Improve GC-MS Sample Prep for Non-Volatile Organics
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Technology Evolution and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved significantly since its inception in the mid-20th century, transforming from a specialized analytical technique into an essential tool across multiple scientific disciplines. The initial development of GC-MS in the 1950s marked a revolutionary advancement in analytical chemistry, combining the separation capabilities of gas chromatography with the identification power of mass spectrometry. This synergistic integration enabled scientists to analyze complex mixtures with unprecedented precision.
Throughout the 1970s and 1980s, GC-MS technology underwent substantial refinements, including improvements in column technology, detector sensitivity, and data processing capabilities. The introduction of capillary columns significantly enhanced separation efficiency, while advances in ionization techniques expanded the range of analyzable compounds. By the 1990s, computerization revolutionized GC-MS operations, enabling automated sample processing and sophisticated data analysis.
Recent technological innovations have focused on miniaturization, portability, and increased sensitivity. Modern GC-MS systems can detect compounds at parts-per-trillion levels, representing a thousand-fold improvement over early instruments. Additionally, the development of two-dimensional GC-MS (GCxGC-MS) has further expanded analytical capabilities by providing enhanced separation of complex mixtures.
Despite these advancements, the analysis of non-volatile organic compounds (NVOCs) remains challenging due to their inherent physical properties. NVOCs typically have high boiling points and molecular weights, making them difficult to vaporize efficiently for GC analysis. Traditional sample preparation methods often fail to adequately prepare these compounds for GC-MS analysis, resulting in poor chromatographic performance, peak tailing, and incomplete separation.
The primary objective of improving GC-MS sample preparation for NVOCs is to develop methodologies that effectively convert these compounds into forms suitable for gas-phase analysis without compromising their structural integrity or analytical accuracy. This includes exploring novel derivatization techniques that can reduce polarity and increase volatility, investigating alternative extraction methods that maximize recovery rates, and optimizing instrumental parameters to accommodate the unique characteristics of derivatized NVOCs.
Additionally, there is a growing need to develop standardized protocols that ensure reproducibility across different laboratories and applications. The ideal solution would combine efficiency, cost-effectiveness, and environmental sustainability while maintaining or enhancing analytical performance. Achieving these objectives would significantly expand the applicability of GC-MS technology across pharmaceutical development, environmental monitoring, food safety, and forensic science sectors.
Throughout the 1970s and 1980s, GC-MS technology underwent substantial refinements, including improvements in column technology, detector sensitivity, and data processing capabilities. The introduction of capillary columns significantly enhanced separation efficiency, while advances in ionization techniques expanded the range of analyzable compounds. By the 1990s, computerization revolutionized GC-MS operations, enabling automated sample processing and sophisticated data analysis.
Recent technological innovations have focused on miniaturization, portability, and increased sensitivity. Modern GC-MS systems can detect compounds at parts-per-trillion levels, representing a thousand-fold improvement over early instruments. Additionally, the development of two-dimensional GC-MS (GCxGC-MS) has further expanded analytical capabilities by providing enhanced separation of complex mixtures.
Despite these advancements, the analysis of non-volatile organic compounds (NVOCs) remains challenging due to their inherent physical properties. NVOCs typically have high boiling points and molecular weights, making them difficult to vaporize efficiently for GC analysis. Traditional sample preparation methods often fail to adequately prepare these compounds for GC-MS analysis, resulting in poor chromatographic performance, peak tailing, and incomplete separation.
The primary objective of improving GC-MS sample preparation for NVOCs is to develop methodologies that effectively convert these compounds into forms suitable for gas-phase analysis without compromising their structural integrity or analytical accuracy. This includes exploring novel derivatization techniques that can reduce polarity and increase volatility, investigating alternative extraction methods that maximize recovery rates, and optimizing instrumental parameters to accommodate the unique characteristics of derivatized NVOCs.
Additionally, there is a growing need to develop standardized protocols that ensure reproducibility across different laboratories and applications. The ideal solution would combine efficiency, cost-effectiveness, and environmental sustainability while maintaining or enhancing analytical performance. Achieving these objectives would significantly expand the applicability of GC-MS technology across pharmaceutical development, environmental monitoring, food safety, and forensic science sectors.
Market Demand Analysis for Advanced Sample Preparation
The global market for advanced sample preparation techniques in GC-MS analysis has experienced significant growth, driven by increasing demands for accurate detection and quantification of non-volatile organic compounds across multiple industries. Current market valuations indicate that the analytical chemistry equipment sector, particularly for sample preparation, exceeds $5 billion annually with a compound annual growth rate of 6.8% projected through 2028.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for approximately 38% of the total demand. These industries require sophisticated sample preparation methods to analyze complex biological matrices containing non-volatile compounds for drug development, quality control, and pharmacokinetic studies. The need for higher throughput and automation in these sectors has created substantial market opportunities for innovative sample preparation technologies.
Environmental monitoring constitutes the second-largest market segment at 27%, where regulatory agencies worldwide have implemented stricter guidelines for detecting trace amounts of persistent organic pollutants and emerging contaminants. This regulatory pressure has intensified the demand for more efficient extraction and derivatization techniques specifically designed for non-volatile compounds in environmental samples.
Food safety testing represents a rapidly growing segment with 18% market share, driven by increasing consumer awareness and regulatory requirements regarding chemical residues in food products. The ability to accurately detect non-volatile pesticides, mycotoxins, and other contaminants has become critical for food producers and testing laboratories.
Clinical diagnostics accounts for 12% of the market, with growing applications in metabolomics and clinical toxicology. The remaining 5% encompasses various industries including forensics, petrochemicals, and academic research.
Geographically, North America leads with 38% market share, followed by Europe (32%), Asia-Pacific (24%), and rest of the world (6%). The Asia-Pacific region demonstrates the fastest growth rate at 8.2% annually, primarily due to expanding pharmaceutical manufacturing and environmental monitoring programs in China and India.
Key market drivers include increasing regulatory requirements for lower detection limits, growing sample complexity requiring more sophisticated preparation techniques, and industry demand for higher throughput and automation. End-users consistently identify sample preparation as the most time-consuming and error-prone step in their analytical workflows, with approximately 60% of analysis time and 30% of total error attributed to this phase.
Market surveys indicate strong willingness to invest in advanced sample preparation technologies that can demonstrate improvements in recovery rates, reproducibility, and processing time for non-volatile compounds, with potential price premiums of 15-25% for solutions that significantly outperform current methods.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for approximately 38% of the total demand. These industries require sophisticated sample preparation methods to analyze complex biological matrices containing non-volatile compounds for drug development, quality control, and pharmacokinetic studies. The need for higher throughput and automation in these sectors has created substantial market opportunities for innovative sample preparation technologies.
Environmental monitoring constitutes the second-largest market segment at 27%, where regulatory agencies worldwide have implemented stricter guidelines for detecting trace amounts of persistent organic pollutants and emerging contaminants. This regulatory pressure has intensified the demand for more efficient extraction and derivatization techniques specifically designed for non-volatile compounds in environmental samples.
Food safety testing represents a rapidly growing segment with 18% market share, driven by increasing consumer awareness and regulatory requirements regarding chemical residues in food products. The ability to accurately detect non-volatile pesticides, mycotoxins, and other contaminants has become critical for food producers and testing laboratories.
Clinical diagnostics accounts for 12% of the market, with growing applications in metabolomics and clinical toxicology. The remaining 5% encompasses various industries including forensics, petrochemicals, and academic research.
Geographically, North America leads with 38% market share, followed by Europe (32%), Asia-Pacific (24%), and rest of the world (6%). The Asia-Pacific region demonstrates the fastest growth rate at 8.2% annually, primarily due to expanding pharmaceutical manufacturing and environmental monitoring programs in China and India.
Key market drivers include increasing regulatory requirements for lower detection limits, growing sample complexity requiring more sophisticated preparation techniques, and industry demand for higher throughput and automation. End-users consistently identify sample preparation as the most time-consuming and error-prone step in their analytical workflows, with approximately 60% of analysis time and 30% of total error attributed to this phase.
Market surveys indicate strong willingness to invest in advanced sample preparation technologies that can demonstrate improvements in recovery rates, reproducibility, and processing time for non-volatile compounds, with potential price premiums of 15-25% for solutions that significantly outperform current methods.
Current Challenges in Non-Volatile Compound Analysis
The analysis of non-volatile organic compounds using GC-MS faces several significant challenges that limit the effectiveness and reliability of current methodologies. Traditional GC-MS techniques were primarily designed for volatile compounds, making the analysis of non-volatile substances inherently problematic. The high molecular weight and low vapor pressure of these compounds often result in poor vaporization in the GC inlet, leading to incomplete transfer to the column and subsequent detection issues.
Sample preparation represents one of the most critical bottlenecks in this analytical process. Current derivatization methods, while effective for increasing volatility, often introduce variability and artifacts that complicate data interpretation. The chemical modification of analytes through derivatization can alter the original molecular structure, potentially masking important chemical information and creating challenges in accurate identification.
Thermal degradation presents another significant obstacle, as many non-volatile compounds decompose at the high temperatures required for GC analysis. This decomposition not only reduces analytical sensitivity but also generates breakdown products that can interfere with target compound identification and quantification. The resulting chromatograms frequently contain complex mixtures of original compounds and their thermal degradation products.
Matrix effects further complicate the analysis, particularly in complex biological or environmental samples. Co-extracted matrix components can cause ion suppression or enhancement, affecting ionization efficiency and leading to inaccurate quantification. The removal of these interfering substances without losing target analytes remains a delicate balance that current sample preparation techniques struggle to achieve.
Instrument contamination represents a persistent challenge, as non-volatile residues can accumulate in the GC inlet, column, and detector systems. This contamination leads to decreased column efficiency, elevated baseline noise, and reduced instrument sensitivity over time. The frequent maintenance required to address these issues increases operational costs and instrument downtime.
Recovery rates for non-volatile compounds using current extraction methods are often inconsistent and sample-dependent. Traditional liquid-liquid extraction and solid-phase extraction techniques may not effectively isolate all target compounds, particularly those with diverse physicochemical properties. This variability in recovery rates compromises method reproducibility and quantitative accuracy.
Standardization across laboratories remains elusive due to the complexity of sample preparation protocols for non-volatile compounds. The multitude of available techniques and lack of harmonized approaches create challenges in comparing results between different research groups and laboratories, hindering collaborative research efforts and the establishment of reliable reference methods.
Sample preparation represents one of the most critical bottlenecks in this analytical process. Current derivatization methods, while effective for increasing volatility, often introduce variability and artifacts that complicate data interpretation. The chemical modification of analytes through derivatization can alter the original molecular structure, potentially masking important chemical information and creating challenges in accurate identification.
Thermal degradation presents another significant obstacle, as many non-volatile compounds decompose at the high temperatures required for GC analysis. This decomposition not only reduces analytical sensitivity but also generates breakdown products that can interfere with target compound identification and quantification. The resulting chromatograms frequently contain complex mixtures of original compounds and their thermal degradation products.
Matrix effects further complicate the analysis, particularly in complex biological or environmental samples. Co-extracted matrix components can cause ion suppression or enhancement, affecting ionization efficiency and leading to inaccurate quantification. The removal of these interfering substances without losing target analytes remains a delicate balance that current sample preparation techniques struggle to achieve.
Instrument contamination represents a persistent challenge, as non-volatile residues can accumulate in the GC inlet, column, and detector systems. This contamination leads to decreased column efficiency, elevated baseline noise, and reduced instrument sensitivity over time. The frequent maintenance required to address these issues increases operational costs and instrument downtime.
Recovery rates for non-volatile compounds using current extraction methods are often inconsistent and sample-dependent. Traditional liquid-liquid extraction and solid-phase extraction techniques may not effectively isolate all target compounds, particularly those with diverse physicochemical properties. This variability in recovery rates compromises method reproducibility and quantitative accuracy.
Standardization across laboratories remains elusive due to the complexity of sample preparation protocols for non-volatile compounds. The multitude of available techniques and lack of harmonized approaches create challenges in comparing results between different research groups and laboratories, hindering collaborative research efforts and the establishment of reliable reference methods.
Current Derivatization and Extraction Methodologies
01 Sample extraction and purification techniques
Various extraction and purification methods are employed to prepare samples for GC-MS analysis. These techniques include solid-phase extraction, liquid-liquid extraction, and filtration processes that help isolate target analytes from complex matrices. The purification steps remove interfering substances and concentrate the analytes of interest, improving detection sensitivity and analytical accuracy. These preparation methods are crucial for environmental, pharmaceutical, and food safety applications.- Sample extraction and purification techniques: Various extraction and purification methods are employed to prepare samples for GC-MS analysis. These techniques include solid-phase extraction, liquid-liquid extraction, and filtration processes that help isolate target analytes from complex matrices. The purification steps remove interfering substances and concentrate the analytes of interest, improving detection sensitivity and analytical accuracy. These methods are critical for environmental, pharmaceutical, and food safety applications where sample matrices are complex.
- Automated sample preparation systems: Automated systems for GC-MS sample preparation improve efficiency and reproducibility while reducing human error. These systems incorporate robotic handling, automated extraction, and precise dispensing of solvents and reagents. Automation allows for high-throughput analysis with standardized protocols, ensuring consistent sample treatment across large batches. These systems often include integrated software for process control and data management, streamlining the workflow from sample receipt to analysis.
- Derivatization methods for GC-MS analysis: Derivatization techniques modify analytes to improve their volatility, thermal stability, and chromatographic behavior for GC-MS analysis. Common derivatization reagents include silylating agents, acylating reagents, and alkylating compounds that react with functional groups in the target molecules. These chemical modifications enhance separation efficiency and detection sensitivity, particularly for polar compounds with hydroxyl, carboxyl, or amine groups that would otherwise be difficult to analyze by GC-MS.
- Microextraction techniques for GC-MS: Microextraction methods provide efficient sample preparation while using minimal solvent volumes and sample amounts. Techniques such as solid-phase microextraction (SPME), single-drop microextraction, and dispersive liquid-liquid microextraction concentrate analytes from various matrices. These approaches are environmentally friendly, cost-effective, and suitable for volatile and semi-volatile compounds. Microextraction techniques can be coupled directly with GC-MS instruments, allowing for streamlined workflows and reduced sample handling.
- Sample preparation for specific applications: Specialized sample preparation protocols are developed for specific GC-MS applications across different industries. These include methods for environmental pollutant analysis, food contaminant detection, pharmaceutical impurity profiling, and biological sample analysis. Each protocol addresses the unique challenges of the sample matrix, such as high lipid content in food samples or complex biological matrices in clinical specimens. These tailored approaches optimize extraction efficiency, minimize matrix effects, and enhance the detection of target compounds at trace levels.
02 Automated sample preparation systems
Automated systems for GC-MS sample preparation enhance efficiency and reproducibility while reducing human error. These systems incorporate robotic handling, precise liquid dispensing, and programmable workflows for consistent sample processing. Automation solutions range from simple autosampler units to fully integrated workstations capable of performing multiple preparation steps. These systems are particularly valuable for high-throughput laboratories analyzing large sample volumes.Expand Specific Solutions03 Derivatization methods for GC-MS analysis
Derivatization techniques modify analyte molecules to improve their volatility, thermal stability, and chromatographic behavior for GC-MS analysis. Common derivatization reagents include silylating agents, acylating compounds, and alkylating reagents that react with functional groups in the target molecules. These chemical modifications enhance separation efficiency and detection sensitivity, particularly for polar compounds with hydroxyl, carboxyl, or amine groups that would otherwise be difficult to analyze by GC-MS.Expand Specific Solutions04 Microextraction and concentration techniques
Microextraction methods provide efficient sample preparation while minimizing solvent use and sample volume requirements. Techniques such as solid-phase microextraction (SPME), stir bar sorptive extraction (SBSE), and headspace extraction concentrate volatile and semi-volatile compounds from various matrices. These approaches offer advantages in terms of sensitivity, simplicity, and environmental friendliness, making them suitable for trace analysis in environmental, forensic, and food safety applications.Expand Specific Solutions05 Matrix-specific sample preparation protocols
Specialized sample preparation protocols are developed for specific sample matrices to address unique challenges in GC-MS analysis. These protocols consider matrix complexity, potential interferences, and analyte stability in materials such as biological fluids, environmental samples, food products, and industrial materials. Matrix-matched calibration standards and internal standardization methods are often incorporated to compensate for matrix effects and ensure accurate quantification. These tailored approaches optimize extraction efficiency and minimize matrix-induced analytical artifacts.Expand Specific Solutions
Key Industry Players and Instrument Manufacturers
The GC-MS sample preparation for non-volatile organic compounds market is in a growth phase, with increasing demand driven by pharmaceutical, environmental, and food safety applications. The global market size is expanding as analytical testing requirements become more stringent across industries. Technologically, the field shows moderate maturity with ongoing innovation focused on improving extraction efficiency and reducing matrix interference. Key players include established analytical instrumentation companies like Shimadzu Corp. and PerkinElmer/Revvity, who offer comprehensive sample preparation solutions, alongside specialized firms such as Entech Instruments focusing on volatile and semi-volatile compound analysis. Academic institutions like Sichuan University and research organizations like CNRS are advancing fundamental methodologies, while tobacco research institutes are developing industry-specific applications for non-volatile compound analysis.
Entech Instruments, Inc.
Technical Solution: Entech Instruments has developed the SIMS (Sorbent Intake Monitoring System) technology specifically for improving GC-MS analysis of non-volatile organic compounds. Their approach utilizes a multi-bed sorbent trap system that allows selective capture and concentration of target analytes while eliminating matrix interferences. The company's Thermal Desorption Autosampler (TDA) incorporates a water management system that effectively removes moisture during the sample preparation process, which is crucial for non-volatile compounds that are often polar and water-soluble. Entech's proprietary SPME Arrow technology provides enhanced surface area compared to traditional SPME fibers, resulting in improved extraction efficiency for non-volatile compounds. Their automated sample preparation systems incorporate sequential desorption steps with programmable temperature profiles, allowing for selective volatilization of different compound classes based on their thermal properties. This approach has proven particularly effective for environmental samples containing complex mixtures of semi-volatile and non-volatile organic compounds.
Strengths: Exceptional water management capabilities improve chromatographic performance for polar non-volatile compounds; modular design allows customization for specific application needs. Weaknesses: Specialized training required for optimal system operation; higher initial investment compared to manual preparation techniques.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences (formerly part of PerkinElmer) has developed the AxION Sample Prep system specifically designed to enhance GC-MS analysis of non-volatile organic compounds. Their technology employs a combination of automated liquid handling and solid-phase extraction (SPE) to efficiently isolate and concentrate target analytes from complex matrices. The system incorporates intelligent pressure monitoring during extraction steps to ensure optimal flow rates and prevent channeling effects that can reduce recovery of non-volatile compounds. Revvity's approach includes automated derivatization protocols using their proprietary reagent delivery system, which precisely controls reaction conditions to maximize conversion efficiency of non-volatile compounds to their volatile derivatives. Their QuEChERS automation platform has been specifically optimized for non-volatile compounds, incorporating specialized dispersive phases and cleanup sorbents that selectively remove matrix interferences while preserving target analytes. The company has also developed application-specific extraction protocols for challenging non-volatile compounds in biological, environmental, and food safety testing.
Strengths: Highly reproducible automated workflows reduce method variability; specialized consumables designed specifically for non-volatile compound classes. Weaknesses: Proprietary consumables may increase operational costs; complex samples may still require method optimization despite automation capabilities.
Breakthrough Technologies for Non-Volatile Compound Detection
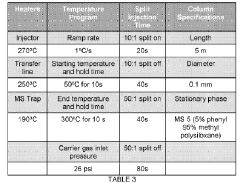
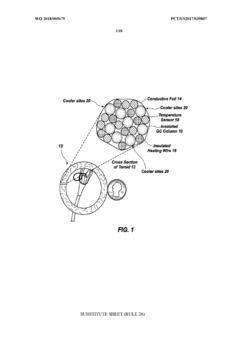
Improved low thermal mass GC module
PatentWO2018005679A1
Innovation
- A new low thermal mass GC module design featuring a single-layer ordered-arrangement GC column wrapped with thin aluminum covers and an insulated heating wire, minimizing cooler spots through uniform heat distribution, and a temperature sensor for precise temperature control.
Performing chemical reactions and/or ionization during gas chromatography-mass spectrometry runs
PatentWO2013119435A1
Innovation
- The method employs an atmospheric pressure ionization source to perform chemical reactions and ionization, allowing for protonation and deuteration conditions to be switched, enabling the detection of specific compounds by mass shift analysis, and the selective addition or inhibition of halogens to aromatic analytes.
Regulatory Compliance and Method Validation
Regulatory compliance and method validation are critical aspects of GC-MS sample preparation for non-volatile organic compounds, particularly in regulated industries such as pharmaceuticals, food safety, environmental monitoring, and clinical diagnostics. These elements ensure that analytical methods produce reliable, reproducible results that meet established standards and regulatory requirements.
The regulatory landscape for GC-MS analysis varies significantly across different sectors and geographical regions. In the pharmaceutical industry, compliance with ICH (International Council for Harmonisation) guidelines, particularly ICH Q2(R1) for analytical method validation, is mandatory. Environmental testing laboratories must adhere to EPA (Environmental Protection Agency) methods in the United States or equivalent standards in other countries. Food safety testing follows regulations set by organizations such as the FDA, EFSA, or Codex Alimentarius.
Method validation for non-volatile organic compound analysis via GC-MS encompasses several key parameters. Specificity ensures that the method accurately identifies and quantifies the analytes in the presence of potential interferences. Linearity verifies the proportional relationship between analyte concentration and instrument response across the working range. Accuracy measures how close the test results come to the true value, while precision evaluates the closeness of agreement between repeated measurements.
Detection limit (LOD) and quantification limit (LOQ) determinations are particularly challenging for non-volatile compounds due to their complex sample preparation requirements. Robustness testing must account for variations in sample preparation procedures, including derivatization efficiency, extraction recovery, and matrix effects. These factors significantly impact method transferability between laboratories.
Standard operating procedures (SOPs) for sample preparation must be meticulously documented to ensure compliance. This documentation should include detailed protocols for sample collection, storage conditions, extraction procedures, derivatization methods, and quality control measures. Traceability of standards, reagents, and reference materials is essential for regulatory acceptance.
Proficiency testing and inter-laboratory comparisons provide external validation of method performance. Participation in such programs helps laboratories demonstrate competence and identify potential areas for improvement in their sample preparation techniques for non-volatile compounds. This external assessment is increasingly becoming a regulatory requirement in many jurisdictions.
The validation of automated sample preparation systems presents additional regulatory considerations. While automation can improve precision and reduce human error, the validation process must demonstrate that the automated system performs equivalently or superiorly to manual methods. Software validation and data integrity measures must comply with regulations such as 21 CFR Part 11 in the pharmaceutical industry.
The regulatory landscape for GC-MS analysis varies significantly across different sectors and geographical regions. In the pharmaceutical industry, compliance with ICH (International Council for Harmonisation) guidelines, particularly ICH Q2(R1) for analytical method validation, is mandatory. Environmental testing laboratories must adhere to EPA (Environmental Protection Agency) methods in the United States or equivalent standards in other countries. Food safety testing follows regulations set by organizations such as the FDA, EFSA, or Codex Alimentarius.
Method validation for non-volatile organic compound analysis via GC-MS encompasses several key parameters. Specificity ensures that the method accurately identifies and quantifies the analytes in the presence of potential interferences. Linearity verifies the proportional relationship between analyte concentration and instrument response across the working range. Accuracy measures how close the test results come to the true value, while precision evaluates the closeness of agreement between repeated measurements.
Detection limit (LOD) and quantification limit (LOQ) determinations are particularly challenging for non-volatile compounds due to their complex sample preparation requirements. Robustness testing must account for variations in sample preparation procedures, including derivatization efficiency, extraction recovery, and matrix effects. These factors significantly impact method transferability between laboratories.
Standard operating procedures (SOPs) for sample preparation must be meticulously documented to ensure compliance. This documentation should include detailed protocols for sample collection, storage conditions, extraction procedures, derivatization methods, and quality control measures. Traceability of standards, reagents, and reference materials is essential for regulatory acceptance.
Proficiency testing and inter-laboratory comparisons provide external validation of method performance. Participation in such programs helps laboratories demonstrate competence and identify potential areas for improvement in their sample preparation techniques for non-volatile compounds. This external assessment is increasingly becoming a regulatory requirement in many jurisdictions.
The validation of automated sample preparation systems presents additional regulatory considerations. While automation can improve precision and reduce human error, the validation process must demonstrate that the automated system performs equivalently or superiorly to manual methods. Software validation and data integrity measures must comply with regulations such as 21 CFR Part 11 in the pharmaceutical industry.
Environmental Impact of Sample Preparation Solvents
The environmental impact of solvents used in GC-MS sample preparation for non-volatile organic compounds represents a significant concern in analytical chemistry. Traditional sample preparation methods often rely on chlorinated solvents such as dichloromethane, chloroform, and carbon tetrachloride, which pose substantial environmental hazards. These solvents contribute to ozone depletion, persist in the environment, and can bioaccumulate in aquatic organisms, creating long-term ecological damage.
Recent environmental assessments indicate that laboratory solvent waste accounts for approximately 10-15% of total hazardous waste generated by research institutions. The disposal of these solvents, particularly halogenated compounds, requires specialized handling procedures and incurs significant costs. Furthermore, accidental releases during sample preparation can contaminate water systems and soil, with remediation efforts often proving both expensive and technically challenging.
The principles of green chemistry have begun to influence sample preparation protocols, driving the development of environmentally benign alternatives. Supercritical fluid extraction using CO2 represents one promising approach, offering a non-toxic, non-flammable medium that can be easily recycled. This technique has demonstrated comparable extraction efficiencies to traditional methods while dramatically reducing environmental impact.
Water-based extraction systems, including subcritical water extraction and micellar systems, have emerged as viable alternatives for certain compound classes. These methods eliminate organic solvent usage entirely, though they may require higher energy inputs for temperature control. The environmental trade-offs between solvent reduction and increased energy consumption require careful life-cycle assessment to determine net environmental benefits.
Ionic liquids present another innovative solution, offering tunable properties and negligible vapor pressure. Their environmental profile, however, remains complex - while they reduce air pollution concerns, questions regarding their biodegradability and aquatic toxicity persist. Current research focuses on developing "greener" ionic liquids with improved environmental characteristics.
Miniaturization of sample preparation techniques, such as micro-extraction methods, significantly reduces solvent volumes. Solid-phase micro-extraction (SPME) and single-drop micro-extraction can decrease solvent consumption by 90-99% compared to conventional liquid-liquid extraction, substantially reducing waste generation and environmental footprint.
Regulatory frameworks worldwide are increasingly restricting the use of environmentally harmful solvents. The European REACH regulation and similar initiatives in North America and Asia are driving analytical laboratories to adopt greener alternatives. This regulatory pressure, combined with corporate sustainability goals, accelerates the transition toward environmentally responsible sample preparation methodologies for GC-MS analysis of non-volatile organic compounds.
Recent environmental assessments indicate that laboratory solvent waste accounts for approximately 10-15% of total hazardous waste generated by research institutions. The disposal of these solvents, particularly halogenated compounds, requires specialized handling procedures and incurs significant costs. Furthermore, accidental releases during sample preparation can contaminate water systems and soil, with remediation efforts often proving both expensive and technically challenging.
The principles of green chemistry have begun to influence sample preparation protocols, driving the development of environmentally benign alternatives. Supercritical fluid extraction using CO2 represents one promising approach, offering a non-toxic, non-flammable medium that can be easily recycled. This technique has demonstrated comparable extraction efficiencies to traditional methods while dramatically reducing environmental impact.
Water-based extraction systems, including subcritical water extraction and micellar systems, have emerged as viable alternatives for certain compound classes. These methods eliminate organic solvent usage entirely, though they may require higher energy inputs for temperature control. The environmental trade-offs between solvent reduction and increased energy consumption require careful life-cycle assessment to determine net environmental benefits.
Ionic liquids present another innovative solution, offering tunable properties and negligible vapor pressure. Their environmental profile, however, remains complex - while they reduce air pollution concerns, questions regarding their biodegradability and aquatic toxicity persist. Current research focuses on developing "greener" ionic liquids with improved environmental characteristics.
Miniaturization of sample preparation techniques, such as micro-extraction methods, significantly reduces solvent volumes. Solid-phase micro-extraction (SPME) and single-drop micro-extraction can decrease solvent consumption by 90-99% compared to conventional liquid-liquid extraction, substantially reducing waste generation and environmental footprint.
Regulatory frameworks worldwide are increasingly restricting the use of environmentally harmful solvents. The European REACH regulation and similar initiatives in North America and Asia are driving analytical laboratories to adopt greener alternatives. This regulatory pressure, combined with corporate sustainability goals, accelerates the transition toward environmentally responsible sample preparation methodologies for GC-MS analysis of non-volatile organic compounds.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!