Impact of High-Pressure Calorimetry on Chemical Reaction Dynamics
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High-Pressure Calorimetry Background and Objectives
High-pressure calorimetry has emerged as a powerful technique in the study of chemical reaction dynamics, offering unprecedented insights into the behavior of materials under extreme conditions. This method combines the principles of calorimetry with high-pressure technology, allowing researchers to investigate thermodynamic properties and reaction kinetics at pressures far beyond atmospheric conditions.
The development of high-pressure calorimetry can be traced back to the early 20th century, with significant advancements occurring in the latter half of the century. As our understanding of matter under extreme conditions grew, so did the need for more sophisticated experimental techniques. The evolution of this field has been driven by both technological innovations and the increasing demand for knowledge about materials behavior in high-pressure environments.
In recent years, high-pressure calorimetry has gained prominence due to its applications in various scientific and industrial domains. From geochemistry to materials science, this technique has proven invaluable in unraveling the mysteries of chemical reactions under pressure. The ability to simulate conditions found deep within the Earth or in industrial processes has opened new avenues for research and development.
The primary objective of high-pressure calorimetry in the context of chemical reaction dynamics is to elucidate the pressure-dependent behavior of chemical systems. This includes understanding how pressure affects reaction rates, equilibrium constants, and thermodynamic parameters. By manipulating pressure as a variable, researchers can gain insights into reaction mechanisms that are not observable under standard conditions.
Another crucial goal is to explore the phase behavior of materials under high pressure. This is particularly relevant in fields such as planetary science, where understanding the behavior of materials under extreme conditions is essential for modeling planetary interiors. High-pressure calorimetry allows scientists to map out phase diagrams and identify new phases of matter that may only exist under specific pressure-temperature conditions.
Furthermore, high-pressure calorimetry aims to bridge the gap between theoretical predictions and experimental observations. As computational models become more sophisticated, the need for accurate experimental data to validate these models grows. High-pressure calorimetry provides a means to test and refine theoretical frameworks, enhancing our predictive capabilities in materials science and chemistry.
The ongoing technological advancements in high-pressure calorimetry are focused on expanding the accessible pressure and temperature ranges, improving measurement accuracy, and developing in situ characterization techniques. These efforts are driven by the need to study increasingly complex systems and to push the boundaries of our understanding of matter under extreme conditions.
The development of high-pressure calorimetry can be traced back to the early 20th century, with significant advancements occurring in the latter half of the century. As our understanding of matter under extreme conditions grew, so did the need for more sophisticated experimental techniques. The evolution of this field has been driven by both technological innovations and the increasing demand for knowledge about materials behavior in high-pressure environments.
In recent years, high-pressure calorimetry has gained prominence due to its applications in various scientific and industrial domains. From geochemistry to materials science, this technique has proven invaluable in unraveling the mysteries of chemical reactions under pressure. The ability to simulate conditions found deep within the Earth or in industrial processes has opened new avenues for research and development.
The primary objective of high-pressure calorimetry in the context of chemical reaction dynamics is to elucidate the pressure-dependent behavior of chemical systems. This includes understanding how pressure affects reaction rates, equilibrium constants, and thermodynamic parameters. By manipulating pressure as a variable, researchers can gain insights into reaction mechanisms that are not observable under standard conditions.
Another crucial goal is to explore the phase behavior of materials under high pressure. This is particularly relevant in fields such as planetary science, where understanding the behavior of materials under extreme conditions is essential for modeling planetary interiors. High-pressure calorimetry allows scientists to map out phase diagrams and identify new phases of matter that may only exist under specific pressure-temperature conditions.
Furthermore, high-pressure calorimetry aims to bridge the gap between theoretical predictions and experimental observations. As computational models become more sophisticated, the need for accurate experimental data to validate these models grows. High-pressure calorimetry provides a means to test and refine theoretical frameworks, enhancing our predictive capabilities in materials science and chemistry.
The ongoing technological advancements in high-pressure calorimetry are focused on expanding the accessible pressure and temperature ranges, improving measurement accuracy, and developing in situ characterization techniques. These efforts are driven by the need to study increasingly complex systems and to push the boundaries of our understanding of matter under extreme conditions.
Market Analysis for High-Pressure Calorimetry Applications
The market for high-pressure calorimetry applications has been experiencing significant growth in recent years, driven by the increasing demand for advanced analytical tools in various industries. The chemical and pharmaceutical sectors are the primary contributors to this market expansion, as these industries rely heavily on precise thermodynamic measurements for process optimization and product development.
In the chemical industry, high-pressure calorimetry is crucial for studying reaction kinetics, phase transitions, and material properties under extreme conditions. This technology enables researchers to simulate industrial processes and develop more efficient catalysts, leading to improved production methods and cost savings. The pharmaceutical sector utilizes high-pressure calorimetry for drug discovery and formulation, as it provides valuable insights into the stability and behavior of compounds under different pressure conditions.
The oil and gas industry is another key market for high-pressure calorimetry applications. As exploration and production activities move into deeper waters and more challenging environments, the need for accurate thermodynamic data at high pressures becomes increasingly important. This technology helps in optimizing extraction processes, developing new drilling fluids, and assessing the behavior of hydrocarbons under reservoir conditions.
The food and beverage industry is also adopting high-pressure calorimetry for various applications, including food preservation, texture modification, and the development of novel processing techniques. This technology allows for the study of pressure-induced changes in food components, leading to improved product quality and shelf life.
Geochemistry and materials science represent emerging markets for high-pressure calorimetry. Researchers in these fields use the technology to study the behavior of minerals and materials under extreme pressure conditions, simulating deep Earth environments or developing new materials with unique properties.
The global market for high-pressure calorimetry equipment and services is expected to grow steadily over the next five years. Factors contributing to this growth include increasing research and development activities in both academia and industry, the need for more accurate and reliable thermodynamic data, and the expansion of high-pressure processing techniques in various sectors.
North America and Europe currently dominate the high-pressure calorimetry market, owing to their well-established research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by increasing investments in research and development, particularly in China, Japan, and South Korea.
As the technology continues to advance, there is a growing trend towards the development of more compact, user-friendly, and automated high-pressure calorimetry systems. This evolution is expected to broaden the user base and open up new application areas, further driving market growth in the coming years.
In the chemical industry, high-pressure calorimetry is crucial for studying reaction kinetics, phase transitions, and material properties under extreme conditions. This technology enables researchers to simulate industrial processes and develop more efficient catalysts, leading to improved production methods and cost savings. The pharmaceutical sector utilizes high-pressure calorimetry for drug discovery and formulation, as it provides valuable insights into the stability and behavior of compounds under different pressure conditions.
The oil and gas industry is another key market for high-pressure calorimetry applications. As exploration and production activities move into deeper waters and more challenging environments, the need for accurate thermodynamic data at high pressures becomes increasingly important. This technology helps in optimizing extraction processes, developing new drilling fluids, and assessing the behavior of hydrocarbons under reservoir conditions.
The food and beverage industry is also adopting high-pressure calorimetry for various applications, including food preservation, texture modification, and the development of novel processing techniques. This technology allows for the study of pressure-induced changes in food components, leading to improved product quality and shelf life.
Geochemistry and materials science represent emerging markets for high-pressure calorimetry. Researchers in these fields use the technology to study the behavior of minerals and materials under extreme pressure conditions, simulating deep Earth environments or developing new materials with unique properties.
The global market for high-pressure calorimetry equipment and services is expected to grow steadily over the next five years. Factors contributing to this growth include increasing research and development activities in both academia and industry, the need for more accurate and reliable thermodynamic data, and the expansion of high-pressure processing techniques in various sectors.
North America and Europe currently dominate the high-pressure calorimetry market, owing to their well-established research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by increasing investments in research and development, particularly in China, Japan, and South Korea.
As the technology continues to advance, there is a growing trend towards the development of more compact, user-friendly, and automated high-pressure calorimetry systems. This evolution is expected to broaden the user base and open up new application areas, further driving market growth in the coming years.
Current Challenges in High-Pressure Calorimetry
High-pressure calorimetry faces several significant challenges that hinder its widespread application and limit the accuracy of measurements in chemical reaction dynamics. One of the primary obstacles is the design and construction of calorimeters capable of withstanding extreme pressures while maintaining precise temperature control. The materials used must be both pressure-resistant and thermally conductive, a combination that often proves difficult to achieve.
Another major challenge lies in the calibration of high-pressure calorimeters. Traditional calibration methods may not be suitable under high-pressure conditions, necessitating the development of new calibration techniques. This process is further complicated by the pressure-dependent behavior of many calibration standards, which can lead to inconsistencies in measurements across different pressure ranges.
The interpretation of data obtained from high-pressure calorimetry experiments presents its own set of challenges. Pressure-induced changes in the physical and chemical properties of both the sample and the surrounding medium can significantly affect the measured heat flows. Separating these effects from the actual reaction dynamics requires sophisticated data analysis techniques and a deep understanding of high-pressure thermodynamics.
Ensuring uniform temperature distribution within the sample chamber under high-pressure conditions is another critical challenge. Pressure gradients can lead to localized heating or cooling effects, potentially skewing the calorimetric measurements. This issue is particularly pronounced in larger sample volumes, limiting the scalability of high-pressure calorimetry experiments.
The time resolution of high-pressure calorimetry measurements is often limited by the thermal inertia of the system. Rapid chemical reactions or phase transitions may occur faster than the calorimeter can respond, leading to incomplete or inaccurate data. Improving the temporal resolution while maintaining high-pressure capabilities remains an ongoing challenge in the field.
Lastly, the integration of high-pressure calorimetry with other analytical techniques poses significant technical hurdles. While combining calorimetry with spectroscopic or diffraction methods could provide invaluable insights into reaction mechanisms, the practical implementation of such multi-technique approaches under high-pressure conditions is extremely challenging. Overcoming these obstacles would greatly enhance our understanding of chemical reaction dynamics in extreme environments.
Another major challenge lies in the calibration of high-pressure calorimeters. Traditional calibration methods may not be suitable under high-pressure conditions, necessitating the development of new calibration techniques. This process is further complicated by the pressure-dependent behavior of many calibration standards, which can lead to inconsistencies in measurements across different pressure ranges.
The interpretation of data obtained from high-pressure calorimetry experiments presents its own set of challenges. Pressure-induced changes in the physical and chemical properties of both the sample and the surrounding medium can significantly affect the measured heat flows. Separating these effects from the actual reaction dynamics requires sophisticated data analysis techniques and a deep understanding of high-pressure thermodynamics.
Ensuring uniform temperature distribution within the sample chamber under high-pressure conditions is another critical challenge. Pressure gradients can lead to localized heating or cooling effects, potentially skewing the calorimetric measurements. This issue is particularly pronounced in larger sample volumes, limiting the scalability of high-pressure calorimetry experiments.
The time resolution of high-pressure calorimetry measurements is often limited by the thermal inertia of the system. Rapid chemical reactions or phase transitions may occur faster than the calorimeter can respond, leading to incomplete or inaccurate data. Improving the temporal resolution while maintaining high-pressure capabilities remains an ongoing challenge in the field.
Lastly, the integration of high-pressure calorimetry with other analytical techniques poses significant technical hurdles. While combining calorimetry with spectroscopic or diffraction methods could provide invaluable insights into reaction mechanisms, the practical implementation of such multi-technique approaches under high-pressure conditions is extremely challenging. Overcoming these obstacles would greatly enhance our understanding of chemical reaction dynamics in extreme environments.
Existing High-Pressure Calorimetry Solutions
01 High-pressure calorimetry apparatus design
Specialized apparatus designs for high-pressure calorimetry to study chemical reaction dynamics. These designs include features for precise temperature control, pressure regulation, and measurement of heat flow under extreme conditions. The apparatus may incorporate advanced sensors, pressure vessels, and thermal management systems to enable accurate measurements of reaction thermodynamics and kinetics at elevated pressures.- High-pressure calorimetry apparatus design: Advanced apparatus designs for high-pressure calorimetry to study chemical reaction dynamics. These designs incorporate features for precise temperature control, pressure regulation, and sample handling to enable accurate measurements of thermodynamic properties and reaction kinetics under high-pressure conditions.
- Reaction kinetics analysis under high pressure: Methods and systems for analyzing reaction kinetics in high-pressure environments. These approaches involve sophisticated data collection and analysis techniques to understand how pressure affects reaction rates, activation energies, and reaction mechanisms, providing insights into chemical reaction dynamics under extreme conditions.
- Thermodynamic property measurements: Techniques for measuring thermodynamic properties such as heat capacity, enthalpy, and entropy using high-pressure calorimetry. These measurements are crucial for understanding the energetics of chemical reactions and phase transitions under high-pressure conditions, contributing to the study of reaction dynamics.
- In-situ spectroscopic analysis: Integration of spectroscopic techniques with high-pressure calorimetry for in-situ analysis of chemical reactions. This combination allows for real-time monitoring of reaction progress, identification of intermediates, and characterization of products under high-pressure conditions, enhancing the understanding of reaction mechanisms.
- Computational modeling and simulation: Development and application of computational models and simulations to complement high-pressure calorimetry experiments. These computational approaches help predict reaction behavior, interpret experimental results, and provide insights into molecular-level processes occurring during high-pressure chemical reactions.
02 Reaction monitoring and data analysis techniques
Advanced methods for monitoring chemical reactions and analyzing data obtained from high-pressure calorimetry experiments. These techniques may include real-time spectroscopic analysis, data processing algorithms, and computational modeling to interpret complex reaction dynamics. The focus is on extracting meaningful kinetic and thermodynamic parameters from experimental data to understand reaction mechanisms under high-pressure conditions.Expand Specific Solutions03 High-pressure synthesis and material characterization
Utilization of high-pressure calorimetry for the synthesis and characterization of novel materials. This approach allows for the exploration of new reaction pathways and the creation of materials with unique properties. The technique is particularly useful for studying phase transitions, polymorphism, and the behavior of materials under extreme conditions, providing insights into their structure-property relationships.Expand Specific Solutions04 Thermodynamic and kinetic studies of high-pressure reactions
Investigation of thermodynamic and kinetic aspects of chemical reactions under high-pressure conditions. This includes the measurement of reaction enthalpies, activation energies, and reaction rates as a function of pressure. The studies aim to elucidate the pressure effects on reaction mechanisms, equilibria, and transition states, contributing to a deeper understanding of chemical processes in extreme environments.Expand Specific Solutions05 Applications in industrial processes and green chemistry
Implementation of high-pressure calorimetry findings in industrial processes and green chemistry applications. This involves optimizing reaction conditions for improved efficiency, developing more sustainable chemical processes, and exploring alternative reaction pathways. The knowledge gained from high-pressure studies is applied to enhance the performance and environmental impact of various chemical manufacturing processes.Expand Specific Solutions
Key Players in High-Pressure Calorimetry Industry
The impact of high-pressure calorimetry on chemical reaction dynamics is at a pivotal stage of development, with the market showing significant growth potential. The technology is advancing rapidly, driven by collaborations between academic institutions like Northeastern University, Zhejiang University, and Harvard College, and industry leaders such as BASF Corp. and Dow Global Technologies. While still evolving, the field is attracting increased attention from both research and commercial sectors. The technology's maturity varies across applications, with some areas nearing commercial viability while others remain in early research phases. This diversity in development stages presents opportunities for innovation and market differentiation among key players.
President & Fellows of Harvard College
Technical Solution: Harvard College has developed advanced high-pressure calorimetry techniques to study chemical reaction dynamics. Their approach utilizes custom-designed high-pressure cells capable of withstanding pressures up to 2 GPa, integrated with sensitive calorimetric sensors[1]. This setup allows for precise measurements of heat flow and enthalpy changes during reactions under extreme conditions. The system is coupled with in-situ spectroscopic methods, including Raman and IR spectroscopy, enabling real-time monitoring of molecular changes[2]. Harvard's research has particularly focused on understanding pressure-induced phase transitions and reaction pathways in complex organic systems, providing insights into reaction mechanisms that are not observable under ambient conditions[3].
Strengths: Cutting-edge instrumentation, multidisciplinary approach combining calorimetry with spectroscopy, and expertise in high-pressure chemistry. Weaknesses: High cost of specialized equipment and potential limitations in studying very fast reactions.
BASF Corp.
Technical Solution: BASF has developed a high-pressure calorimetry platform for studying industrially relevant chemical reactions. Their system incorporates a series of flow-through reactors capable of operating at pressures up to 300 bar and temperatures up to 400°C[4]. The setup allows for continuous monitoring of reaction kinetics and thermodynamics under conditions that closely mimic industrial processes. BASF's approach integrates online analytics, including GC-MS and HPLC, for real-time product analysis[5]. This technology has been particularly valuable in optimizing catalytic processes, where pressure plays a crucial role in reaction selectivity and yield. The company has successfully applied this method to improve the efficiency of hydrogenation reactions and other pressure-sensitive transformations in fine chemical synthesis[6].
Strengths: Direct applicability to industrial processes, comprehensive analytical capabilities, and expertise in catalysis. Weaknesses: Limited to moderate pressure ranges compared to some academic setups, potentially less suitable for fundamental studies of extreme conditions.
Core Innovations in High-Pressure Calorimetry
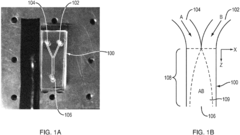
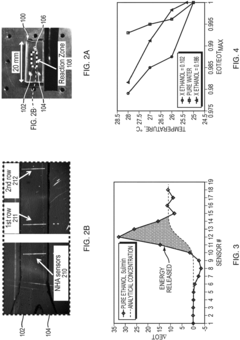
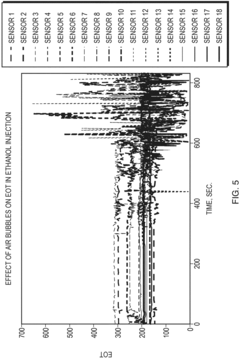
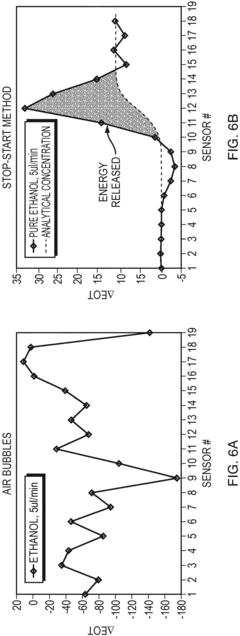
Stop-start method in a microfluidic calorimeter
PatentInactiveEP3321652A1
Innovation
- The method involves a Stop-Start process using a co-flow reactor microchannel with Nano Hole Array (NHA) sensors, where a first fluid is flowed, measured, and then stopped and evacuated to prevent bubble formation, allowing a second fluid to flow for reaction measurement, calculating calorimetry based on extraordinary optical transmission (EOT) differences.
Use of transition metal catalyst to produce linear ethylene/polar copolymers in a high pressure process
PatentWO2025006554A1
Innovation
- A high-pressure polymerization process using a transition metal catalyst system, specifically nickel(II) or palladium(II), in a supercritical ethylene environment at temperatures above 100°C to produce highly linear ethylene/alkylacrylate copolymers with improved molecular weight distribution and melt temperatures.
Safety Considerations in High-Pressure Experiments
High-pressure calorimetry experiments, while offering valuable insights into chemical reaction dynamics, present significant safety challenges that must be carefully addressed. The primary concern in these experiments is the potential for catastrophic failure of pressure vessels, which can result in explosive decompression and the release of hazardous materials. To mitigate these risks, stringent safety protocols and engineering controls are essential.
Pressure vessel design and material selection are critical factors in ensuring experimental safety. Vessels must be constructed from high-strength materials capable of withstanding extreme pressures, such as specialized alloys or reinforced composites. Regular inspection and maintenance of these vessels are crucial to detect any signs of wear, corrosion, or fatigue that could compromise their integrity. Additionally, pressure relief systems, such as burst discs or safety valves, should be incorporated to prevent over-pressurization.
Personal protective equipment (PPE) plays a vital role in safeguarding researchers. This includes, but is not limited to, impact-resistant face shields, pressure-resistant gloves, and protective clothing. The experimental area should be equipped with blast shields and containment systems to minimize the risk of injury or exposure in case of vessel failure.
Remote operation and monitoring systems are increasingly being employed to enhance safety. By allowing researchers to control experiments from a safe distance, these systems significantly reduce the risk of personal injury. Advanced sensors and real-time data acquisition systems can provide early warning of potential issues, enabling timely intervention.
Training and education are fundamental to maintaining a safe experimental environment. All personnel involved in high-pressure calorimetry experiments must receive comprehensive training on equipment operation, emergency procedures, and the specific hazards associated with high-pressure systems. Regular safety drills and refresher courses should be conducted to ensure that all team members are prepared to respond effectively to potential incidents.
Environmental considerations are also crucial in high-pressure experiments. Proper ventilation systems must be in place to manage potential releases of gases or vapors. Containment and disposal protocols for hazardous materials used or produced during experiments should be strictly followed to prevent environmental contamination.
Lastly, a comprehensive risk assessment should be conducted before undertaking any high-pressure calorimetry experiment. This assessment should identify potential hazards, evaluate their likelihood and severity, and outline appropriate control measures. Continuous monitoring and periodic review of safety procedures are essential to adapt to new findings or changes in experimental conditions, ensuring the ongoing safety of all personnel involved in this critical area of research.
Pressure vessel design and material selection are critical factors in ensuring experimental safety. Vessels must be constructed from high-strength materials capable of withstanding extreme pressures, such as specialized alloys or reinforced composites. Regular inspection and maintenance of these vessels are crucial to detect any signs of wear, corrosion, or fatigue that could compromise their integrity. Additionally, pressure relief systems, such as burst discs or safety valves, should be incorporated to prevent over-pressurization.
Personal protective equipment (PPE) plays a vital role in safeguarding researchers. This includes, but is not limited to, impact-resistant face shields, pressure-resistant gloves, and protective clothing. The experimental area should be equipped with blast shields and containment systems to minimize the risk of injury or exposure in case of vessel failure.
Remote operation and monitoring systems are increasingly being employed to enhance safety. By allowing researchers to control experiments from a safe distance, these systems significantly reduce the risk of personal injury. Advanced sensors and real-time data acquisition systems can provide early warning of potential issues, enabling timely intervention.
Training and education are fundamental to maintaining a safe experimental environment. All personnel involved in high-pressure calorimetry experiments must receive comprehensive training on equipment operation, emergency procedures, and the specific hazards associated with high-pressure systems. Regular safety drills and refresher courses should be conducted to ensure that all team members are prepared to respond effectively to potential incidents.
Environmental considerations are also crucial in high-pressure experiments. Proper ventilation systems must be in place to manage potential releases of gases or vapors. Containment and disposal protocols for hazardous materials used or produced during experiments should be strictly followed to prevent environmental contamination.
Lastly, a comprehensive risk assessment should be conducted before undertaking any high-pressure calorimetry experiment. This assessment should identify potential hazards, evaluate their likelihood and severity, and outline appropriate control measures. Continuous monitoring and periodic review of safety procedures are essential to adapt to new findings or changes in experimental conditions, ensuring the ongoing safety of all personnel involved in this critical area of research.
Environmental Impact of High-Pressure Calorimetry
High-pressure calorimetry, while a powerful tool for studying chemical reaction dynamics, has potential environmental impacts that must be carefully considered. The use of high-pressure systems in calorimetric experiments can lead to increased energy consumption, as maintaining elevated pressures often requires substantial power input. This energy demand may contribute to greenhouse gas emissions if sourced from non-renewable energy sources.
The materials used in high-pressure calorimetry equipment, such as specialized alloys and reinforced vessels, may have environmental implications in their production and disposal. These materials often require energy-intensive manufacturing processes and may not be easily recyclable, potentially contributing to waste accumulation.
Chemical reagents used in high-pressure calorimetry experiments can pose environmental risks if not properly handled and disposed of. Some reactions conducted under high pressure may produce byproducts or waste streams that require specialized treatment before release into the environment. Proper containment and disposal protocols are essential to mitigate potential contamination of soil, water, or air.
The safety measures required for high-pressure calorimetry operations can have indirect environmental impacts. Enhanced ventilation systems, safety barriers, and backup power supplies may increase the overall energy footprint of laboratories employing these techniques. Additionally, the production and maintenance of safety equipment contribute to resource consumption and potential waste generation.
On a positive note, high-pressure calorimetry can contribute to environmental sustainability by enabling the study and optimization of chemical processes under extreme conditions. This research can lead to the development of more efficient industrial processes, potentially reducing energy consumption and waste production on a larger scale. For instance, insights gained from high-pressure studies may inform the design of more environmentally friendly catalysts or reaction pathways.
The environmental impact of high-pressure calorimetry extends to the broader scientific community through knowledge dissemination. Research findings may inspire innovations in green chemistry and sustainable technology development. However, the increased resource demands of high-pressure experiments may also lead to a higher carbon footprint for scientific publications and conferences in this field.
Balancing the environmental costs and benefits of high-pressure calorimetry requires a holistic approach. Researchers and institutions should strive to implement energy-efficient practices, explore renewable energy sources for power-intensive experiments, and develop recycling strategies for specialized materials. Additionally, incorporating life cycle assessments into research planning can help identify and mitigate the long-term environmental impacts of this valuable scientific technique.
The materials used in high-pressure calorimetry equipment, such as specialized alloys and reinforced vessels, may have environmental implications in their production and disposal. These materials often require energy-intensive manufacturing processes and may not be easily recyclable, potentially contributing to waste accumulation.
Chemical reagents used in high-pressure calorimetry experiments can pose environmental risks if not properly handled and disposed of. Some reactions conducted under high pressure may produce byproducts or waste streams that require specialized treatment before release into the environment. Proper containment and disposal protocols are essential to mitigate potential contamination of soil, water, or air.
The safety measures required for high-pressure calorimetry operations can have indirect environmental impacts. Enhanced ventilation systems, safety barriers, and backup power supplies may increase the overall energy footprint of laboratories employing these techniques. Additionally, the production and maintenance of safety equipment contribute to resource consumption and potential waste generation.
On a positive note, high-pressure calorimetry can contribute to environmental sustainability by enabling the study and optimization of chemical processes under extreme conditions. This research can lead to the development of more efficient industrial processes, potentially reducing energy consumption and waste production on a larger scale. For instance, insights gained from high-pressure studies may inform the design of more environmentally friendly catalysts or reaction pathways.
The environmental impact of high-pressure calorimetry extends to the broader scientific community through knowledge dissemination. Research findings may inspire innovations in green chemistry and sustainable technology development. However, the increased resource demands of high-pressure experiments may also lead to a higher carbon footprint for scientific publications and conferences in this field.
Balancing the environmental costs and benefits of high-pressure calorimetry requires a holistic approach. Researchers and institutions should strive to implement energy-efficient practices, explore renewable energy sources for power-intensive experiments, and develop recycling strategies for specialized materials. Additionally, incorporating life cycle assessments into research planning can help identify and mitigate the long-term environmental impacts of this valuable scientific technique.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!