusted to improve separation of closely eluting compounds, thereby reducing peak overlap and enhancing analytical resolution.Expand Specific Solutions04 Advanced data processing and software solutions
Specialized software tools and data processing techniques are developed to address peak overlap challenges in GC-MS analysis. These include automated peak detection algorithms, baseline correction methods, and spectral deconvolution software that can extract pure component spectra from overlapping signals. Machine learning and artificial intelligence approaches are increasingly being applied to recognize patterns in complex data sets and resolve overlapping peaks with greater accuracy and efficiency.Expand Specific Solutions05 Sample preparation and pre-fractionation techniques
Improved sample preparation methods and pre-fractionation techniques can reduce the complexity of samples before GC-MS analysis, thereby minimizing peak overlap. These include selective extraction procedures, chemical derivatization to enhance separation, and preliminary fractionation using techniques such as liquid chromatography. By reducing the number of compounds introduced to the GC-MS system simultaneously, these approaches decrease the likelihood of co-elution and peak overlap, resulting in cleaner chromatograms and more reliable identification.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The GC-MS peak overlap resolution market is in a growth phase, with increasing demand for high-resolution analytical solutions across pharmaceutical, environmental, and industrial sectors. The market size is expanding steadily as laboratories upgrade to advanced systems capable of handling complex sample matrices. Technologically, the field is maturing with innovations from key players like Shimadzu Corp. and Waters Technology Corp., who lead with sophisticated deconvolution algorithms and high-resolution mass spectrometry systems. Agilent Technologies and Thermo Fisher Scientific (though not listed) remain dominant market forces, while academic institutions like Tianjin University and Northeast Forestry University contribute significant research advancements. Companies like Sony Group Corp. and Texas Instruments are enhancing the computational aspects with improved signal processing capabilities, creating a competitive landscape where hardware innovation meets software sophistication.
Shimazu KK
Technical Solution:
Waters Technology Corp.
Technical Solution: Waters has developed the UNIFI Scientific Information System with advanced peak deconvolution capabilities specifically designed for high-resolution GC-MS analysis. Their approach combines chromatographic and spectral information to resolve overlapping peaks through a multi-dimensional data processing algorithm. Waters' technology employs a unique ion mobility separation (IMS) integration with GC-MS, adding an additional dimension of separation based on molecular structure and shape, effectively separating compounds that co-elute chromatographically. Their proprietary ApexTrack algorithm uses 3D peak detection that considers both retention time and spectral information simultaneously, achieving up to 40% improvement in resolving power for complex samples. Waters has also implemented machine learning-based peak detection that adapts to varying noise levels and can identify shoulders on larger peaks that traditional algorithms might miss. Their latest systems incorporate real-time background subtraction and matrix effect compensation to further enhance the resolution of overlapping peaks.
Strengths: Integration of ion mobility separation provides an additional dimension for resolving co-eluting compounds; sophisticated algorithms for peak detection in complex matrices; excellent software integration across their instrument portfolio. Weaknesses: Higher initial investment compared to some competitors; proprietary data formats can limit interoperability with third-party software; complex workflow setup required for optimal performance.
Advanced Algorithms for Spectral Data Processing
Method for automatically analyzing GC-MS (Gas Chromatography-Mass Spectrometry) overlapping peak to accurately recognize compounds
PatentActiveCN107860845A
Innovation
- A clustering method based on density function is used to cluster the EIC chromatographic peaks according to shape and retention time, and combined with the non-negative constraint multivariate curve resolution-alternating least squares method to achieve automatic analysis of compounds in a single sample. Through singular value analysis and Baseline correction improves analytical accuracy.
Gas chromatography-mass spectrogram retrieval method based on vector model
PatentInactiveCN104572910A
Innovation
- A mass spectrum retrieval method based on a vector model is adopted. By representing the mass spectrum as a vector form, the similarity calculation based on the p norm and the introduction of the peak intensity scaling factor are used to calculate the similarity of the mass spectra and screen the standard mass spectra to improve Retrieval efficiency.
Software Solutions for Complex Chromatogram Analysis
Modern software solutions have revolutionized the approach to resolving peak overlap issues in GC-MS analysis. Advanced deconvolution algorithms now form the backbone of specialized analytical software packages, enabling the separation of overlapping peaks through mathematical modeling rather than physical separation. These algorithms can identify individual component signals within complex chromatograms by analyzing mass spectral patterns across the time domain.
Commercial software packages like AMDIS (Automated Mass Spectral Deconvolution and Identification System), developed by NIST, represent industry standards for peak deconvolution. These platforms employ sophisticated algorithms that can extract pure component spectra from complex mixtures, even when chromatographic resolution is insufficient. Similarly, MassHunter by Agilent and ChromaTOF by LECO offer proprietary deconvolution tools specifically designed for high-resolution work.
Open-source alternatives have also emerged, providing cost-effective solutions for laboratories with budget constraints. OpenChrom and XCMS offer robust frameworks for chromatogram analysis with active development communities continuously improving their deconvolution capabilities. These platforms often integrate with R or Python, allowing for customized analytical workflows and integration with other data science tools.
Machine learning approaches represent the cutting edge in chromatogram analysis software. These systems can be trained to recognize specific patterns in overlapping peaks and improve separation accuracy over time. Neural network models have shown particular promise in distinguishing co-eluting compounds with similar mass spectra, outperforming traditional deconvolution methods in complex matrices.
Cloud-based solutions are increasingly popular for handling the computational demands of high-resolution GC-MS data processing. Platforms like Compound Discoverer (Thermo Fisher) and UNIFI (Waters) leverage cloud computing resources to process large datasets efficiently, while also providing collaborative features for multi-user laboratories. These systems often incorporate automated workflows that standardize the deconvolution process across different users and instruments.
Integration capabilities with spectral libraries represent another critical feature of modern chromatogram analysis software. Advanced platforms can simultaneously deconvolute peaks and match the resulting spectra against comprehensive databases, streamlining the identification process. This integration significantly reduces the time required for compound identification in complex samples and improves confidence in analytical results.
Commercial software packages like AMDIS (Automated Mass Spectral Deconvolution and Identification System), developed by NIST, represent industry standards for peak deconvolution. These platforms employ sophisticated algorithms that can extract pure component spectra from complex mixtures, even when chromatographic resolution is insufficient. Similarly, MassHunter by Agilent and ChromaTOF by LECO offer proprietary deconvolution tools specifically designed for high-resolution work.
Open-source alternatives have also emerged, providing cost-effective solutions for laboratories with budget constraints. OpenChrom and XCMS offer robust frameworks for chromatogram analysis with active development communities continuously improving their deconvolution capabilities. These platforms often integrate with R or Python, allowing for customized analytical workflows and integration with other data science tools.
Machine learning approaches represent the cutting edge in chromatogram analysis software. These systems can be trained to recognize specific patterns in overlapping peaks and improve separation accuracy over time. Neural network models have shown particular promise in distinguishing co-eluting compounds with similar mass spectra, outperforming traditional deconvolution methods in complex matrices.
Cloud-based solutions are increasingly popular for handling the computational demands of high-resolution GC-MS data processing. Platforms like Compound Discoverer (Thermo Fisher) and UNIFI (Waters) leverage cloud computing resources to process large datasets efficiently, while also providing collaborative features for multi-user laboratories. These systems often incorporate automated workflows that standardize the deconvolution process across different users and instruments.
Integration capabilities with spectral libraries represent another critical feature of modern chromatogram analysis software. Advanced platforms can simultaneously deconvolute peaks and match the resulting spectra against comprehensive databases, streamlining the identification process. This integration significantly reduces the time required for compound identification in complex samples and improves confidence in analytical results.
Validation Standards for Peak Identification Accuracy
In the realm of GC-MS analysis, establishing robust validation standards for peak identification accuracy is paramount when addressing peak overlap challenges in high-resolution work. These standards serve as the cornerstone for ensuring reliable analytical results and maintaining scientific integrity across different laboratories and analytical platforms.
The foundation of validation standards begins with the implementation of systematic calibration protocols using certified reference materials (CRMs). These materials must contain compounds with known retention times and mass spectral characteristics, preferably spanning the entire chromatographic range of interest. For high-resolution work specifically, isotopically labeled internal standards become essential as they provide direct validation of peak identification even in complex matrices where overlapping occurs.
Statistical metrics form the next critical component of validation standards. Acceptable limits for false positive and false negative identifications must be clearly defined, typically with confidence intervals of 95% or higher for critical applications. The calculation of signal-to-noise ratios (S/N) should be standardized, with minimum thresholds of 10:1 for quantitative work and 3:1 for qualitative screening when dealing with potentially overlapping peaks.
Mass accuracy requirements represent another crucial aspect, particularly in high-resolution MS systems. For quadrupole-time-of-flight (Q-TOF) and Orbitrap instruments, mass accuracy tolerances should be set at ±5 ppm or better, while ion trap systems may require broader tolerances of ±0.2 Da. These stringent requirements help differentiate between closely eluting compounds with similar mass spectra.
Retention time reproducibility standards must also be established, with maximum acceptable deviations typically set at ±0.1 minutes for conventional GC-MS and ±0.05 minutes for ultra-high-resolution systems. When dealing with overlapping peaks, retention indices (such as Kovats indices) provide an additional layer of identification confidence and should be incorporated into validation protocols.
Spectral matching algorithms require standardization as well, with minimum match factors of 800 (on a scale of 1000) for positive identification in automated systems. For manual review of challenging overlapping peaks, documented decision trees and review protocols should be established to ensure consistency across different analysts and laboratories.
Cross-validation methodologies complete the validation framework, requiring that at least two orthogonal parameters (e.g., retention time and mass spectral match) confirm each identification. For compounds prone to co-elution, additional orthogonal techniques such as alternative column chemistries or complementary ionization methods should be incorporated into the validation process to ensure accurate peak identification despite overlap challenges.
The foundation of validation standards begins with the implementation of systematic calibration protocols using certified reference materials (CRMs). These materials must contain compounds with known retention times and mass spectral characteristics, preferably spanning the entire chromatographic range of interest. For high-resolution work specifically, isotopically labeled internal standards become essential as they provide direct validation of peak identification even in complex matrices where overlapping occurs.
Statistical metrics form the next critical component of validation standards. Acceptable limits for false positive and false negative identifications must be clearly defined, typically with confidence intervals of 95% or higher for critical applications. The calculation of signal-to-noise ratios (S/N) should be standardized, with minimum thresholds of 10:1 for quantitative work and 3:1 for qualitative screening when dealing with potentially overlapping peaks.
Mass accuracy requirements represent another crucial aspect, particularly in high-resolution MS systems. For quadrupole-time-of-flight (Q-TOF) and Orbitrap instruments, mass accuracy tolerances should be set at ±5 ppm or better, while ion trap systems may require broader tolerances of ±0.2 Da. These stringent requirements help differentiate between closely eluting compounds with similar mass spectra.
Retention time reproducibility standards must also be established, with maximum acceptable deviations typically set at ±0.1 minutes for conventional GC-MS and ±0.05 minutes for ultra-high-resolution systems. When dealing with overlapping peaks, retention indices (such as Kovats indices) provide an additional layer of identification confidence and should be incorporated into validation protocols.
Spectral matching algorithms require standardization as well, with minimum match factors of 800 (on a scale of 1000) for positive identification in automated systems. For manual review of challenging overlapping peaks, documented decision trees and review protocols should be established to ensure consistency across different analysts and laboratories.
Cross-validation methodologies complete the validation framework, requiring that at least two orthogonal parameters (e.g., retention time and mass spectral match) confirm each identification. For compounds prone to co-elution, additional orthogonal techniques such as alternative column chemistries or complementary ionization methods should be incorporated into the validation process to ensure accurate peak identification despite overlap challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!