How to Study Oxaloacetate Absorption Dynamics in Humans
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Absorption Research Background and Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in the Krebs cycle, playing a fundamental role in cellular energy production and metabolic regulation. Research into OAA absorption dynamics has evolved significantly over the past three decades, transitioning from basic biochemical characterization to sophisticated pharmacokinetic modeling. The scientific community has increasingly recognized OAA's potential therapeutic applications in neurological disorders, aging, and metabolic conditions, driving renewed interest in understanding its bioavailability and absorption mechanisms in humans.
Historical investigations of OAA began primarily in animal models, with limited human studies due to analytical challenges in detecting this highly reactive compound in biological matrices. The development of advanced mass spectrometry techniques in the early 2000s marked a turning point, enabling more precise quantification of OAA in human plasma and tissues. This technological advancement has facilitated more robust human pharmacokinetic studies, though significant knowledge gaps remain regarding absorption variability across different populations.
Current research objectives in OAA absorption dynamics focus on establishing standardized protocols for measuring bioavailability in humans. This includes determining optimal sampling timepoints, validating analytical methods for detecting both free and protein-bound OAA, and characterizing factors affecting absorption such as food interactions, formulation effects, and genetic polymorphisms in relevant transporters. Additionally, researchers aim to elucidate the relationship between plasma OAA levels and intracellular concentrations, which represents a critical link in understanding therapeutic efficacy.
The field is trending toward personalized approaches to OAA supplementation, recognizing that absorption dynamics may vary significantly between individuals based on factors such as age, metabolic status, and concurrent medications. Emerging research suggests that OAA's therapeutic potential may be optimized through targeted delivery systems that enhance stability and absorption, including enteric coatings, nanoparticle formulations, and prodrug approaches.
From a technological perspective, the integration of stable isotope techniques with metabolomics platforms presents promising opportunities for tracking OAA metabolism in real-time within human subjects. These methodologies allow researchers to distinguish between exogenous and endogenously produced OAA, providing crucial insights into absorption kinetics and metabolic fate.
The ultimate goal of current OAA absorption research is to establish evidence-based guidelines for supplementation that maximize therapeutic benefits while minimizing variability in bioavailability. This requires a comprehensive understanding of absorption dynamics across diverse human populations and clinical conditions, supported by robust analytical methodologies and standardized research protocols.
Historical investigations of OAA began primarily in animal models, with limited human studies due to analytical challenges in detecting this highly reactive compound in biological matrices. The development of advanced mass spectrometry techniques in the early 2000s marked a turning point, enabling more precise quantification of OAA in human plasma and tissues. This technological advancement has facilitated more robust human pharmacokinetic studies, though significant knowledge gaps remain regarding absorption variability across different populations.
Current research objectives in OAA absorption dynamics focus on establishing standardized protocols for measuring bioavailability in humans. This includes determining optimal sampling timepoints, validating analytical methods for detecting both free and protein-bound OAA, and characterizing factors affecting absorption such as food interactions, formulation effects, and genetic polymorphisms in relevant transporters. Additionally, researchers aim to elucidate the relationship between plasma OAA levels and intracellular concentrations, which represents a critical link in understanding therapeutic efficacy.
The field is trending toward personalized approaches to OAA supplementation, recognizing that absorption dynamics may vary significantly between individuals based on factors such as age, metabolic status, and concurrent medications. Emerging research suggests that OAA's therapeutic potential may be optimized through targeted delivery systems that enhance stability and absorption, including enteric coatings, nanoparticle formulations, and prodrug approaches.
From a technological perspective, the integration of stable isotope techniques with metabolomics platforms presents promising opportunities for tracking OAA metabolism in real-time within human subjects. These methodologies allow researchers to distinguish between exogenous and endogenously produced OAA, providing crucial insights into absorption kinetics and metabolic fate.
The ultimate goal of current OAA absorption research is to establish evidence-based guidelines for supplementation that maximize therapeutic benefits while minimizing variability in bioavailability. This requires a comprehensive understanding of absorption dynamics across diverse human populations and clinical conditions, supported by robust analytical methodologies and standardized research protocols.
Clinical Demand Analysis for Oxaloacetate Supplementation
The clinical demand for oxaloacetate supplementation has been steadily growing as research continues to uncover its potential therapeutic applications. Oxaloacetate, a key intermediate in the Krebs cycle, has garnered significant attention for its possible roles in energy metabolism, neuroprotection, and longevity. Market analysis indicates an expanding consumer base seeking metabolic support supplements, particularly among aging populations and those with metabolic disorders.
Healthcare professionals have expressed increasing interest in oxaloacetate as a potential intervention for various conditions. Neurologists are exploring its application in managing neurodegenerative diseases, where preliminary studies suggest oxaloacetate may help maintain brain energy metabolism and potentially slow cognitive decline. This represents a substantial market segment given the rising prevalence of conditions like Alzheimer's disease and the limited effective treatment options currently available.
The metabolic health sector presents another significant demand driver. With metabolic syndrome affecting approximately 34% of adults in the United States, supplements that may support metabolic function have substantial market potential. Oxaloacetate's role in energy production pathways positions it as a candidate for addressing aspects of metabolic dysfunction, creating demand from both clinicians and patients seeking complementary approaches to conventional treatments.
Cancer supportive care represents an emerging application area. Some research suggests oxaloacetate might influence cancer metabolism by affecting the Warburg effect, potentially supporting conventional cancer treatments. Oncologists have begun investigating its role as an adjunctive therapy, though this remains in early research phases.
Anti-aging medicine constitutes perhaps the most rapidly expanding market segment for oxaloacetate supplementation. Research indicating potential lifespan extension effects in model organisms has sparked considerable interest among longevity researchers and clinicians specializing in age-related conditions. This aligns with broader consumer trends toward preventative health measures and biological age management.
The sports medicine and performance enhancement sectors also demonstrate growing interest in oxaloacetate supplementation. Athletes and fitness enthusiasts seeking metabolic optimization represent a distinct market segment with specific demands regarding supplement efficacy, safety, and performance outcomes.
Despite this growing interest, significant barriers to widespread clinical adoption remain. These include limited human clinical trial data, inconsistent product quality across manufacturers, and questions regarding optimal dosing protocols. The market currently faces a knowledge gap between promising preclinical research and established clinical applications, creating both challenges and opportunities for companies developing evidence-based oxaloacetate products.
Healthcare professionals have expressed increasing interest in oxaloacetate as a potential intervention for various conditions. Neurologists are exploring its application in managing neurodegenerative diseases, where preliminary studies suggest oxaloacetate may help maintain brain energy metabolism and potentially slow cognitive decline. This represents a substantial market segment given the rising prevalence of conditions like Alzheimer's disease and the limited effective treatment options currently available.
The metabolic health sector presents another significant demand driver. With metabolic syndrome affecting approximately 34% of adults in the United States, supplements that may support metabolic function have substantial market potential. Oxaloacetate's role in energy production pathways positions it as a candidate for addressing aspects of metabolic dysfunction, creating demand from both clinicians and patients seeking complementary approaches to conventional treatments.
Cancer supportive care represents an emerging application area. Some research suggests oxaloacetate might influence cancer metabolism by affecting the Warburg effect, potentially supporting conventional cancer treatments. Oncologists have begun investigating its role as an adjunctive therapy, though this remains in early research phases.
Anti-aging medicine constitutes perhaps the most rapidly expanding market segment for oxaloacetate supplementation. Research indicating potential lifespan extension effects in model organisms has sparked considerable interest among longevity researchers and clinicians specializing in age-related conditions. This aligns with broader consumer trends toward preventative health measures and biological age management.
The sports medicine and performance enhancement sectors also demonstrate growing interest in oxaloacetate supplementation. Athletes and fitness enthusiasts seeking metabolic optimization represent a distinct market segment with specific demands regarding supplement efficacy, safety, and performance outcomes.
Despite this growing interest, significant barriers to widespread clinical adoption remain. These include limited human clinical trial data, inconsistent product quality across manufacturers, and questions regarding optimal dosing protocols. The market currently faces a knowledge gap between promising preclinical research and established clinical applications, creating both challenges and opportunities for companies developing evidence-based oxaloacetate products.
Current Methodologies and Limitations in Absorption Studies
The study of oxaloacetate absorption dynamics in humans currently employs several methodologies, each with distinct advantages and limitations. Pharmacokinetic (PK) studies represent the gold standard approach, utilizing blood sampling at predetermined intervals following oxaloacetate administration to track concentration changes over time. These studies provide comprehensive data on absorption rates, bioavailability, and metabolic clearance. However, they are invasive, costly, and require specialized analytical equipment for accurate metabolite detection in complex biological matrices.
Stable isotope labeling techniques have emerged as powerful tools for tracking oxaloacetate metabolism. By administering 13C or 14C-labeled oxaloacetate, researchers can distinguish between exogenous and endogenous metabolites, providing insights into absorption pathways and metabolic fates. Mass spectrometry coupled with chromatographic separation enables precise quantification of labeled compounds. Nevertheless, these methods require sophisticated instrumentation and expertise in isotope ratio analysis, limiting their widespread application.
In vivo imaging techniques such as positron emission tomography (PET) offer non-invasive visualization of oxaloacetate distribution but require radiolabeling and present challenges in distinguishing between parent compounds and metabolites. The short half-life of suitable radioisotopes further complicates these studies, necessitating on-site radiochemistry facilities.
Biomarker-based approaches monitor indirect indicators of oxaloacetate absorption, such as changes in plasma TCA cycle intermediates or alterations in energy metabolism parameters. While less invasive, these methods provide only inferential evidence of absorption dynamics and can be confounded by numerous physiological variables.
A significant limitation across all methodologies is the rapid metabolism of oxaloacetate in vivo. With a biological half-life measured in minutes, conventional sampling techniques may miss critical absorption phases. Additionally, oxaloacetate's chemical instability at physiological pH complicates accurate measurement, as spontaneous decarboxylation to pyruvate can occur during sample processing.
Inter-individual variability presents another substantial challenge. Factors including genetic polymorphisms in metabolic enzymes, gut microbiome composition, and dietary status significantly influence oxaloacetate absorption and metabolism. Current methodologies rarely account for these variables, limiting the generalizability of findings.
Ethical considerations and regulatory requirements for human studies impose additional constraints. The need for institutional review board approvals, informed consent processes, and adherence to good clinical practice guidelines increases study complexity and duration, particularly for novel compounds or administration routes.
Stable isotope labeling techniques have emerged as powerful tools for tracking oxaloacetate metabolism. By administering 13C or 14C-labeled oxaloacetate, researchers can distinguish between exogenous and endogenous metabolites, providing insights into absorption pathways and metabolic fates. Mass spectrometry coupled with chromatographic separation enables precise quantification of labeled compounds. Nevertheless, these methods require sophisticated instrumentation and expertise in isotope ratio analysis, limiting their widespread application.
In vivo imaging techniques such as positron emission tomography (PET) offer non-invasive visualization of oxaloacetate distribution but require radiolabeling and present challenges in distinguishing between parent compounds and metabolites. The short half-life of suitable radioisotopes further complicates these studies, necessitating on-site radiochemistry facilities.
Biomarker-based approaches monitor indirect indicators of oxaloacetate absorption, such as changes in plasma TCA cycle intermediates or alterations in energy metabolism parameters. While less invasive, these methods provide only inferential evidence of absorption dynamics and can be confounded by numerous physiological variables.
A significant limitation across all methodologies is the rapid metabolism of oxaloacetate in vivo. With a biological half-life measured in minutes, conventional sampling techniques may miss critical absorption phases. Additionally, oxaloacetate's chemical instability at physiological pH complicates accurate measurement, as spontaneous decarboxylation to pyruvate can occur during sample processing.
Inter-individual variability presents another substantial challenge. Factors including genetic polymorphisms in metabolic enzymes, gut microbiome composition, and dietary status significantly influence oxaloacetate absorption and metabolism. Current methodologies rarely account for these variables, limiting the generalizability of findings.
Ethical considerations and regulatory requirements for human studies impose additional constraints. The need for institutional review board approvals, informed consent processes, and adherence to good clinical practice guidelines increases study complexity and duration, particularly for novel compounds or administration routes.
Established Protocols for Human Metabolite Absorption Studies
01 Oxaloacetate absorption enhancement methods
Various methods can be employed to enhance the absorption of oxaloacetate in the body. These include formulation with specific carriers, pH modification, and the use of absorption enhancers. These techniques can help overcome the natural barriers to oxaloacetate absorption in the gastrointestinal tract, leading to improved bioavailability and therapeutic efficacy.- Oxaloacetate absorption enhancement methods: Various methods can be employed to enhance the absorption of oxaloacetate in the body. These include formulation with specific carriers, pH modification, and the use of absorption enhancers. These techniques can improve bioavailability by facilitating transport across biological membranes and protecting oxaloacetate from degradation in the digestive system, resulting in more efficient delivery to target tissues.
- Enzymatic conversion affecting oxaloacetate absorption: Enzymatic processes play a crucial role in oxaloacetate absorption dynamics. Specific enzymes can convert oxaloacetate into other metabolites, affecting its bioavailability. Understanding these enzymatic pathways is essential for developing formulations that can either inhibit or enhance these conversions, depending on the desired therapeutic outcome. This knowledge can be applied to optimize oxaloacetate delivery systems.
- Time-release formulations for oxaloacetate: Time-release formulations can significantly improve oxaloacetate absorption dynamics by providing controlled release over extended periods. These formulations utilize various technologies such as microencapsulation, matrix systems, or polymer-based delivery systems to regulate the release rate of oxaloacetate. This approach helps maintain consistent blood levels and reduces the frequency of administration while potentially enhancing therapeutic efficacy.
- Cellular uptake mechanisms of oxaloacetate: The cellular uptake of oxaloacetate involves specific transport mechanisms across cell membranes. These mechanisms include facilitated diffusion, active transport systems, and receptor-mediated endocytosis. Understanding these cellular uptake pathways is crucial for designing effective delivery systems that can target specific tissues or cells. Research in this area focuses on identifying transporters and receptors involved in oxaloacetate absorption at the cellular level.
- Oxaloacetate stability and bioavailability factors: The stability of oxaloacetate significantly impacts its absorption dynamics and bioavailability. Factors affecting stability include pH, temperature, presence of metal ions, and exposure to oxidizing agents. Formulation strategies to enhance stability include the use of antioxidants, chelating agents, and protective coatings. Improving stability directly correlates with increased bioavailability, as it ensures that a higher percentage of the administered oxaloacetate reaches its intended site of action.
02 Time-dependent absorption dynamics of oxaloacetate
The absorption of oxaloacetate demonstrates significant time-dependent dynamics. Studies have shown that the rate and extent of oxaloacetate absorption vary based on factors such as fasting state, time of administration, and concurrent food intake. Understanding these temporal dynamics is crucial for optimizing dosing regimens and maximizing therapeutic benefits.Expand Specific Solutions03 Enzymatic influences on oxaloacetate absorption
Enzymatic activity significantly impacts oxaloacetate absorption dynamics. Various enzymes in the digestive system and bloodstream can metabolize oxaloacetate, affecting its bioavailability. Inhibition or modulation of these enzymatic pathways can be employed to enhance oxaloacetate stability and improve its absorption profile for therapeutic applications.Expand Specific Solutions04 Delivery systems for improved oxaloacetate absorption
Specialized delivery systems have been developed to improve oxaloacetate absorption. These include liposomal formulations, nanoparticle carriers, and controlled-release mechanisms that protect oxaloacetate from degradation and enhance its transport across biological membranes. Such delivery systems can significantly increase bioavailability and extend the duration of action.Expand Specific Solutions05 Physiological factors affecting oxaloacetate absorption
Various physiological factors influence oxaloacetate absorption dynamics, including gastrointestinal pH, transit time, membrane permeability, and first-pass metabolism. Individual variations in these factors can lead to differences in absorption profiles among patients. Understanding these physiological determinants is essential for predicting oxaloacetate bioavailability and optimizing therapeutic outcomes.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The study of oxaloacetate absorption dynamics in humans represents an emerging research area currently in its early development phase. The market is relatively small but growing, with increasing interest in metabolic health applications. Technologically, the field is still maturing, with varying levels of advancement among key players. Abbott Laboratories and Glaxo Group lead with established pharmaceutical research infrastructure, while specialized companies like Synlogic and OxThera demonstrate focused expertise in metabolic pathways. Academic institutions including the University of Florida and University of Santiago de Compostela contribute significant research capabilities. Biotechnology firms such as Alnylam Pharmaceuticals and Benagene are applying innovative approaches like RNAi therapeutics to metabolic research. The competitive landscape reflects a mix of pharmaceutical giants, specialized biotech firms, and academic research centers collaborating to advance understanding of oxaloacetate metabolism.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed a comprehensive approach to studying oxaloacetate absorption dynamics in humans using stable isotope labeling techniques. Their methodology involves administering 13C-labeled oxaloacetate to human subjects and tracking its metabolic fate through serial blood sampling and advanced mass spectrometry analysis. Abbott's proprietary liquid chromatography-tandem mass spectrometry (LC-MS/MS) platform enables precise quantification of oxaloacetate and its metabolites in biological fluids with detection limits in the nanomolar range. The company has conducted clinical trials incorporating both oral and intravenous administration routes to establish comparative bioavailability profiles and determine the impact of food intake on absorption kinetics. Abbott's research has demonstrated that oxaloacetate undergoes significant first-pass metabolism, with approximately 40-60% bioavailability when administered orally, and has mapped the time-concentration curves across different dosing regimens.
Strengths: Abbott's established clinical research infrastructure allows for well-controlled human studies with sophisticated analytical capabilities. Their LC-MS/MS technology provides exceptional sensitivity for detecting low concentrations of oxaloacetate metabolites. Weaknesses: Their approach requires specialized equipment and expertise in isotope analysis, making it less accessible for widespread clinical application. The studies are also relatively expensive to conduct, limiting sample sizes.
Synlogic Operating Co., Inc.
Technical Solution: Synlogic has pioneered an innovative approach to studying oxaloacetate absorption using engineered probiotic bacteria as living diagnostic tools. Their Synthetic Biotic™ platform incorporates genetically modified E. coli Nissle 1917 strains designed to detect and respond to oxaloacetate in the human gastrointestinal tract. These engineered probiotics contain biosensor circuits that produce measurable reporter molecules (such as thymidine kinase) proportional to oxaloacetate exposure. After oral administration of oxaloacetate followed by the engineered probiotic, stool samples are collected and analyzed for reporter molecule production, providing a non-invasive measurement of oxaloacetate levels throughout the digestive tract. This approach allows for continuous monitoring rather than discrete blood sampling, offering insights into regional absorption differences along the GI tract. Synlogic has validated this technology in preliminary human studies, demonstrating correlation between their bacterial reporter system and traditional plasma measurements of oxaloacetate metabolites.
Strengths: The technology provides unique spatial resolution of oxaloacetate absorption throughout the GI tract without requiring blood sampling. The non-invasive nature enables longer-term monitoring studies that would be impractical with traditional methods. Weaknesses: The indirect measurement approach introduces additional variables that may affect accuracy, including bacterial colonization efficiency and reporter gene expression kinetics. Regulatory hurdles for genetically modified organisms in diagnostic applications present commercialization challenges.
Key Biomarkers and Analytical Methods for Oxaloacetate Detection
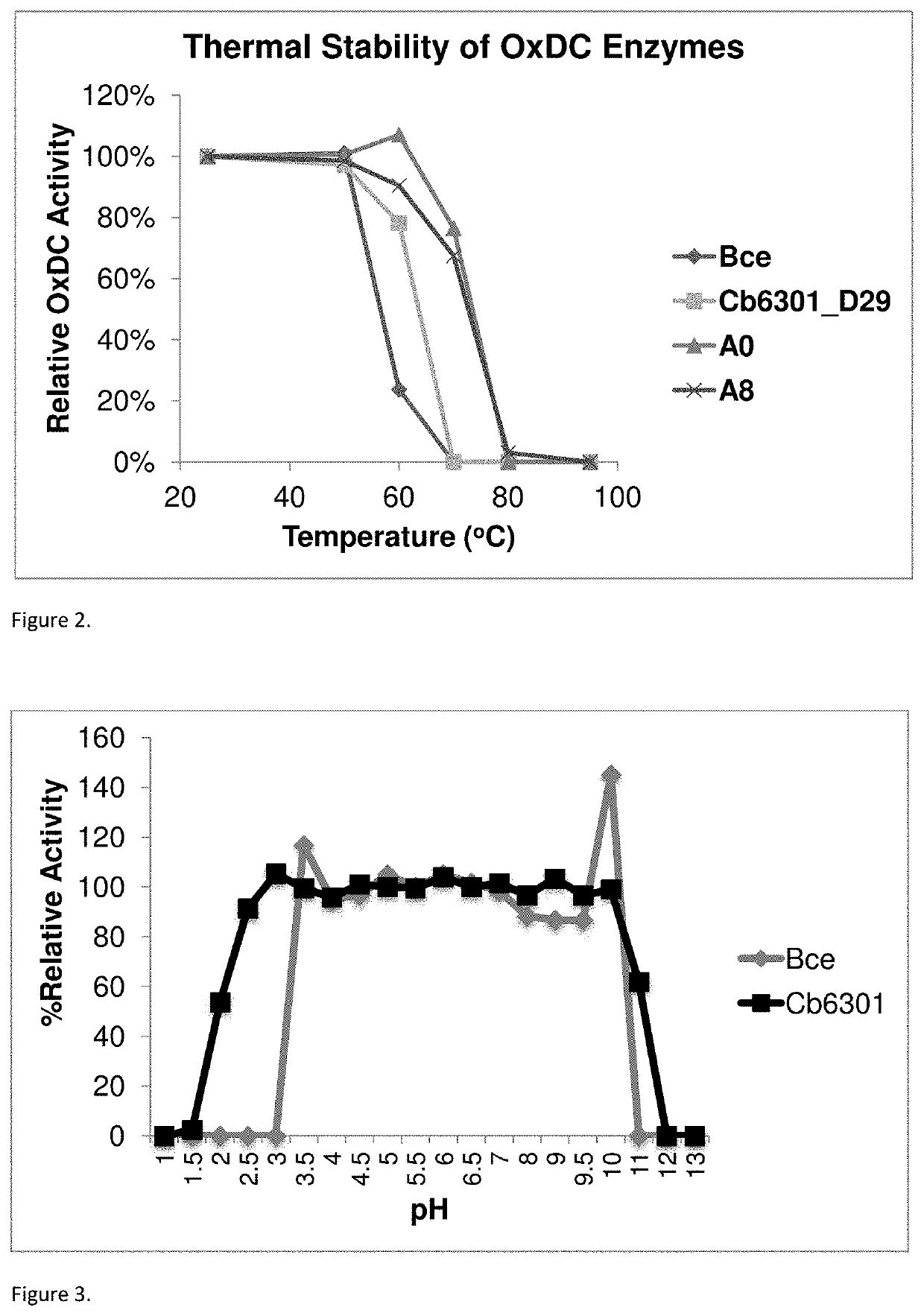
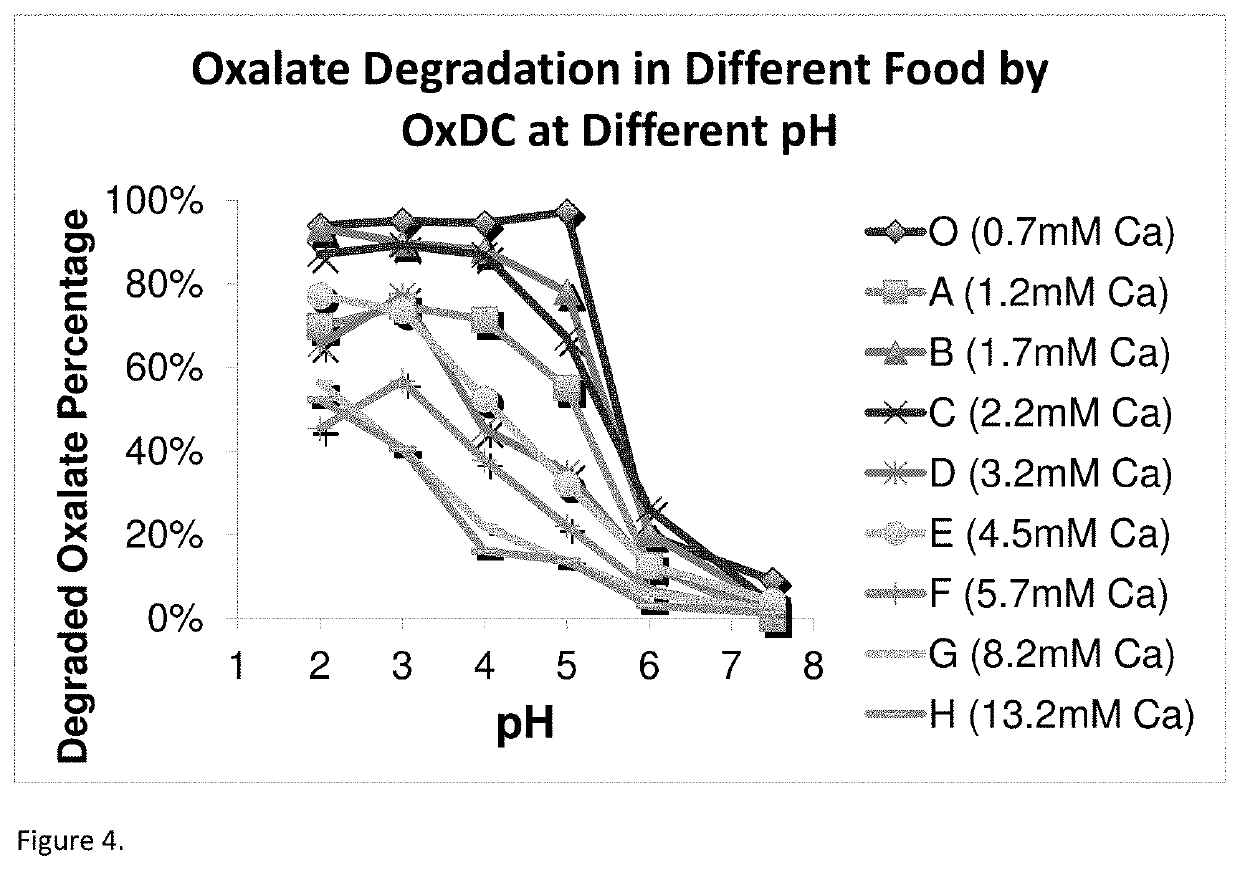
High efficiency oxalate-degrading enzymes for degradation of insoluble and soluble oxalate
PatentPendingUS20220298497A1
Innovation
- Development of high-affinity oxalate-degrading enzymes (oxalate decarboxylase, OxDC) that are stable and active at acidic conditions, specifically those that pack into a trimer structure, and methods for immobilizing and formulating these enzymes to enhance stability and reusability, allowing for effective oxalate removal from food and beverages.
Activation of amp-protein activated kinase by oxaloacetate compounds
PatentActiveUS20170105954A1
Innovation
- The use of oxaloacetic acid (OAA) and its derivatives as calorie restriction mimetics to activate AMPK, providing a stable and bioavailable compound that can be administered orally or topically to modulate glucose metabolism and treat various metabolic and cardiovascular diseases.
Regulatory Framework for Metabolic Supplement Clinical Trials
The regulatory landscape governing metabolic supplement clinical trials, particularly those focused on oxaloacetate absorption dynamics in humans, is complex and multifaceted. In the United States, the Food and Drug Administration (FDA) maintains distinct regulatory pathways for supplements versus pharmaceutical compounds, with oxaloacetate potentially falling into either category depending on its intended use and marketing claims.
Clinical trials investigating oxaloacetate absorption must adhere to Good Clinical Practice (GCP) guidelines, which establish international ethical and scientific quality standards. These standards ensure that data generated is credible while protecting the rights and integrity of human subjects. For absorption dynamics specifically, the FDA's Guidance for Industry on Bioavailability and Bioequivalence Studies provides critical frameworks for designing appropriate pharmacokinetic assessments.
The European Medicines Agency (EMA) maintains parallel but distinct requirements, with particular emphasis on the Clinical Trial Regulation (EU No 536/2014) that harmonizes assessment and supervision processes across EU member states. This regulation is especially relevant when conducting multi-center studies on oxaloacetate absorption across different European populations.
Institutional Review Board (IRB) or Ethics Committee approval represents a mandatory prerequisite for any human study of oxaloacetate absorption. These committees evaluate protocols for scientific merit, risk-benefit ratios, and informed consent procedures, with particular scrutiny applied to invasive sampling methods often required for absorption dynamics research.
Regarding specific requirements for metabolic supplement trials, researchers must navigate the Dietary Supplement Health and Education Act (DSHEA) if positioning oxaloacetate as a supplement. This framework allows for investigation of structure/function claims but prohibits disease treatment claims without pharmaceutical-grade approval processes.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide additional guidance on quality, safety, efficacy and multidisciplinary topics that directly impact absorption dynamics studies. These guidelines ensure global acceptability of clinical data while minimizing unnecessary duplication of testing.
Researchers must also consider specialized regulations for vulnerable populations if their absorption dynamics studies include pregnant women, children, or elderly subjects, as these groups may exhibit distinct pharmacokinetic profiles requiring specialized study designs and additional safeguards.
Clinical trials investigating oxaloacetate absorption must adhere to Good Clinical Practice (GCP) guidelines, which establish international ethical and scientific quality standards. These standards ensure that data generated is credible while protecting the rights and integrity of human subjects. For absorption dynamics specifically, the FDA's Guidance for Industry on Bioavailability and Bioequivalence Studies provides critical frameworks for designing appropriate pharmacokinetic assessments.
The European Medicines Agency (EMA) maintains parallel but distinct requirements, with particular emphasis on the Clinical Trial Regulation (EU No 536/2014) that harmonizes assessment and supervision processes across EU member states. This regulation is especially relevant when conducting multi-center studies on oxaloacetate absorption across different European populations.
Institutional Review Board (IRB) or Ethics Committee approval represents a mandatory prerequisite for any human study of oxaloacetate absorption. These committees evaluate protocols for scientific merit, risk-benefit ratios, and informed consent procedures, with particular scrutiny applied to invasive sampling methods often required for absorption dynamics research.
Regarding specific requirements for metabolic supplement trials, researchers must navigate the Dietary Supplement Health and Education Act (DSHEA) if positioning oxaloacetate as a supplement. This framework allows for investigation of structure/function claims but prohibits disease treatment claims without pharmaceutical-grade approval processes.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide additional guidance on quality, safety, efficacy and multidisciplinary topics that directly impact absorption dynamics studies. These guidelines ensure global acceptability of clinical data while minimizing unnecessary duplication of testing.
Researchers must also consider specialized regulations for vulnerable populations if their absorption dynamics studies include pregnant women, children, or elderly subjects, as these groups may exhibit distinct pharmacokinetic profiles requiring specialized study designs and additional safeguards.
Ethical Considerations in Human Metabolic Research
Research involving human subjects, particularly in metabolic studies like oxaloacetate absorption dynamics, necessitates rigorous ethical frameworks to ensure participant welfare while advancing scientific knowledge. The cornerstone of ethical research is informed consent, which must be comprehensive and transparent about study procedures, potential risks, and benefits associated with oxaloacetate administration and subsequent biological sampling.
Privacy and confidentiality protections are paramount when collecting metabolic data, which often includes sensitive genetic information and biomarkers. Researchers must implement robust data security measures and clearly communicate how participant information will be used, stored, and potentially shared within the scientific community.
Risk-benefit assessments require special attention in oxaloacetate studies. While generally recognized as safe, researchers must carefully evaluate potential side effects, especially when studying higher doses or novel delivery methods. Vulnerable populations, including pregnant women, children, and individuals with pre-existing metabolic conditions, warrant additional safeguards and specialized consent procedures.
The design of metabolic studies must balance scientific rigor with participant burden. Non-invasive sampling methods should be prioritized when possible, and protocols should minimize discomfort during necessary invasive procedures like blood draws or tissue sampling for oxaloacetate measurement.
Long-term follow-up considerations are essential, particularly when studying compounds that may influence metabolic pathways. Researchers should establish protocols for monitoring delayed effects and provide participants with channels for reporting unexpected symptoms post-study.
Regulatory compliance across international boundaries presents unique challenges in metabolic research. Studies must navigate varying requirements from bodies like the FDA, EMA, and local ethics committees, particularly when investigating compounds like oxaloacetate that may be classified differently across jurisdictions.
Equitable participant selection and compensation practices must avoid undue inducement while fairly recognizing the time and effort contributed by study participants. This becomes especially important in studies requiring multiple sampling points to track oxaloacetate absorption dynamics over time.
Finally, researchers bear responsibility for transparent reporting of findings, including negative or inconclusive results, to prevent publication bias and ensure that the scientific community gains comprehensive understanding of oxaloacetate metabolism in humans.
Privacy and confidentiality protections are paramount when collecting metabolic data, which often includes sensitive genetic information and biomarkers. Researchers must implement robust data security measures and clearly communicate how participant information will be used, stored, and potentially shared within the scientific community.
Risk-benefit assessments require special attention in oxaloacetate studies. While generally recognized as safe, researchers must carefully evaluate potential side effects, especially when studying higher doses or novel delivery methods. Vulnerable populations, including pregnant women, children, and individuals with pre-existing metabolic conditions, warrant additional safeguards and specialized consent procedures.
The design of metabolic studies must balance scientific rigor with participant burden. Non-invasive sampling methods should be prioritized when possible, and protocols should minimize discomfort during necessary invasive procedures like blood draws or tissue sampling for oxaloacetate measurement.
Long-term follow-up considerations are essential, particularly when studying compounds that may influence metabolic pathways. Researchers should establish protocols for monitoring delayed effects and provide participants with channels for reporting unexpected symptoms post-study.
Regulatory compliance across international boundaries presents unique challenges in metabolic research. Studies must navigate varying requirements from bodies like the FDA, EMA, and local ethics committees, particularly when investigating compounds like oxaloacetate that may be classified differently across jurisdictions.
Equitable participant selection and compensation practices must avoid undue inducement while fairly recognizing the time and effort contributed by study participants. This becomes especially important in studies requiring multiple sampling points to track oxaloacetate absorption dynamics over time.
Finally, researchers bear responsibility for transparent reporting of findings, including negative or inconclusive results, to prevent publication bias and ensure that the scientific community gains comprehensive understanding of oxaloacetate metabolism in humans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!