Interoperability Between MAPs And External LIMS/ELN Systems
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MAP-LIMS Integration Background and Objectives
The integration of Manufacturing Automation Platforms (MAPs) with Laboratory Information Management Systems (LIMS) and Electronic Laboratory Notebooks (ELN) represents a critical technological evolution in modern manufacturing and research environments. This interoperability challenge has emerged from the increasing digitalization of both manufacturing processes and laboratory operations, creating a need for seamless data exchange between previously isolated systems.
Historically, MAPs evolved from basic manufacturing execution systems to sophisticated platforms managing complex production workflows, while LIMS and ELN systems developed independently within laboratory settings to manage samples, tests, and experimental data. The disconnection between these systems has created significant inefficiencies, data redundancies, and quality control challenges across industries including pharmaceuticals, biotechnology, and advanced materials manufacturing.
The primary objective of MAP-LIMS/ELN integration is to establish bidirectional, real-time data exchange capabilities that eliminate manual data transfer processes while maintaining data integrity and regulatory compliance. This integration aims to create a continuous digital thread from research and development through manufacturing and quality control, supporting the broader industry trends toward digital transformation and Industry 4.0 implementation.
Current integration efforts face several technical challenges, including disparate data formats, varying communication protocols, and complex validation requirements, particularly in regulated industries. The evolution of these integration approaches has progressed from simple file-based transfers to API-driven architectures and now toward more sophisticated middleware solutions and cloud-based integration platforms.
Market drivers for this integration include increasing regulatory pressures for data integrity, growing demand for manufacturing agility, and the need to accelerate time-to-market for new products. Organizations implementing successful MAP-LIMS/ELN integrations have reported significant improvements in operational efficiency, with some studies indicating up to 30% reduction in batch release times and 25% improvement in laboratory productivity.
The technological landscape continues to evolve with emerging standards like ASTM E1578 for laboratory information management and ISA-95 for enterprise-control system integration providing frameworks for more standardized approaches. Additionally, the rise of cloud-native architectures and containerization technologies is creating new opportunities for more flexible and scalable integration solutions.
This technical research aims to comprehensively evaluate the current state of MAP-LIMS/ELN integration technologies, identify best practices and emerging solutions, and provide strategic guidance for organizations seeking to implement or enhance these critical system interconnections.
Historically, MAPs evolved from basic manufacturing execution systems to sophisticated platforms managing complex production workflows, while LIMS and ELN systems developed independently within laboratory settings to manage samples, tests, and experimental data. The disconnection between these systems has created significant inefficiencies, data redundancies, and quality control challenges across industries including pharmaceuticals, biotechnology, and advanced materials manufacturing.
The primary objective of MAP-LIMS/ELN integration is to establish bidirectional, real-time data exchange capabilities that eliminate manual data transfer processes while maintaining data integrity and regulatory compliance. This integration aims to create a continuous digital thread from research and development through manufacturing and quality control, supporting the broader industry trends toward digital transformation and Industry 4.0 implementation.
Current integration efforts face several technical challenges, including disparate data formats, varying communication protocols, and complex validation requirements, particularly in regulated industries. The evolution of these integration approaches has progressed from simple file-based transfers to API-driven architectures and now toward more sophisticated middleware solutions and cloud-based integration platforms.
Market drivers for this integration include increasing regulatory pressures for data integrity, growing demand for manufacturing agility, and the need to accelerate time-to-market for new products. Organizations implementing successful MAP-LIMS/ELN integrations have reported significant improvements in operational efficiency, with some studies indicating up to 30% reduction in batch release times and 25% improvement in laboratory productivity.
The technological landscape continues to evolve with emerging standards like ASTM E1578 for laboratory information management and ISA-95 for enterprise-control system integration providing frameworks for more standardized approaches. Additionally, the rise of cloud-native architectures and containerization technologies is creating new opportunities for more flexible and scalable integration solutions.
This technical research aims to comprehensively evaluate the current state of MAP-LIMS/ELN integration technologies, identify best practices and emerging solutions, and provide strategic guidance for organizations seeking to implement or enhance these critical system interconnections.
Market Demand Analysis for Laboratory System Integration
The laboratory informatics market is experiencing significant growth, with a projected CAGR of 7.5% from 2023 to 2028, driven primarily by the increasing demand for integrated laboratory systems. This growth reflects the urgent need for seamless interoperability between Modern Analytical Platforms (MAPs) and Laboratory Information Management Systems (LIMS) or Electronic Laboratory Notebooks (ELN).
Research indicates that over 70% of pharmaceutical and biotechnology organizations currently struggle with data silos created by disconnected laboratory systems. These disconnects result in manual data transfer processes that introduce errors, delay decision-making, and impede regulatory compliance efforts. The demand for integration solutions is particularly acute in regulated industries where data integrity and traceability are paramount.
Market surveys reveal that laboratory managers and IT directors consistently rank system integration capabilities among their top three priorities when evaluating new laboratory informatics investments. This prioritization stems from the recognition that integrated systems can reduce operational costs by approximately 30% through elimination of redundant data entry and improved resource utilization.
The COVID-19 pandemic has accelerated this market demand, as remote work requirements highlighted the limitations of isolated systems. Organizations with well-integrated laboratory informatics ecosystems demonstrated 40% greater operational resilience during pandemic disruptions compared to those with fragmented systems.
Geographically, North America leads the demand for integration solutions, accounting for approximately 40% of the global market. However, the Asia-Pacific region is showing the fastest growth rate at 9.2% annually, driven by rapid laboratory modernization initiatives in China, India, and Singapore.
By industry vertical, pharmaceutical companies represent the largest market segment seeking integration solutions, followed by academic research institutions and contract research organizations. The biopharmaceutical sector specifically shows the highest willingness to invest in advanced integration technologies, with average implementation budgets 25% higher than other sectors.
Small and medium-sized laboratories are emerging as a significant growth segment, as cloud-based integration solutions have reduced implementation barriers. This democratization of integration capabilities is expanding the total addressable market beyond traditional enterprise customers.
Looking forward, the demand for real-time data exchange capabilities between systems is expected to intensify as organizations pursue digital transformation initiatives. The ability to support artificial intelligence and machine learning applications through integrated data ecosystems has become a key differentiator in purchase decisions, with 65% of decision-makers citing this capability as "very important" in recent market surveys.
Research indicates that over 70% of pharmaceutical and biotechnology organizations currently struggle with data silos created by disconnected laboratory systems. These disconnects result in manual data transfer processes that introduce errors, delay decision-making, and impede regulatory compliance efforts. The demand for integration solutions is particularly acute in regulated industries where data integrity and traceability are paramount.
Market surveys reveal that laboratory managers and IT directors consistently rank system integration capabilities among their top three priorities when evaluating new laboratory informatics investments. This prioritization stems from the recognition that integrated systems can reduce operational costs by approximately 30% through elimination of redundant data entry and improved resource utilization.
The COVID-19 pandemic has accelerated this market demand, as remote work requirements highlighted the limitations of isolated systems. Organizations with well-integrated laboratory informatics ecosystems demonstrated 40% greater operational resilience during pandemic disruptions compared to those with fragmented systems.
Geographically, North America leads the demand for integration solutions, accounting for approximately 40% of the global market. However, the Asia-Pacific region is showing the fastest growth rate at 9.2% annually, driven by rapid laboratory modernization initiatives in China, India, and Singapore.
By industry vertical, pharmaceutical companies represent the largest market segment seeking integration solutions, followed by academic research institutions and contract research organizations. The biopharmaceutical sector specifically shows the highest willingness to invest in advanced integration technologies, with average implementation budgets 25% higher than other sectors.
Small and medium-sized laboratories are emerging as a significant growth segment, as cloud-based integration solutions have reduced implementation barriers. This democratization of integration capabilities is expanding the total addressable market beyond traditional enterprise customers.
Looking forward, the demand for real-time data exchange capabilities between systems is expected to intensify as organizations pursue digital transformation initiatives. The ability to support artificial intelligence and machine learning applications through integrated data ecosystems has become a key differentiator in purchase decisions, with 65% of decision-makers citing this capability as "very important" in recent market surveys.
Current Interoperability Challenges and Limitations
Despite significant advancements in laboratory automation, the integration between Manufacturing Automation Platforms (MAPs) and external Laboratory Information Management Systems (LIMS) or Electronic Laboratory Notebooks (ELN) remains fraught with substantial challenges. The fundamental issue stems from the heterogeneous nature of these systems, which were often developed independently with proprietary data formats, communication protocols, and architectural frameworks.
Data format incompatibility represents one of the most persistent barriers to seamless interoperability. MAPs typically utilize industrial automation protocols such as OPC UA, MQTT, or proprietary formats, while LIMS and ELN systems commonly employ scientific data standards like ASTM, HL7, or custom XML schemas. This divergence necessitates complex data transformation processes that are prone to information loss or misinterpretation.
Authentication and security mechanisms present another significant hurdle. Manufacturing environments implement strict access controls and security protocols that often conflict with the more collaborative and research-oriented security models of LIMS/ELN systems. Establishing trusted connections between these disparate security domains without compromising either system's integrity remains problematic.
Real-time data synchronization capabilities are frequently inadequate. Manufacturing processes generate continuous data streams requiring immediate analysis and response, whereas laboratory systems are traditionally designed for batch processing and deliberate workflows. This temporal mismatch creates bottlenecks in data flow and impedes timely decision-making processes.
Regulatory compliance requirements further complicate integration efforts. Both manufacturing and laboratory domains are subject to stringent but different regulatory frameworks (e.g., GMP, 21 CFR Part 11, ISO standards). Integration solutions must satisfy multiple compliance requirements simultaneously, often leading to compromised functionality or excessive validation overhead.
Semantic interoperability remains perhaps the most challenging aspect. Even when syntactic integration is achieved, ensuring that data maintains consistent meaning across systems requires sophisticated ontologies and mapping mechanisms. The contextual nuances of manufacturing parameters versus laboratory measurements frequently lead to misinterpretation when data crosses system boundaries.
Legacy system constraints significantly impede modernization efforts. Many established manufacturing facilities and laboratories operate decades-old systems with limited connectivity options. Retrofitting these systems for modern integration capabilities often requires substantial investment or custom middleware development that introduces additional points of failure.
Vendor lock-in strategies further exacerbate interoperability challenges. System providers frequently implement proprietary interfaces that discourage third-party integration, forcing organizations to either commit to single-vendor ecosystems or invest in costly custom integration solutions that may become obsolete with system upgrades.
Data format incompatibility represents one of the most persistent barriers to seamless interoperability. MAPs typically utilize industrial automation protocols such as OPC UA, MQTT, or proprietary formats, while LIMS and ELN systems commonly employ scientific data standards like ASTM, HL7, or custom XML schemas. This divergence necessitates complex data transformation processes that are prone to information loss or misinterpretation.
Authentication and security mechanisms present another significant hurdle. Manufacturing environments implement strict access controls and security protocols that often conflict with the more collaborative and research-oriented security models of LIMS/ELN systems. Establishing trusted connections between these disparate security domains without compromising either system's integrity remains problematic.
Real-time data synchronization capabilities are frequently inadequate. Manufacturing processes generate continuous data streams requiring immediate analysis and response, whereas laboratory systems are traditionally designed for batch processing and deliberate workflows. This temporal mismatch creates bottlenecks in data flow and impedes timely decision-making processes.
Regulatory compliance requirements further complicate integration efforts. Both manufacturing and laboratory domains are subject to stringent but different regulatory frameworks (e.g., GMP, 21 CFR Part 11, ISO standards). Integration solutions must satisfy multiple compliance requirements simultaneously, often leading to compromised functionality or excessive validation overhead.
Semantic interoperability remains perhaps the most challenging aspect. Even when syntactic integration is achieved, ensuring that data maintains consistent meaning across systems requires sophisticated ontologies and mapping mechanisms. The contextual nuances of manufacturing parameters versus laboratory measurements frequently lead to misinterpretation when data crosses system boundaries.
Legacy system constraints significantly impede modernization efforts. Many established manufacturing facilities and laboratories operate decades-old systems with limited connectivity options. Retrofitting these systems for modern integration capabilities often requires substantial investment or custom middleware development that introduces additional points of failure.
Vendor lock-in strategies further exacerbate interoperability challenges. System providers frequently implement proprietary interfaces that discourage third-party integration, forcing organizations to either commit to single-vendor ecosystems or invest in costly custom integration solutions that may become obsolete with system upgrades.
Existing Integration Architectures and Protocols
01 Data integration frameworks for MAPs and LIMS/ELN systems
Integration frameworks enable seamless data exchange between Manufacturing Automation Platforms (MAPs) and Laboratory Information Management Systems (LIMS) or Electronic Laboratory Notebooks (ELN). These frameworks provide standardized interfaces and protocols for connecting disparate systems, allowing for real-time data synchronization and unified access to manufacturing and laboratory data. Such integration eliminates data silos, reduces manual data entry errors, and enables end-to-end process visibility across laboratory testing and production environments.- Data integration frameworks between MAPs and LIMS/ELN systems: Integration frameworks enable seamless data exchange between Manufacturing Automation Platforms (MAPs) and Laboratory Information Management Systems (LIMS) or Electronic Laboratory Notebooks (ELN). These frameworks establish standardized communication protocols and data formats to ensure consistent information flow across manufacturing and laboratory environments. By implementing middleware solutions and API-based architectures, organizations can achieve real-time synchronization of manufacturing process data with laboratory testing results, enabling more efficient decision-making and quality control processes.
- Cloud-based platforms for MAP and LIMS/ELN connectivity: Cloud-based solutions provide scalable infrastructure for connecting Manufacturing Automation Platforms with laboratory systems. These platforms offer centralized data repositories accessible from multiple locations, facilitating collaboration between manufacturing and laboratory teams. Cloud architectures support distributed manufacturing environments while maintaining data integrity and security across integrated systems. The implementation of cloud-based connectivity enables organizations to deploy standardized integration patterns across multiple facilities while reducing on-premise infrastructure requirements.
- Standardized data exchange protocols for system interoperability: Standardized protocols establish common communication methods between manufacturing and laboratory systems. These protocols define data structures, messaging formats, and exchange mechanisms that enable different vendors' systems to communicate effectively. By implementing industry standards like OPC UA, MQTT, or RESTful APIs, organizations can reduce custom integration efforts and ensure long-term sustainability of system connections. Standardized approaches also facilitate validation processes by providing well-documented and widely accepted methods for system integration.
- Real-time data synchronization between manufacturing and laboratory systems: Real-time synchronization mechanisms ensure that critical data is immediately available across manufacturing and laboratory environments. These solutions implement event-driven architectures that trigger data transfers when specific manufacturing or testing activities occur. By maintaining synchronized datasets between MAPs and LIMS/ELN systems, organizations can accelerate quality decisions, reduce production delays, and improve overall manufacturing efficiency. Real-time capabilities are particularly important for continuous manufacturing processes where laboratory results directly impact ongoing production parameters.
- Security frameworks for integrated MAP and LIMS/ELN environments: Security frameworks protect sensitive manufacturing and laboratory data across integrated systems. These solutions implement authentication mechanisms, encryption protocols, and access controls to maintain data integrity throughout the information exchange process. By establishing comprehensive security models that span both manufacturing and laboratory domains, organizations can comply with regulatory requirements while enabling appropriate data sharing. Security frameworks also include audit capabilities to track data lineage and system interactions for compliance documentation and issue investigation.
02 API-based connectivity solutions for system interoperability
Application Programming Interfaces (APIs) serve as critical connectivity points between Manufacturing Automation Platforms and laboratory systems. These APIs enable standardized communication protocols that allow different systems to exchange information regardless of their underlying architecture. RESTful APIs, SOAP services, and GraphQL implementations facilitate real-time data transfer, automated workflows, and system-to-system notifications. This approach provides flexibility in integration while maintaining security and data integrity across manufacturing and laboratory environments.Expand Specific Solutions03 Cloud-based platforms for unified laboratory and manufacturing data management
Cloud-based solutions provide a centralized platform for integrating Manufacturing Automation Platforms with LIMS/ELN systems. These platforms offer scalable infrastructure that enables real-time data sharing, collaborative workflows, and remote access capabilities. By leveraging cloud technologies, organizations can implement unified data repositories that bridge the gap between laboratory testing and manufacturing processes, while supporting compliance requirements through comprehensive audit trails and data governance features.Expand Specific Solutions04 Middleware solutions for legacy system integration
Middleware technologies enable interoperability between modern Manufacturing Automation Platforms and legacy LIMS/ELN systems. These solutions act as translation layers that convert data formats, protocols, and communication methods between disparate systems. By implementing middleware connectors, organizations can extend the useful life of existing laboratory systems while gradually transitioning to more advanced manufacturing platforms. This approach minimizes disruption to ongoing operations while enabling incremental modernization of the technology landscape.Expand Specific Solutions05 Standardized data models for cross-system compatibility
Standardized data models and ontologies facilitate seamless information exchange between Manufacturing Automation Platforms and laboratory systems. These models define common vocabularies, data structures, and relationship frameworks that ensure consistent interpretation of information across different systems. By implementing industry standards like ASTM, ISO, or custom semantic models, organizations can achieve higher levels of data consistency, improve search capabilities, and enable advanced analytics that span both manufacturing and laboratory domains.Expand Specific Solutions
Key Players in MAP and LIMS/ELN Ecosystem
The interoperability between Manufacturing Automation Protocols (MAPs) and external Laboratory Information Management Systems/Electronic Laboratory Notebooks (LIMS/ELN) is currently in a growth phase, with the market expanding as organizations seek seamless data integration across laboratory environments. The global market is projected to grow significantly as digital transformation in laboratories accelerates. From a technical maturity perspective, established technology leaders like Samsung Electronics, Huawei Technologies, and Cisco are driving standardization efforts, while companies including LG Electronics, Canon, and NEC are developing specialized integration solutions. Telecom infrastructure providers such as Ericsson and British Telecommunications are contributing networking expertise to enhance connectivity between systems. The competitive landscape features both large enterprise solution providers and specialized laboratory technology companies working to address interoperability challenges through open standards and API-driven approaches.
Cisco Technology, Inc.
Technical Solution: Cisco has developed comprehensive interoperability solutions between Manufacturing Automation Platforms (MAPs) and Laboratory Information Management Systems (LIMS)/Electronic Laboratory Notebooks (ELN) through their Industrial Network Architecture framework. Their approach leverages secure API gateways and middleware connectors that facilitate real-time data exchange between operational technology (OT) and information technology (IT) environments. Cisco's Industrial IoT (IIoT) platform incorporates edge computing capabilities that pre-process manufacturing data before integration with laboratory systems, reducing latency and bandwidth requirements. Their solution implements the ISA-95 standard for enterprise-control system integration, allowing for standardized data mapping between manufacturing execution systems and laboratory platforms. Additionally, Cisco has developed specialized security protocols that maintain data integrity while enabling cross-system authentication through their Identity Services Engine (ISE) specifically tailored for regulated manufacturing environments.
Strengths: Robust security architecture specifically designed for regulated industries; extensive experience with industrial network protocols; strong edge computing capabilities for real-time processing. Weaknesses: Solutions may require significant customization for specific LIMS/ELN systems; higher implementation costs compared to specialized vendors; potential complexity in deployment across diverse manufacturing environments.
British Telecommunications Plc
Technical Solution: British Telecommunications (BT) has engineered an interoperability framework for connecting Manufacturing Automation Platforms (MAPs) with Laboratory Information Management Systems (LIMS) and Electronic Laboratory Notebooks (ELN) through their BT Connect platform. Their solution employs a service-oriented architecture (SOA) that creates virtualized data services between manufacturing and laboratory environments. BT's approach includes specialized data transformation modules that handle the conversion between industrial automation protocols (such as OPC UA, MQTT) and laboratory data formats (typically XML, JSON, or proprietary formats). The platform incorporates event-driven integration patterns that enable real-time notification of critical manufacturing events to laboratory systems, facilitating immediate quality testing and documentation. BT has also implemented blockchain-based data provenance tracking to maintain audit trails across system boundaries, which is particularly valuable for regulated manufacturing environments requiring complete traceability from raw materials through laboratory testing to finished products.
Strengths: Strong telecommunications infrastructure expertise enabling reliable data transfer across distributed sites; advanced data transformation capabilities for handling diverse protocols; robust audit trail mechanisms. Weaknesses: Less specialized in laboratory-specific workflows compared to dedicated LIMS vendors; may require third-party partnerships for deep laboratory system integration; solutions may be more communication-focused than process-oriented.
Core Technologies for Seamless Data Transfer
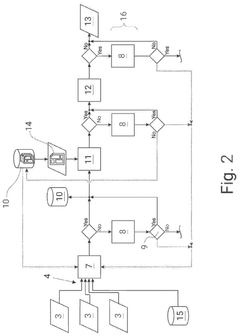
Method for automatically documenting a laboratory workflow
PatentPendingUS20240311727A1
Innovation
- A control system that uses a classification engine with trained AI models to automatically document laboratory workflows by analyzing unstructured data from various devices, filling predefined templates, and prompting a second user for classification when certainty thresholds are not met, allowing for continuous improvement of the automation process.
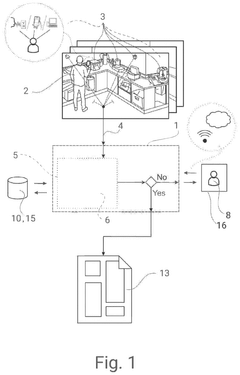
Parsing, evaluating leaf, and branch nodes, and navigating the nodes based on the evaluation
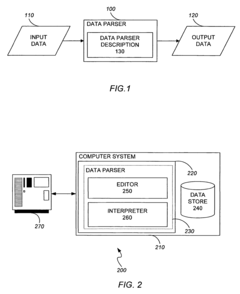
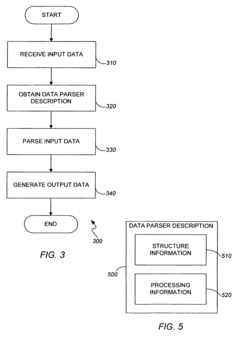
PatentActiveUS7818666B2
Innovation
- A data parsing and processing system that uses a data parser description with a tree structure, including leaf and branch nodes, to extract and translate data into a common format, enabling flexible processing and storage of data across different formats and sources.
Data Security and Compliance Requirements
Data security and compliance requirements represent critical considerations in the integration of Manufacturing Automation Platforms (MAPs) with Laboratory Information Management Systems (LIMS) and Electronic Laboratory Notebooks (ELN). These systems frequently handle sensitive intellectual property, proprietary manufacturing processes, and regulated data that demand robust protection mechanisms.
The interoperability between these systems must adhere to multiple regulatory frameworks, including FDA 21 CFR Part 11, GDPR, HIPAA, and ISO 27001, depending on the industry and geographical location. These regulations establish specific requirements for data integrity, electronic signatures, audit trails, and access controls that directly impact system integration approaches.
Authentication and authorization mechanisms require particular attention when establishing connections between MAPs and external laboratory systems. Implementation of Single Sign-On (SSO) solutions, multi-factor authentication, and role-based access controls helps maintain security while facilitating seamless user experiences across integrated platforms. The authentication protocols must ensure that only authorized personnel can access specific data sets or functionalities.
Data encryption represents another fundamental security requirement, with both data-in-transit and data-at-rest requiring protection. Current industry standards recommend TLS 1.3 for communications and AES-256 for stored data. When MAPs and LIMS/ELN systems exchange information, encrypted communication channels must be established, often through secure APIs or encrypted file transfers.
Audit trail capabilities must span across integrated systems to maintain complete records of data creation, modification, and deletion events. This cross-system traceability presents significant technical challenges but remains essential for regulatory compliance, particularly in GxP environments where data provenance is critical for validation purposes.
Data residency and sovereignty considerations add complexity to interoperability solutions, especially for multinational organizations. Different jurisdictions impose varying requirements regarding where data can be stored and processed, necessitating flexible architecture designs that can accommodate regional compliance needs while maintaining system integration.
Vendor validation documentation plays a crucial role in compliance efforts. Organizations implementing interoperability solutions must obtain and maintain comprehensive documentation from technology providers demonstrating adherence to relevant regulations. This documentation becomes particularly important during regulatory inspections and audits.
Risk assessment methodologies should be applied specifically to integration points between systems, as these often represent potential security vulnerabilities. Continuous monitoring and periodic security testing of these integration interfaces help identify and remediate potential weaknesses before they can be exploited.
The interoperability between these systems must adhere to multiple regulatory frameworks, including FDA 21 CFR Part 11, GDPR, HIPAA, and ISO 27001, depending on the industry and geographical location. These regulations establish specific requirements for data integrity, electronic signatures, audit trails, and access controls that directly impact system integration approaches.
Authentication and authorization mechanisms require particular attention when establishing connections between MAPs and external laboratory systems. Implementation of Single Sign-On (SSO) solutions, multi-factor authentication, and role-based access controls helps maintain security while facilitating seamless user experiences across integrated platforms. The authentication protocols must ensure that only authorized personnel can access specific data sets or functionalities.
Data encryption represents another fundamental security requirement, with both data-in-transit and data-at-rest requiring protection. Current industry standards recommend TLS 1.3 for communications and AES-256 for stored data. When MAPs and LIMS/ELN systems exchange information, encrypted communication channels must be established, often through secure APIs or encrypted file transfers.
Audit trail capabilities must span across integrated systems to maintain complete records of data creation, modification, and deletion events. This cross-system traceability presents significant technical challenges but remains essential for regulatory compliance, particularly in GxP environments where data provenance is critical for validation purposes.
Data residency and sovereignty considerations add complexity to interoperability solutions, especially for multinational organizations. Different jurisdictions impose varying requirements regarding where data can be stored and processed, necessitating flexible architecture designs that can accommodate regional compliance needs while maintaining system integration.
Vendor validation documentation plays a crucial role in compliance efforts. Organizations implementing interoperability solutions must obtain and maintain comprehensive documentation from technology providers demonstrating adherence to relevant regulations. This documentation becomes particularly important during regulatory inspections and audits.
Risk assessment methodologies should be applied specifically to integration points between systems, as these often represent potential security vulnerabilities. Continuous monitoring and periodic security testing of these integration interfaces help identify and remediate potential weaknesses before they can be exploited.
ROI Assessment for Integration Implementation
Implementing integration between Manufacturing Automation Platforms (MAPs) and external Laboratory Information Management Systems (LIMS) or Electronic Laboratory Notebooks (ELN) requires significant investment. This ROI assessment provides a comprehensive analysis of the financial benefits and costs associated with such integration projects.
Initial implementation costs typically range from $150,000 to $500,000 depending on the complexity of systems involved and the depth of integration required. These costs encompass software development, system configuration, data migration, validation, and staff training. Organizations should anticipate allocating 15-20% of the initial implementation cost for annual maintenance and updates.
The tangible benefits of MAP-LIMS/ELN integration manifest in multiple operational areas. Data transcription error reduction alone can save pharmaceutical companies an estimated $200,000-$300,000 annually by preventing costly manufacturing errors and compliance issues. Labor efficiency improvements typically result in 20-30% reduction in manual data entry time, translating to approximately $80,000-$120,000 in annual labor cost savings for mid-sized operations.
Accelerated batch release processes represent another significant ROI factor. Integration can reduce review and approval cycles by 40-60%, potentially increasing production capacity by 15-25%. For organizations with high-value products, this acceleration can generate $500,000-$2,000,000 in additional annual revenue through increased production throughput.
The payback period for integration projects varies based on implementation scope and organizational size. Small to medium enterprises typically achieve ROI within 18-24 months, while larger organizations with more complex implementations may see returns within 12-18 months due to greater economies of scale.
Intangible benefits, though harder to quantify, significantly contribute to long-term ROI. Enhanced data integrity and improved compliance reduce regulatory risks and associated remediation costs. Organizations implementing integrated systems report 40-50% fewer compliance findings during audits. Additionally, improved data accessibility enables more informed decision-making and supports continuous improvement initiatives.
When calculating ROI, organizations should consider both direct cost savings and opportunity costs. The formula (Net Gain from Integration ÷ Cost of Integration) × 100 provides a percentage return, with successful implementations typically yielding 150-300% ROI over a five-year period. Companies should establish clear KPIs before implementation to accurately measure post-integration performance improvements and validate ROI projections.
Initial implementation costs typically range from $150,000 to $500,000 depending on the complexity of systems involved and the depth of integration required. These costs encompass software development, system configuration, data migration, validation, and staff training. Organizations should anticipate allocating 15-20% of the initial implementation cost for annual maintenance and updates.
The tangible benefits of MAP-LIMS/ELN integration manifest in multiple operational areas. Data transcription error reduction alone can save pharmaceutical companies an estimated $200,000-$300,000 annually by preventing costly manufacturing errors and compliance issues. Labor efficiency improvements typically result in 20-30% reduction in manual data entry time, translating to approximately $80,000-$120,000 in annual labor cost savings for mid-sized operations.
Accelerated batch release processes represent another significant ROI factor. Integration can reduce review and approval cycles by 40-60%, potentially increasing production capacity by 15-25%. For organizations with high-value products, this acceleration can generate $500,000-$2,000,000 in additional annual revenue through increased production throughput.
The payback period for integration projects varies based on implementation scope and organizational size. Small to medium enterprises typically achieve ROI within 18-24 months, while larger organizations with more complex implementations may see returns within 12-18 months due to greater economies of scale.
Intangible benefits, though harder to quantify, significantly contribute to long-term ROI. Enhanced data integrity and improved compliance reduce regulatory risks and associated remediation costs. Organizations implementing integrated systems report 40-50% fewer compliance findings during audits. Additionally, improved data accessibility enables more informed decision-making and supports continuous improvement initiatives.
When calculating ROI, organizations should consider both direct cost savings and opportunity costs. The formula (Net Gain from Integration ÷ Cost of Integration) × 100 provides a percentage return, with successful implementations typically yielding 150-300% ROI over a five-year period. Companies should establish clear KPIs before implementation to accurately measure post-integration performance improvements and validate ROI projections.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!