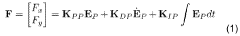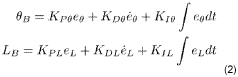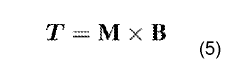Implementing Ferrofluid Strategies in Healthcare Innovation Projects
JUL 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ferrofluid in Healthcare: Background and Objectives
Ferrofluids, a unique class of magnetic nanomaterials, have emerged as a promising frontier in healthcare innovation. These colloidal suspensions of magnetic nanoparticles in a carrier fluid exhibit remarkable properties that bridge the gap between fluid dynamics and magnetism. The evolution of ferrofluid technology can be traced back to the 1960s when NASA first developed them for controlling liquids in zero gravity. Since then, their potential applications have expanded far beyond aerospace, with healthcare becoming a particularly exciting domain for exploration.
The primary objective of implementing ferrofluid strategies in healthcare innovation projects is to leverage the unique characteristics of these smart materials to address critical challenges in medical diagnostics, drug delivery, and therapeutic interventions. By harnessing the ability of ferrofluids to respond to external magnetic fields, researchers aim to develop more precise, targeted, and minimally invasive medical treatments.
One of the key trends driving ferrofluid research in healthcare is the growing demand for personalized medicine. As the medical community shifts towards tailored treatments based on individual patient profiles, ferrofluids offer a versatile platform for developing customizable therapeutic solutions. Their potential to be manipulated at the nanoscale opens up new possibilities for targeted drug delivery, enhancing treatment efficacy while minimizing side effects.
Another significant trend is the integration of ferrofluids with existing medical imaging technologies. The magnetic properties of ferrofluids make them excellent contrast agents for magnetic resonance imaging (MRI), potentially improving the resolution and diagnostic accuracy of this widely used imaging technique. This synergy between ferrofluids and medical imaging is expected to play a crucial role in early disease detection and treatment planning.
The development of ferrofluid-based biosensors represents another promising avenue for healthcare innovation. These advanced sensors could enable rapid, sensitive, and cost-effective detection of various biomarkers, pathogens, and environmental toxins, potentially revolutionizing point-of-care diagnostics and environmental health monitoring.
As research in this field progresses, the technical goals for ferrofluid applications in healthcare are becoming increasingly ambitious. These include developing biocompatible ferrofluids with enhanced stability and magnetic responsiveness, optimizing their interaction with biological systems, and creating novel ferrofluid-based devices for minimally invasive surgeries and targeted therapies.
The primary objective of implementing ferrofluid strategies in healthcare innovation projects is to leverage the unique characteristics of these smart materials to address critical challenges in medical diagnostics, drug delivery, and therapeutic interventions. By harnessing the ability of ferrofluids to respond to external magnetic fields, researchers aim to develop more precise, targeted, and minimally invasive medical treatments.
One of the key trends driving ferrofluid research in healthcare is the growing demand for personalized medicine. As the medical community shifts towards tailored treatments based on individual patient profiles, ferrofluids offer a versatile platform for developing customizable therapeutic solutions. Their potential to be manipulated at the nanoscale opens up new possibilities for targeted drug delivery, enhancing treatment efficacy while minimizing side effects.
Another significant trend is the integration of ferrofluids with existing medical imaging technologies. The magnetic properties of ferrofluids make them excellent contrast agents for magnetic resonance imaging (MRI), potentially improving the resolution and diagnostic accuracy of this widely used imaging technique. This synergy between ferrofluids and medical imaging is expected to play a crucial role in early disease detection and treatment planning.
The development of ferrofluid-based biosensors represents another promising avenue for healthcare innovation. These advanced sensors could enable rapid, sensitive, and cost-effective detection of various biomarkers, pathogens, and environmental toxins, potentially revolutionizing point-of-care diagnostics and environmental health monitoring.
As research in this field progresses, the technical goals for ferrofluid applications in healthcare are becoming increasingly ambitious. These include developing biocompatible ferrofluids with enhanced stability and magnetic responsiveness, optimizing their interaction with biological systems, and creating novel ferrofluid-based devices for minimally invasive surgeries and targeted therapies.
Market Analysis for Ferrofluid-Based Medical Solutions
The ferrofluid-based medical solutions market is experiencing significant growth, driven by the increasing demand for innovative healthcare technologies and the unique properties of ferrofluids. These magnetic nanofluids offer a wide range of potential applications in medical diagnostics, drug delivery, and therapeutic interventions, making them a promising area for healthcare innovation projects.
The global market for ferrofluid-based medical solutions is expected to expand rapidly in the coming years. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for more efficient and targeted drug delivery systems, and the continuous advancements in nanotechnology and materials science. The market is segmented into various applications, including magnetic resonance imaging (MRI) contrast agents, targeted drug delivery, hyperthermia treatment for cancer, and biosensors.
In the field of medical imaging, ferrofluids are gaining traction as contrast agents for MRI, offering enhanced image quality and diagnostic accuracy. This segment is projected to witness substantial growth due to the increasing number of MRI procedures performed globally and the demand for more precise imaging techniques.
The targeted drug delivery segment is another key area driving market growth. Ferrofluid-based nanocarriers can be precisely guided to specific locations in the body using external magnetic fields, potentially improving treatment efficacy while reducing side effects. This application is particularly promising for cancer therapies and the treatment of neurological disorders.
Hyperthermia treatment using ferrofluids is emerging as a novel approach in cancer therapy. By applying an alternating magnetic field to ferrofluid nanoparticles localized in tumor tissues, it is possible to generate localized heat, selectively destroying cancer cells while minimizing damage to healthy tissues. This innovative treatment method is attracting significant research interest and investment.
The biosensor market is also benefiting from ferrofluid technology, with applications in rapid and sensitive detection of various biomarkers. These advanced biosensors have potential uses in point-of-care diagnostics, environmental monitoring, and food safety testing.
Geographically, North America and Europe are currently the leading markets for ferrofluid-based medical solutions, owing to their advanced healthcare infrastructure, high R&D investments, and early adoption of innovative technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare systems, increasing healthcare expenditure, and growing awareness of advanced medical technologies.
Despite the promising outlook, the market faces challenges such as the need for extensive clinical trials to prove safety and efficacy, regulatory hurdles, and the high cost of research and development. Overcoming these obstacles will be crucial for the widespread adoption of ferrofluid-based medical solutions and the realization of their full market potential.
The global market for ferrofluid-based medical solutions is expected to expand rapidly in the coming years. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for more efficient and targeted drug delivery systems, and the continuous advancements in nanotechnology and materials science. The market is segmented into various applications, including magnetic resonance imaging (MRI) contrast agents, targeted drug delivery, hyperthermia treatment for cancer, and biosensors.
In the field of medical imaging, ferrofluids are gaining traction as contrast agents for MRI, offering enhanced image quality and diagnostic accuracy. This segment is projected to witness substantial growth due to the increasing number of MRI procedures performed globally and the demand for more precise imaging techniques.
The targeted drug delivery segment is another key area driving market growth. Ferrofluid-based nanocarriers can be precisely guided to specific locations in the body using external magnetic fields, potentially improving treatment efficacy while reducing side effects. This application is particularly promising for cancer therapies and the treatment of neurological disorders.
Hyperthermia treatment using ferrofluids is emerging as a novel approach in cancer therapy. By applying an alternating magnetic field to ferrofluid nanoparticles localized in tumor tissues, it is possible to generate localized heat, selectively destroying cancer cells while minimizing damage to healthy tissues. This innovative treatment method is attracting significant research interest and investment.
The biosensor market is also benefiting from ferrofluid technology, with applications in rapid and sensitive detection of various biomarkers. These advanced biosensors have potential uses in point-of-care diagnostics, environmental monitoring, and food safety testing.
Geographically, North America and Europe are currently the leading markets for ferrofluid-based medical solutions, owing to their advanced healthcare infrastructure, high R&D investments, and early adoption of innovative technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare systems, increasing healthcare expenditure, and growing awareness of advanced medical technologies.
Despite the promising outlook, the market faces challenges such as the need for extensive clinical trials to prove safety and efficacy, regulatory hurdles, and the high cost of research and development. Overcoming these obstacles will be crucial for the widespread adoption of ferrofluid-based medical solutions and the realization of their full market potential.
Current Challenges in Ferrofluid Healthcare Applications
The implementation of ferrofluid strategies in healthcare innovation projects faces several significant challenges that hinder widespread adoption and clinical application. One of the primary obstacles is the complexity of synthesizing biocompatible ferrofluids that can safely interact with biological systems. The development of nanoparticles with appropriate surface coatings to prevent aggregation and ensure stability in physiological environments remains a critical hurdle.
Another major challenge lies in the precise control and manipulation of ferrofluids within the human body. While external magnetic fields can guide these fluids, achieving accurate localization and targeted delivery in complex anatomical structures presents substantial difficulties. The heterogeneity of biological tissues and the dynamic nature of physiological processes further complicate the predictable behavior of ferrofluids in vivo.
The long-term safety and toxicity of ferrofluids in medical applications are also areas of concern. Although initial studies have shown promising results, comprehensive long-term studies on the potential adverse effects of prolonged exposure to ferrofluids and their breakdown products are still lacking. This gap in knowledge creates regulatory hurdles and slows down the clinical translation of ferrofluid-based technologies.
Furthermore, the scalability and reproducibility of ferrofluid production for healthcare applications pose significant challenges. Ensuring consistent quality and performance across different batches of ferrofluids is crucial for their reliable use in medical settings. The development of standardized manufacturing processes and quality control measures remains an ongoing challenge in the field.
Integrating ferrofluid-based technologies with existing medical devices and imaging systems presents another set of obstacles. Compatibility issues with current diagnostic and therapeutic equipment need to be addressed to facilitate seamless integration into clinical workflows. Additionally, the development of specialized instruments and protocols for handling and administering ferrofluids in medical procedures requires significant investment and collaboration across multiple disciplines.
Lastly, the cost-effectiveness of ferrofluid-based healthcare solutions remains a challenge. The production of high-quality, biocompatible ferrofluids and the development of associated technologies often involve expensive materials and complex processes. Demonstrating clear clinical benefits and cost advantages over existing treatments is crucial for gaining acceptance from healthcare providers and payers, and ultimately achieving widespread adoption in the medical field.
Another major challenge lies in the precise control and manipulation of ferrofluids within the human body. While external magnetic fields can guide these fluids, achieving accurate localization and targeted delivery in complex anatomical structures presents substantial difficulties. The heterogeneity of biological tissues and the dynamic nature of physiological processes further complicate the predictable behavior of ferrofluids in vivo.
The long-term safety and toxicity of ferrofluids in medical applications are also areas of concern. Although initial studies have shown promising results, comprehensive long-term studies on the potential adverse effects of prolonged exposure to ferrofluids and their breakdown products are still lacking. This gap in knowledge creates regulatory hurdles and slows down the clinical translation of ferrofluid-based technologies.
Furthermore, the scalability and reproducibility of ferrofluid production for healthcare applications pose significant challenges. Ensuring consistent quality and performance across different batches of ferrofluids is crucial for their reliable use in medical settings. The development of standardized manufacturing processes and quality control measures remains an ongoing challenge in the field.
Integrating ferrofluid-based technologies with existing medical devices and imaging systems presents another set of obstacles. Compatibility issues with current diagnostic and therapeutic equipment need to be addressed to facilitate seamless integration into clinical workflows. Additionally, the development of specialized instruments and protocols for handling and administering ferrofluids in medical procedures requires significant investment and collaboration across multiple disciplines.
Lastly, the cost-effectiveness of ferrofluid-based healthcare solutions remains a challenge. The production of high-quality, biocompatible ferrofluids and the development of associated technologies often involve expensive materials and complex processes. Demonstrating clear clinical benefits and cost advantages over existing treatments is crucial for gaining acceptance from healthcare providers and payers, and ultimately achieving widespread adoption in the medical field.
Existing Ferrofluid Strategies in Medical Projects
01 Composition and preparation of ferrofluids
Ferrofluids are colloidal suspensions of magnetic nanoparticles in a carrier fluid. They are typically composed of magnetite or other ferromagnetic materials coated with a surfactant to prevent agglomeration. The preparation process involves careful control of particle size and distribution to maintain stability and magnetic properties.- Composition and preparation of ferrofluids: Ferrofluids are colloidal suspensions of magnetic nanoparticles in a carrier fluid. They are typically composed of magnetite or other ferromagnetic materials coated with surfactants to prevent agglomeration. The preparation process involves careful control of particle size and distribution to maintain stability and magnetic properties.
- Applications in sealing and lubrication: Ferrofluids are widely used in sealing and lubrication applications, particularly in rotating shaft seals. They provide a liquid barrier that can be controlled by magnetic fields, offering advantages in terms of low friction, minimal leakage, and adaptability to various operating conditions.
- Thermal management and cooling systems: Ferrofluids have unique heat transfer properties that make them suitable for thermal management applications. They can be used in cooling systems for electronic devices, where their magnetic properties allow for enhanced heat dissipation and controlled fluid movement.
- Sensor and actuator technologies: The responsive nature of ferrofluids to magnetic fields makes them valuable in sensor and actuator technologies. They can be used in accelerometers, tilt sensors, and various types of actuators where precise control of fluid movement is required.
- Medical and biomedical applications: Ferrofluids have potential applications in medical and biomedical fields. They can be used for targeted drug delivery, magnetic hyperthermia treatment of cancer, and as contrast agents in magnetic resonance imaging (MRI). The ability to control these fluids using external magnetic fields offers new possibilities in minimally invasive medical procedures.
02 Applications in sealing and lubrication
Ferrofluids are widely used in sealing and lubrication applications, particularly in rotating shaft seals. They provide a liquid barrier that can be controlled by magnetic fields, offering advantages in terms of low friction, long life, and the ability to operate in vacuum environments.Expand Specific Solutions03 Magnetic field-responsive devices
Ferrofluids are utilized in various devices that respond to magnetic fields. These include actuators, sensors, and dampers. The unique properties of ferrofluids allow for precise control and manipulation of fluid behavior using external magnetic fields, enabling novel applications in areas such as robotics and vibration control.Expand Specific Solutions04 Heat transfer and cooling applications
Ferrofluids have thermal management applications, particularly in electronic cooling. Their ability to be manipulated by magnetic fields allows for enhanced heat transfer and targeted cooling in compact spaces. This property is exploited in the design of more efficient cooling systems for electronic devices and power equipment.Expand Specific Solutions05 Measurement and analysis techniques
Various techniques have been developed for measuring and analyzing the properties of ferrofluids. These include methods for determining particle size distribution, magnetic susceptibility, and rheological characteristics. Advanced imaging and spectroscopic techniques are employed to study the behavior of ferrofluids under different conditions.Expand Specific Solutions
Key Players in Ferrofluid Healthcare Innovation
The implementation of ferrofluid strategies in healthcare innovation projects is in an early developmental stage, with a growing market potential as research progresses. The technology's maturity is still evolving, with key players from academia and industry driving advancements. Universities like Yale, Duke, and MIT are at the forefront of research, while companies such as Ferrotec and Advanced Liquid Logic are developing practical applications. The involvement of major healthcare corporations like Philips indicates increasing commercial interest. Collaborations between research institutions and industry partners are crucial for translating ferrofluid technology into viable healthcare solutions, suggesting a competitive landscape that is both cooperative and diverse.
Advanced Liquid Logic, Inc.
Technical Solution: Advanced Liquid Logic has focused on integrating ferrofluid technology into their digital microfluidics platform for healthcare applications. They have developed systems that use ferrofluids for precise liquid handling and manipulation in diagnostic assays, enabling miniaturization and automation of complex laboratory procedures[10]. The company has also explored the use of ferrofluids in creating adaptive optical elements for medical imaging devices, potentially improving resolution and adaptability in various imaging modalities[11].
Strengths: Specialized in microfluidics, potential for high-precision diagnostic tools. Weaknesses: Narrow focus may limit broader healthcare applications.
Duke University
Technical Solution: Duke University researchers have made notable contributions to ferrofluid applications in healthcare. They have developed ferrofluid-based magnetic particle imaging (MPI) techniques for non-invasive, real-time tracking of cells and biomolecules in the body, with potential applications in cancer detection and treatment monitoring[12]. Duke has also explored the use of ferrofluids in creating adaptive prosthetics and orthotic devices, leveraging the fluid's unique properties to enhance comfort and functionality[13]. Furthermore, their work on ferrofluid-enhanced drug delivery systems has shown promise in improving the efficacy of cancer treatments while reducing side effects[14].
Strengths: Strong research foundation, collaborations with medical institutions. Weaknesses: May face challenges in translating academic research to clinical applications.
Breakthrough Ferrofluid Technologies for Healthcare
Systems and methods for controlling shape and position of a ferrofluid droplet
PatentWO2021041471A1
Innovation
- A system comprising a ferrofluid droplet and an electromagnetic field generation system with a controller that determines and applies necessary magnetic field parameters to control the position and shape of the ferrofluid droplet, using PID controllers for precise manipulation, allowing for shape and position control, and simultaneous position and shape control through the generation and manipulation of controlled magnetic fields.
Method for moving a fluid of interest in a capillary tube and fluidic microsystem
PatentInactiveEP1444042A1
Innovation
- A method involving the placement of a train of ferrofluid with a cap of ferrofluid and a liquid immiscible with the fluid of interest within capillaries, controlled by a magnetic field generated externally, which prevents contamination and allows precise movement of fluid plugs by using ionic ferrofluids and hydrophobic capillary walls, along with oil plugs for pre-wetting and separation.
Regulatory Framework for Ferrofluid Medical Devices
The regulatory framework for ferrofluid medical devices is a complex and evolving landscape that requires careful navigation by healthcare innovators. As ferrofluids gain traction in medical applications, regulatory bodies worldwide are adapting their guidelines to ensure the safety and efficacy of these novel technologies.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating ferrofluid-based medical devices. These devices typically fall under Class II or Class III classifications, depending on their intended use and potential risks. Class II devices may require a 510(k) premarket notification, while Class III devices often necessitate a more rigorous premarket approval (PMA) process. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for overseeing the evaluation and approval of ferrofluid medical devices.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2021 and May 2022, respectively. These regulations have significant implications for ferrofluid medical devices, introducing more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to market their devices in the EU, which involves demonstrating compliance with essential requirements and undergoing conformity assessment procedures.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices, including those incorporating ferrofluids. The regulatory pathway depends on the device's classification, with higher-risk devices requiring more extensive clinical data and review processes. Similarly, China's National Medical Products Administration (NMPA) has established a regulatory framework that categorizes medical devices into three classes based on risk levels, with ferrofluid devices likely falling into Class II or III.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different jurisdictions. These initiatives can potentially reduce the regulatory burden for manufacturers of ferrofluid medical devices seeking global market access.
Key considerations for regulatory compliance of ferrofluid medical devices include biocompatibility testing, stability studies, and performance evaluations under various environmental conditions. Manufacturers must also address potential safety concerns related to magnetic field interactions and long-term exposure to ferrofluid materials. Additionally, quality management systems compliant with ISO 13485 standards are typically required to ensure consistent production and maintain regulatory approval.
As the field of ferrofluid medical devices continues to advance, regulatory frameworks are likely to evolve. Innovators must stay informed about emerging guidelines and engage proactively with regulatory bodies to navigate the approval process successfully. Early consultation with regulatory experts and careful planning of clinical studies can significantly streamline the path to market for novel ferrofluid-based healthcare technologies.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating ferrofluid-based medical devices. These devices typically fall under Class II or Class III classifications, depending on their intended use and potential risks. Class II devices may require a 510(k) premarket notification, while Class III devices often necessitate a more rigorous premarket approval (PMA) process. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for overseeing the evaluation and approval of ferrofluid medical devices.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2021 and May 2022, respectively. These regulations have significant implications for ferrofluid medical devices, introducing more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to market their devices in the EU, which involves demonstrating compliance with essential requirements and undergoing conformity assessment procedures.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices, including those incorporating ferrofluids. The regulatory pathway depends on the device's classification, with higher-risk devices requiring more extensive clinical data and review processes. Similarly, China's National Medical Products Administration (NMPA) has established a regulatory framework that categorizes medical devices into three classes based on risk levels, with ferrofluid devices likely falling into Class II or III.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different jurisdictions. These initiatives can potentially reduce the regulatory burden for manufacturers of ferrofluid medical devices seeking global market access.
Key considerations for regulatory compliance of ferrofluid medical devices include biocompatibility testing, stability studies, and performance evaluations under various environmental conditions. Manufacturers must also address potential safety concerns related to magnetic field interactions and long-term exposure to ferrofluid materials. Additionally, quality management systems compliant with ISO 13485 standards are typically required to ensure consistent production and maintain regulatory approval.
As the field of ferrofluid medical devices continues to advance, regulatory frameworks are likely to evolve. Innovators must stay informed about emerging guidelines and engage proactively with regulatory bodies to navigate the approval process successfully. Early consultation with regulatory experts and careful planning of clinical studies can significantly streamline the path to market for novel ferrofluid-based healthcare technologies.
Biocompatibility and Safety Considerations
The implementation of ferrofluid strategies in healthcare innovation projects necessitates a thorough examination of biocompatibility and safety considerations. Ferrofluids, composed of nanoscale magnetic particles suspended in a carrier fluid, present unique challenges when introduced into biological systems.
Biocompatibility is a primary concern, as these nanoparticles must interact with living tissues without causing adverse reactions. The size, shape, and surface properties of the magnetic particles play crucial roles in determining their biological interactions. Particles with diameters below 100 nm can potentially cross biological barriers, necessitating careful design to prevent unintended distribution within the body.
Surface modification of ferrofluid nanoparticles is a key strategy to enhance biocompatibility. Coating the particles with biocompatible materials such as polyethylene glycol (PEG) or dextran can reduce protein adsorption and minimize immune system recognition, thereby increasing circulation time and reducing potential toxicity.
The potential for long-term accumulation of magnetic nanoparticles in organs, particularly the liver and spleen, raises concerns about chronic toxicity. Comprehensive studies on the biodistribution, metabolism, and clearance of ferrofluids are essential to assess their long-term safety profile. Additionally, the potential for these particles to generate reactive oxygen species (ROS) under certain conditions must be carefully evaluated and mitigated.
Safety considerations extend beyond the nanoparticles themselves to the carrier fluid and any additional components in the ferrofluid formulation. The choice of carrier fluid must balance the desired magnetic properties with biological inertness. Biocompatible oils or water-based solutions are often preferred, with careful attention paid to pH, osmolarity, and potential interactions with biological molecules.
The application of external magnetic fields to manipulate ferrofluids in vivo introduces additional safety considerations. While these fields are generally considered safe, potential effects on cardiac pacemakers, metallic implants, or sensitive electronic devices must be thoroughly assessed. Moreover, the heat generated by alternating magnetic fields, while potentially beneficial in some applications like hyperthermia treatment, must be carefully controlled to prevent thermal damage to healthy tissues.
Regulatory compliance is a critical aspect of ferrofluid implementation in healthcare. Developers must navigate complex regulatory frameworks, such as those set by the FDA or EMA, which may require extensive preclinical and clinical testing to demonstrate safety and efficacy. This process often involves rigorous toxicology studies, biodistribution analyses, and long-term follow-up studies to monitor for potential delayed effects.
Biocompatibility is a primary concern, as these nanoparticles must interact with living tissues without causing adverse reactions. The size, shape, and surface properties of the magnetic particles play crucial roles in determining their biological interactions. Particles with diameters below 100 nm can potentially cross biological barriers, necessitating careful design to prevent unintended distribution within the body.
Surface modification of ferrofluid nanoparticles is a key strategy to enhance biocompatibility. Coating the particles with biocompatible materials such as polyethylene glycol (PEG) or dextran can reduce protein adsorption and minimize immune system recognition, thereby increasing circulation time and reducing potential toxicity.
The potential for long-term accumulation of magnetic nanoparticles in organs, particularly the liver and spleen, raises concerns about chronic toxicity. Comprehensive studies on the biodistribution, metabolism, and clearance of ferrofluids are essential to assess their long-term safety profile. Additionally, the potential for these particles to generate reactive oxygen species (ROS) under certain conditions must be carefully evaluated and mitigated.
Safety considerations extend beyond the nanoparticles themselves to the carrier fluid and any additional components in the ferrofluid formulation. The choice of carrier fluid must balance the desired magnetic properties with biological inertness. Biocompatible oils or water-based solutions are often preferred, with careful attention paid to pH, osmolarity, and potential interactions with biological molecules.
The application of external magnetic fields to manipulate ferrofluids in vivo introduces additional safety considerations. While these fields are generally considered safe, potential effects on cardiac pacemakers, metallic implants, or sensitive electronic devices must be thoroughly assessed. Moreover, the heat generated by alternating magnetic fields, while potentially beneficial in some applications like hyperthermia treatment, must be carefully controlled to prevent thermal damage to healthy tissues.
Regulatory compliance is a critical aspect of ferrofluid implementation in healthcare. Developers must navigate complex regulatory frameworks, such as those set by the FDA or EMA, which may require extensive preclinical and clinical testing to demonstrate safety and efficacy. This process often involves rigorous toxicology studies, biodistribution analyses, and long-term follow-up studies to monitor for potential delayed effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!