Scaling MAPs From Benchtop To Pilot Manufacturing Validation
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MAPs Scale-up Background and Objectives
Monoclonal antibody products (MAPs) have revolutionized the biopharmaceutical industry since their introduction in the 1980s. These highly specific therapeutic proteins have become essential treatments for various diseases including cancer, autoimmune disorders, and infectious diseases. The global market for MAPs has experienced exponential growth, reaching approximately $150 billion in 2020 and projected to exceed $300 billion by 2027, highlighting their critical importance in modern medicine.
The transition from laboratory-scale production to pilot manufacturing represents one of the most challenging aspects in MAPs development. This scale-up process involves increasing production volume from milliliters to hundreds of liters while maintaining product quality, efficacy, and safety. Historically, this transition has been fraught with technical difficulties, resulting in significant product loss, extended development timelines, and increased costs.
The evolution of MAPs manufacturing technology has progressed through several distinct phases. Initial production relied on murine hybridoma technology with limited yield and significant immunogenicity concerns. This was followed by the development of chimeric and humanized antibodies in the 1990s, which improved clinical outcomes but still presented manufacturing challenges. The current era features fully human antibodies produced in optimized expression systems, enabling higher yields and better quality.
Despite technological advancements, the scale-up process continues to present significant challenges. These include maintaining consistent glycosylation patterns, preventing protein aggregation, optimizing cell culture conditions at larger volumes, and ensuring robust viral clearance. The industry has established that even minor changes in manufacturing parameters can significantly impact critical quality attributes of the final product.
The primary objective of this technical research is to comprehensively evaluate current methodologies for scaling MAPs from benchtop to pilot manufacturing, with particular focus on maintaining critical quality attributes throughout the transition. Secondary objectives include identifying key process parameters that influence successful scale-up, assessing innovative technologies that may improve scale-up efficiency, and developing predictive models to anticipate and mitigate common scale-up challenges.
This research aims to establish a systematic approach to MAPs scale-up that reduces development time, minimizes product loss, and ensures consistent product quality. By analyzing historical data, current best practices, and emerging technologies, we seek to provide a roadmap for more efficient translation of promising antibody candidates from research to clinical manufacturing.
The transition from laboratory-scale production to pilot manufacturing represents one of the most challenging aspects in MAPs development. This scale-up process involves increasing production volume from milliliters to hundreds of liters while maintaining product quality, efficacy, and safety. Historically, this transition has been fraught with technical difficulties, resulting in significant product loss, extended development timelines, and increased costs.
The evolution of MAPs manufacturing technology has progressed through several distinct phases. Initial production relied on murine hybridoma technology with limited yield and significant immunogenicity concerns. This was followed by the development of chimeric and humanized antibodies in the 1990s, which improved clinical outcomes but still presented manufacturing challenges. The current era features fully human antibodies produced in optimized expression systems, enabling higher yields and better quality.
Despite technological advancements, the scale-up process continues to present significant challenges. These include maintaining consistent glycosylation patterns, preventing protein aggregation, optimizing cell culture conditions at larger volumes, and ensuring robust viral clearance. The industry has established that even minor changes in manufacturing parameters can significantly impact critical quality attributes of the final product.
The primary objective of this technical research is to comprehensively evaluate current methodologies for scaling MAPs from benchtop to pilot manufacturing, with particular focus on maintaining critical quality attributes throughout the transition. Secondary objectives include identifying key process parameters that influence successful scale-up, assessing innovative technologies that may improve scale-up efficiency, and developing predictive models to anticipate and mitigate common scale-up challenges.
This research aims to establish a systematic approach to MAPs scale-up that reduces development time, minimizes product loss, and ensures consistent product quality. By analyzing historical data, current best practices, and emerging technologies, we seek to provide a roadmap for more efficient translation of promising antibody candidates from research to clinical manufacturing.
Market Analysis for MAPs Manufacturing
The global market for Monoclonal Antibody Products (MAPs) has experienced remarkable growth over the past decade, with a current market valuation exceeding $150 billion. This segment represents approximately 30% of the entire biopharmaceutical market, making it one of the most significant and rapidly expanding sectors in the pharmaceutical industry. The compound annual growth rate (CAGR) for MAPs is projected at 12.5% through 2028, substantially outpacing traditional pharmaceutical products.
Key market drivers include the increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and inflammatory conditions, which collectively represent the primary therapeutic applications for monoclonal antibodies. Oncology applications alone account for nearly 40% of the MAPs market, followed by autoimmune diseases at 25% and inflammatory conditions at 15%.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, emerging markets in Asia, particularly China and India, are demonstrating the fastest growth rates, exceeding 18% annually, driven by expanding healthcare infrastructure, increasing R&D investments, and growing patient populations.
The manufacturing landscape is characterized by significant concentration, with the top 10 biopharmaceutical companies controlling roughly 70% of production capacity. However, contract manufacturing organizations (CMOs) are gaining market share, currently representing about 25% of total production capacity and growing at 15% annually.
From a technological perspective, the market is witnessing a transition from traditional stainless steel bioreactors to single-use systems, particularly in early-stage manufacturing. This shift is driven by the need for greater flexibility, reduced cross-contamination risks, and lower capital investments. Single-use technologies now account for approximately 35% of early-stage manufacturing processes.
Pricing pressures and manufacturing efficiency have become critical market factors as biosimilars enter the market following patent expirations of blockbuster antibodies. The average cost reduction achieved through manufacturing optimization ranges from 15-25%, representing a significant competitive advantage in an increasingly crowded marketplace.
Regulatory considerations continue to shape market dynamics, with accelerated approval pathways in major markets reducing time-to-market by an average of 1-2 years for breakthrough therapies. This acceleration has significant implications for manufacturing scale-up strategies, requiring more agile and responsive production capabilities.
Key market drivers include the increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and inflammatory conditions, which collectively represent the primary therapeutic applications for monoclonal antibodies. Oncology applications alone account for nearly 40% of the MAPs market, followed by autoimmune diseases at 25% and inflammatory conditions at 15%.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, emerging markets in Asia, particularly China and India, are demonstrating the fastest growth rates, exceeding 18% annually, driven by expanding healthcare infrastructure, increasing R&D investments, and growing patient populations.
The manufacturing landscape is characterized by significant concentration, with the top 10 biopharmaceutical companies controlling roughly 70% of production capacity. However, contract manufacturing organizations (CMOs) are gaining market share, currently representing about 25% of total production capacity and growing at 15% annually.
From a technological perspective, the market is witnessing a transition from traditional stainless steel bioreactors to single-use systems, particularly in early-stage manufacturing. This shift is driven by the need for greater flexibility, reduced cross-contamination risks, and lower capital investments. Single-use technologies now account for approximately 35% of early-stage manufacturing processes.
Pricing pressures and manufacturing efficiency have become critical market factors as biosimilars enter the market following patent expirations of blockbuster antibodies. The average cost reduction achieved through manufacturing optimization ranges from 15-25%, representing a significant competitive advantage in an increasingly crowded marketplace.
Regulatory considerations continue to shape market dynamics, with accelerated approval pathways in major markets reducing time-to-market by an average of 1-2 years for breakthrough therapies. This acceleration has significant implications for manufacturing scale-up strategies, requiring more agile and responsive production capabilities.
Current Challenges in MAPs Scale-up
The scale-up of Monoclonal Antibody Products (MAPs) from laboratory to pilot manufacturing represents one of the most complex challenges in biopharmaceutical development. Current manufacturing processes face significant hurdles that impact product quality, yield, and regulatory compliance. These challenges are multifaceted and require comprehensive solutions that balance scientific rigor with practical manufacturing considerations.
Cell line development and stability present persistent challenges during scale-up. Laboratory-optimized cell lines often demonstrate altered growth kinetics, productivity, and metabolic profiles when transferred to larger bioreactors. This variability can lead to inconsistent product quality attributes and reduced yields, necessitating extensive characterization and adaptation protocols to ensure consistent performance across scales.
Bioreactor conditions represent another critical challenge area. The transition from small-scale shake flasks or bench-top bioreactors to pilot-scale vessels introduces significant changes in mixing dynamics, oxygen transfer, and nutrient distribution. These parameters directly impact cell growth, metabolism, and product quality. Maintaining consistent dissolved oxygen levels, pH, and temperature becomes increasingly difficult as scale increases, often requiring sophisticated control systems and monitoring technologies.
Process parameter sensitivity poses substantial challenges during scale-up. Minor variations in critical process parameters that were negligible at laboratory scale can significantly impact product quality at larger scales. Identifying these critical parameters and understanding their acceptable ranges requires extensive characterization studies and robust risk assessment methodologies.
Downstream processing faces equally significant challenges during scale-up. Chromatography operations that performed efficiently at bench scale often encounter issues with flow distribution, pressure drops, and resin compression at larger scales. Filtration operations face increased fouling and reduced flux rates, while viral clearance steps must demonstrate consistent performance across scales to ensure product safety.
Analytical method transfer and validation represent another major hurdle. Methods optimized for laboratory-scale samples may require significant adaptation to accommodate the increased sample throughput and potentially different impurity profiles encountered at pilot scale. Ensuring method comparability across scales is essential for meaningful process monitoring and control.
Regulatory considerations add complexity to the scale-up process. Demonstrating comparability between products manufactured at different scales requires comprehensive characterization of critical quality attributes and thorough understanding of process-product relationships. Documentation requirements increase substantially as processes move toward commercial manufacturing, necessitating robust quality systems and data management practices.
Economic factors also influence scale-up strategies. The significant capital investment required for pilot-scale manufacturing facilities must be balanced against the need for process flexibility and the uncertainty inherent in early-stage product development. Optimizing resource utilization while maintaining product quality presents ongoing challenges for manufacturing organizations.
Cell line development and stability present persistent challenges during scale-up. Laboratory-optimized cell lines often demonstrate altered growth kinetics, productivity, and metabolic profiles when transferred to larger bioreactors. This variability can lead to inconsistent product quality attributes and reduced yields, necessitating extensive characterization and adaptation protocols to ensure consistent performance across scales.
Bioreactor conditions represent another critical challenge area. The transition from small-scale shake flasks or bench-top bioreactors to pilot-scale vessels introduces significant changes in mixing dynamics, oxygen transfer, and nutrient distribution. These parameters directly impact cell growth, metabolism, and product quality. Maintaining consistent dissolved oxygen levels, pH, and temperature becomes increasingly difficult as scale increases, often requiring sophisticated control systems and monitoring technologies.
Process parameter sensitivity poses substantial challenges during scale-up. Minor variations in critical process parameters that were negligible at laboratory scale can significantly impact product quality at larger scales. Identifying these critical parameters and understanding their acceptable ranges requires extensive characterization studies and robust risk assessment methodologies.
Downstream processing faces equally significant challenges during scale-up. Chromatography operations that performed efficiently at bench scale often encounter issues with flow distribution, pressure drops, and resin compression at larger scales. Filtration operations face increased fouling and reduced flux rates, while viral clearance steps must demonstrate consistent performance across scales to ensure product safety.
Analytical method transfer and validation represent another major hurdle. Methods optimized for laboratory-scale samples may require significant adaptation to accommodate the increased sample throughput and potentially different impurity profiles encountered at pilot scale. Ensuring method comparability across scales is essential for meaningful process monitoring and control.
Regulatory considerations add complexity to the scale-up process. Demonstrating comparability between products manufactured at different scales requires comprehensive characterization of critical quality attributes and thorough understanding of process-product relationships. Documentation requirements increase substantially as processes move toward commercial manufacturing, necessitating robust quality systems and data management practices.
Economic factors also influence scale-up strategies. The significant capital investment required for pilot-scale manufacturing facilities must be balanced against the need for process flexibility and the uncertainty inherent in early-stage product development. Optimizing resource utilization while maintaining product quality presents ongoing challenges for manufacturing organizations.
Current Scale-up Methodologies and Approaches
01 Production and purification methods for monoclonal antibodies
Various methods for the production and purification of monoclonal antibodies are described, including cell culture techniques, chromatography, and filtration processes. These methods aim to increase yield and purity while maintaining the biological activity of the antibodies. Optimization of culture conditions, media composition, and downstream processing steps are critical for efficient large-scale production of monoclonal antibody products.- Production and purification methods for monoclonal antibodies: Various methods for the production and purification of monoclonal antibodies are disclosed, including cell culture techniques, chromatography, and filtration processes. These methods aim to increase the yield and purity of monoclonal antibody products while maintaining their biological activity. The techniques involve optimizing culture conditions, developing efficient purification protocols, and implementing quality control measures to ensure consistent product quality.
- Scale-up strategies for MAPs manufacturing: Scale-up strategies for monoclonal antibody production involve transitioning from laboratory-scale to industrial-scale manufacturing. These strategies address challenges related to bioreactor design, process parameters optimization, and maintaining product quality during scale-up. Approaches include the use of perfusion bioreactors, fed-batch cultivation, and continuous processing technologies to achieve higher cell densities and product titers while ensuring consistent product characteristics.
- Cell line development and optimization for antibody production: Development and optimization of cell lines for monoclonal antibody production focus on creating stable, high-producing cell lines with desired product quality attributes. Techniques include genetic engineering, cell line selection, and adaptation to serum-free media. Methods for improving cell growth, productivity, and longevity in culture are described, along with approaches to minimize cellular stress and optimize protein expression.
- Process analytical technology and quality control for MAPs: Process analytical technology and quality control methods for monoclonal antibody products involve real-time monitoring and control of manufacturing processes. These technologies enable continuous assessment of critical quality attributes, process parameters, and product characteristics. Implementation of advanced analytical techniques, automation, and data management systems helps ensure consistent product quality, reduce batch failures, and comply with regulatory requirements.
- Formulation and stability enhancement of monoclonal antibodies: Formulation strategies for monoclonal antibodies focus on enhancing stability, preventing aggregation, and maintaining biological activity during manufacturing, storage, and administration. Approaches include the use of stabilizing excipients, pH optimization, and lyophilization techniques. Advanced formulation methods aim to extend shelf life, enable alternative delivery routes, and improve patient compliance while preserving the therapeutic efficacy of the antibody products.
02 Scale-up strategies for MAPs manufacturing
Scale-up strategies for monoclonal antibody production involve transitioning from laboratory-scale to industrial-scale manufacturing while maintaining product quality and consistency. These strategies include the use of bioreactors, fed-batch or perfusion culture systems, and process parameter optimization. Considerations for equipment selection, process control, and validation are essential for successful scale-up of monoclonal antibody production.Expand Specific Solutions03 Cell line development and optimization for antibody expression
Development and optimization of cell lines for enhanced monoclonal antibody expression involves genetic engineering, selection of high-producing clones, and adaptation to serum-free media. Techniques such as gene amplification, vector optimization, and cell line stability assessment are employed to increase antibody yields. The selection of appropriate host cells and expression systems is crucial for achieving high productivity in large-scale manufacturing.Expand Specific Solutions04 Process analytical technology and quality control in MAPs scaling
Process analytical technology and quality control measures are implemented throughout the manufacturing process to ensure consistent product quality during scale-up. These include in-process monitoring, analytical methods for product characterization, and control strategies for critical process parameters. Real-time monitoring and feedback control systems help maintain product quality attributes and process consistency in large-scale monoclonal antibody production.Expand Specific Solutions05 Formulation and stability considerations for scaled-up MAPs
Formulation and stability considerations for scaled-up monoclonal antibody products include the selection of appropriate excipients, buffer systems, and stabilizers to maintain antibody integrity during processing, storage, and transportation. Factors such as pH, ionic strength, and protein concentration are optimized to prevent aggregation, denaturation, and other degradation pathways. Stability studies and accelerated aging tests are conducted to ensure product quality throughout its shelf life.Expand Specific Solutions
Key Industry Players in MAPs Production
The monoclonal antibody products (MAPs) manufacturing scale-up landscape is currently in a mature growth phase, with an estimated global market size exceeding $150 billion. Technical maturity varies significantly among key players, with Regeneron Pharmaceuticals, Roche, and Janssen Biotech demonstrating advanced capabilities in transitioning from benchtop to pilot manufacturing. AstraZeneca, MedImmune, and Sanofi have established robust validation protocols, while emerging competitors like Zydus Lifesciences and Dr. Reddy's are rapidly closing technological gaps. The competitive environment is characterized by increasing focus on process intensification and continuous manufacturing technologies, with industry leaders investing heavily in automated systems and real-time monitoring solutions to address scale-up challenges while maintaining product quality and regulatory compliance.
Regeneron Pharmaceuticals, Inc.
Technical Solution: Regeneron has developed a proprietary VelocImmune® platform for MAPs scale-up that integrates high-throughput antibody generation with rapid manufacturing validation. Their approach employs a continuous processing methodology that maintains consistent product quality attributes from bench to commercial scale. The platform incorporates in-line Process Analytical Technology (PAT) monitoring systems that provide real-time data on critical quality attributes during production. Regeneron's scale-up strategy includes the use of single-use bioreactors ranging from 2L to 2000L with comparable cell culture performance and product quality profiles across scales. Their process validation incorporates Design of Experiments (DoE) methodology to establish robust operating parameters and define the design space for critical process parameters. The company has implemented automated sampling and analysis systems that reduce manual intervention and increase process consistency during scale-up[1]. Regeneron also employs advanced cell line development techniques that ensure high productivity and stability during the transition from research to manufacturing scales.
Strengths: Proprietary platform technology enables rapid transition from discovery to manufacturing with consistent product quality. The integrated PAT systems provide superior process control and reduce batch failures during scale-up. Weaknesses: The highly specialized equipment and systems require significant capital investment and specialized technical expertise, potentially limiting flexibility for process modifications during late-stage development.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has pioneered a comprehensive MAPs scale-up platform called "Sustainable Process Development" that focuses on maintaining product quality while optimizing manufacturing efficiency from bench to commercial scale. Their approach incorporates a Quality by Design (QbD) framework with extensive risk assessment at each scale transition point. Roche employs scale-down models that accurately predict large-scale performance, allowing for efficient process characterization and troubleshooting. Their platform includes advanced cell culture media optimization strategies that maintain consistent cellular metabolism across different scales, resulting in comparable glycosylation profiles in the final product. Roche has implemented a modular approach to process validation, with standardized protocols for critical process steps that can be adapted to different antibody products. The company utilizes sophisticated multivariate data analysis tools to identify critical process parameters and establish robust control strategies during scale-up[2]. Their manufacturing validation includes comprehensive comparability studies between clinical and commercial batches, focusing on structural characterization, biological activity, and impurity profiles to ensure consistent product quality throughout the product lifecycle.
Strengths: Extensive experience with diverse antibody formats enables rapid scale-up of complex molecules with predictable outcomes. Their modular approach allows for standardization while maintaining flexibility for product-specific requirements. Weaknesses: The highly structured approach may result in longer development timelines compared to more agile competitors, potentially delaying time-to-market for novel products.
Critical Technologies in MAPs Scale-up
Methods for identifying and quantitating host cell protein
PatentWO2020237095A1
Innovation
- The method employs capillary electrophoresis to separate and immobilize protein components within capillaries, followed by the use of specific primary antibodies labeled with detectable labels to detect and quantify protein contaminants, allowing for discrimination between charge and size variants, and the use of multiple antibodies to detect multiple contaminants simultaneously.
Method for manufacturing monoclonal antibody using yeast, and screening method
PatentPendingUS20220275417A1
Innovation
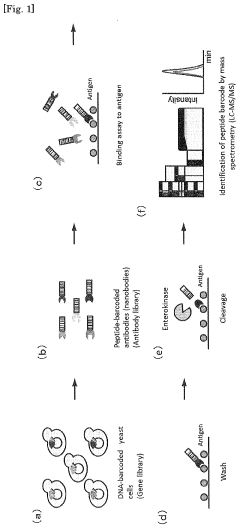
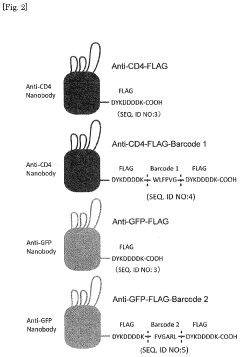
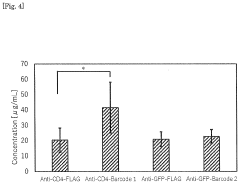
- A method involving the introduction of a DNA fragment encoding a secretory signal, a nanobody, and a peptide barcode into yeast cells for expression and secretion, followed by antigen binding and peptide barcode cleavage for specific antibody identification using mass spectrometry, allowing for the efficient production and identification of monoclonal antibodies with improved sensitivity and specificity.
Regulatory Compliance Framework for MAPs
The regulatory landscape for Monoclonal Antibody Products (MAPs) is complex and multifaceted, requiring manufacturers to navigate a web of international, national, and regional requirements. At the global level, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides critical guidelines that form the foundation of MAP regulatory compliance, particularly ICH Q5A through Q5E, which address quality aspects of biotechnology products.
In the United States, the FDA's regulatory framework for MAPs includes 21 CFR Parts 210 and 211 for Good Manufacturing Practices, alongside specific guidance documents for monoclonal antibodies. The Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER) share oversight responsibilities, with the determination depending on the specific therapeutic application of the MAP.
The European Medicines Agency (EMA) implements a comprehensive framework through Directive 2001/83/EC and Regulation (EC) No 726/2004, supplemented by specific guidelines for biological medicinal products. The EMA's Committee for Medicinal Products for Human Use (CHMP) plays a central role in the scientific assessment of MAPs intended for the European market.
When scaling from benchtop to pilot manufacturing, compliance with Good Manufacturing Practices (GMP) becomes increasingly stringent. The transition necessitates implementation of robust quality systems, including quality risk management approaches as outlined in ICH Q9 and pharmaceutical quality systems described in ICH Q10.
Process validation requirements represent a critical regulatory consideration during scale-up activities. The FDA's guidance on Process Validation emphasizes a lifecycle approach with three stages: process design, process qualification, and continued process verification. Similarly, the EMA's guideline on process validation focuses on a continuous verification approach that is particularly relevant for MAPs.
Environmental monitoring regulations become more demanding as production scales up, with requirements for monitoring viable and non-viable particles, pressure differentials, and other environmental parameters in classified clean rooms according to ISO 14644 standards and regional GMP annexes.
Documentation requirements expand significantly during scale-up, necessitating comprehensive batch records, standard operating procedures, and validation protocols that demonstrate consistent quality and process control. These must be established in accordance with predefined acceptance criteria and must demonstrate that the manufacturing process consistently produces product meeting predetermined quality attributes.
Regulatory agencies increasingly emphasize Quality by Design (QbD) principles as outlined in ICH Q8, encouraging manufacturers to build quality into products through thorough understanding of product and process characteristics. This approach facilitates more efficient scale-up by establishing design spaces within which process changes can occur without requiring additional regulatory approval.
In the United States, the FDA's regulatory framework for MAPs includes 21 CFR Parts 210 and 211 for Good Manufacturing Practices, alongside specific guidance documents for monoclonal antibodies. The Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER) share oversight responsibilities, with the determination depending on the specific therapeutic application of the MAP.
The European Medicines Agency (EMA) implements a comprehensive framework through Directive 2001/83/EC and Regulation (EC) No 726/2004, supplemented by specific guidelines for biological medicinal products. The EMA's Committee for Medicinal Products for Human Use (CHMP) plays a central role in the scientific assessment of MAPs intended for the European market.
When scaling from benchtop to pilot manufacturing, compliance with Good Manufacturing Practices (GMP) becomes increasingly stringent. The transition necessitates implementation of robust quality systems, including quality risk management approaches as outlined in ICH Q9 and pharmaceutical quality systems described in ICH Q10.
Process validation requirements represent a critical regulatory consideration during scale-up activities. The FDA's guidance on Process Validation emphasizes a lifecycle approach with three stages: process design, process qualification, and continued process verification. Similarly, the EMA's guideline on process validation focuses on a continuous verification approach that is particularly relevant for MAPs.
Environmental monitoring regulations become more demanding as production scales up, with requirements for monitoring viable and non-viable particles, pressure differentials, and other environmental parameters in classified clean rooms according to ISO 14644 standards and regional GMP annexes.
Documentation requirements expand significantly during scale-up, necessitating comprehensive batch records, standard operating procedures, and validation protocols that demonstrate consistent quality and process control. These must be established in accordance with predefined acceptance criteria and must demonstrate that the manufacturing process consistently produces product meeting predetermined quality attributes.
Regulatory agencies increasingly emphasize Quality by Design (QbD) principles as outlined in ICH Q8, encouraging manufacturers to build quality into products through thorough understanding of product and process characteristics. This approach facilitates more efficient scale-up by establishing design spaces within which process changes can occur without requiring additional regulatory approval.
Risk Management Strategies in Scale-up Processes
Risk management is a critical component in the scale-up process of Monoclonal Antibody Products (MAPs), requiring systematic approaches to identify, assess, and mitigate potential risks. The transition from benchtop to pilot manufacturing introduces numerous variables that can impact product quality, process efficiency, and regulatory compliance.
A comprehensive risk assessment framework should be established at the outset of scale-up activities. This typically involves implementing Quality Risk Management (QRM) principles as outlined in ICH Q9 guidelines, utilizing tools such as Failure Mode and Effects Analysis (FMEA), Hazard Analysis and Critical Control Points (HACCP), and Risk Ranking and Filtering (RRF) to systematically evaluate potential failure points.
Process parameter sensitivity analysis represents a crucial strategy in risk management. By conducting Design of Experiments (DoE) studies during early development phases, manufacturers can identify Critical Process Parameters (CPPs) that significantly impact Critical Quality Attributes (CQAs). This knowledge enables the establishment of appropriate control strategies and acceptable operating ranges for scaled-up processes.
Material variability management constitutes another essential risk mitigation approach. Raw material variability, particularly in complex biological components like media and chromatography resins, can significantly impact process performance at larger scales. Implementing robust supplier qualification programs and establishing material specifications with appropriate acceptance criteria helps minimize these risks.
Equipment-related risks require particular attention during scale-up. Differences in mixing dynamics, heat transfer characteristics, and mass transfer properties between laboratory and pilot-scale equipment can lead to unexpected process performance variations. Computational fluid dynamics modeling and scale-down models can help predict and address these challenges before full implementation.
Contamination control strategies must be enhanced proportionally with scale increases. As batch values rise significantly at pilot scale, the financial impact of contamination events becomes more severe. Implementing robust cleaning validation protocols, environmental monitoring programs, and appropriate facility design considerations helps mitigate these risks.
Regulatory compliance risk management should be integrated throughout the scale-up process. Early engagement with regulatory authorities, thorough documentation of process changes, and implementation of change control procedures ensure that scale-up activities remain compliant with current Good Manufacturing Practices (cGMP) requirements and facilitate smoother regulatory submissions.
Human factor considerations represent an often-overlooked aspect of risk management. Training programs, clear standard operating procedures, and effective communication channels between development and manufacturing teams are essential to minimize operator-related variability and ensure consistent process execution.
A comprehensive risk assessment framework should be established at the outset of scale-up activities. This typically involves implementing Quality Risk Management (QRM) principles as outlined in ICH Q9 guidelines, utilizing tools such as Failure Mode and Effects Analysis (FMEA), Hazard Analysis and Critical Control Points (HACCP), and Risk Ranking and Filtering (RRF) to systematically evaluate potential failure points.
Process parameter sensitivity analysis represents a crucial strategy in risk management. By conducting Design of Experiments (DoE) studies during early development phases, manufacturers can identify Critical Process Parameters (CPPs) that significantly impact Critical Quality Attributes (CQAs). This knowledge enables the establishment of appropriate control strategies and acceptable operating ranges for scaled-up processes.
Material variability management constitutes another essential risk mitigation approach. Raw material variability, particularly in complex biological components like media and chromatography resins, can significantly impact process performance at larger scales. Implementing robust supplier qualification programs and establishing material specifications with appropriate acceptance criteria helps minimize these risks.
Equipment-related risks require particular attention during scale-up. Differences in mixing dynamics, heat transfer characteristics, and mass transfer properties between laboratory and pilot-scale equipment can lead to unexpected process performance variations. Computational fluid dynamics modeling and scale-down models can help predict and address these challenges before full implementation.
Contamination control strategies must be enhanced proportionally with scale increases. As batch values rise significantly at pilot scale, the financial impact of contamination events becomes more severe. Implementing robust cleaning validation protocols, environmental monitoring programs, and appropriate facility design considerations helps mitigate these risks.
Regulatory compliance risk management should be integrated throughout the scale-up process. Early engagement with regulatory authorities, thorough documentation of process changes, and implementation of change control procedures ensure that scale-up activities remain compliant with current Good Manufacturing Practices (cGMP) requirements and facilitate smoother regulatory submissions.
Human factor considerations represent an often-overlooked aspect of risk management. Training programs, clear standard operating procedures, and effective communication channels between development and manufacturing teams are essential to minimize operator-related variability and ensure consistent process execution.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!