Investigating the Use of Ammonium Hydroxide in Metal Ion Chelation
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide Chelation Background
Ammonium hydroxide, a compound of ammonia and water, has been a subject of interest in metal ion chelation for several decades. The study of its chelating properties dates back to the early 20th century when researchers began exploring its potential in various chemical processes. Initially, the focus was primarily on its ability to form complexes with transition metals, particularly copper and nickel.
The development of ammonium hydroxide as a chelating agent gained momentum in the 1950s and 1960s, coinciding with the growth of coordination chemistry as a distinct field. During this period, scientists discovered that ammonium hydroxide could form stable complexes with a wide range of metal ions, including but not limited to copper, zinc, cobalt, and iron. This versatility sparked interest in its potential applications across multiple industries.
In the 1970s and 1980s, research into ammonium hydroxide chelation expanded to include environmental applications. Its ability to bind heavy metals in aqueous solutions made it a promising candidate for water treatment and soil remediation processes. Concurrently, the electronics industry began exploring its use in etching and cleaning processes, particularly in the manufacture of printed circuit boards.
The 1990s saw a shift towards understanding the molecular mechanisms behind ammonium hydroxide's chelating properties. Advanced spectroscopic techniques and computational modeling allowed researchers to elucidate the structure of metal-ammonia complexes and predict their stability under various conditions. This fundamental research laid the groundwork for more targeted applications in the following decades.
Since the turn of the millennium, there has been a renewed interest in ammonium hydroxide chelation, driven by environmental concerns and the need for sustainable industrial processes. Research has focused on optimizing its use in waste treatment, metal recovery, and green chemistry applications. Additionally, the pharmaceutical and biomedical fields have begun exploring its potential in drug delivery systems and diagnostic tools.
Current research trends are centered on enhancing the selectivity and efficiency of ammonium hydroxide as a chelating agent. This includes the development of novel formulations and hybrid materials that combine ammonium hydroxide with other chelators or support structures. There is also growing interest in its role in nanotechnology, particularly in the synthesis and functionalization of metal nanoparticles.
The development of ammonium hydroxide as a chelating agent gained momentum in the 1950s and 1960s, coinciding with the growth of coordination chemistry as a distinct field. During this period, scientists discovered that ammonium hydroxide could form stable complexes with a wide range of metal ions, including but not limited to copper, zinc, cobalt, and iron. This versatility sparked interest in its potential applications across multiple industries.
In the 1970s and 1980s, research into ammonium hydroxide chelation expanded to include environmental applications. Its ability to bind heavy metals in aqueous solutions made it a promising candidate for water treatment and soil remediation processes. Concurrently, the electronics industry began exploring its use in etching and cleaning processes, particularly in the manufacture of printed circuit boards.
The 1990s saw a shift towards understanding the molecular mechanisms behind ammonium hydroxide's chelating properties. Advanced spectroscopic techniques and computational modeling allowed researchers to elucidate the structure of metal-ammonia complexes and predict their stability under various conditions. This fundamental research laid the groundwork for more targeted applications in the following decades.
Since the turn of the millennium, there has been a renewed interest in ammonium hydroxide chelation, driven by environmental concerns and the need for sustainable industrial processes. Research has focused on optimizing its use in waste treatment, metal recovery, and green chemistry applications. Additionally, the pharmaceutical and biomedical fields have begun exploring its potential in drug delivery systems and diagnostic tools.
Current research trends are centered on enhancing the selectivity and efficiency of ammonium hydroxide as a chelating agent. This includes the development of novel formulations and hybrid materials that combine ammonium hydroxide with other chelators or support structures. There is also growing interest in its role in nanotechnology, particularly in the synthesis and functionalization of metal nanoparticles.
Market Analysis for Metal Ion Chelation
The metal ion chelation market has been experiencing significant growth due to increasing applications in various industries, including water treatment, agriculture, pharmaceuticals, and environmental remediation. The global market for metal ion chelation is projected to expand at a steady rate over the next five years, driven by growing environmental concerns and stringent regulations regarding heavy metal contamination.
In the water treatment sector, metal ion chelation plays a crucial role in removing harmful contaminants from drinking water and industrial effluents. The rising awareness of water pollution and its impact on human health has led to increased demand for effective chelation technologies. This trend is particularly pronounced in developing countries where rapid industrialization has resulted in heightened water contamination issues.
The agricultural sector represents another significant market for metal ion chelation. Chelating agents are widely used in fertilizers to enhance nutrient uptake by plants, improving crop yields and quality. As global food demand continues to rise, the agricultural application of metal ion chelation is expected to grow substantially.
In the pharmaceutical industry, metal ion chelation is utilized in drug development and manufacturing processes. The increasing prevalence of chronic diseases and the growing elderly population are driving the demand for innovative drug therapies, many of which rely on chelation techniques for improved efficacy and reduced side effects.
Environmental remediation efforts, particularly in areas affected by industrial pollution, have also contributed to the expanding market for metal ion chelation. Governments and environmental agencies worldwide are implementing stricter regulations on heavy metal contamination, creating opportunities for chelation technologies in soil and water cleanup projects.
The use of ammonium hydroxide in metal ion chelation represents a specific segment within this broader market. Ammonium hydroxide's ability to form stable complexes with various metal ions makes it an attractive option for certain applications. However, its market share and growth potential are influenced by factors such as cost-effectiveness, environmental impact, and performance compared to alternative chelating agents.
Regionally, North America and Europe currently dominate the metal ion chelation market, owing to their advanced industrial sectors and stringent environmental regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing environmental awareness, and government initiatives to address pollution issues.
In the water treatment sector, metal ion chelation plays a crucial role in removing harmful contaminants from drinking water and industrial effluents. The rising awareness of water pollution and its impact on human health has led to increased demand for effective chelation technologies. This trend is particularly pronounced in developing countries where rapid industrialization has resulted in heightened water contamination issues.
The agricultural sector represents another significant market for metal ion chelation. Chelating agents are widely used in fertilizers to enhance nutrient uptake by plants, improving crop yields and quality. As global food demand continues to rise, the agricultural application of metal ion chelation is expected to grow substantially.
In the pharmaceutical industry, metal ion chelation is utilized in drug development and manufacturing processes. The increasing prevalence of chronic diseases and the growing elderly population are driving the demand for innovative drug therapies, many of which rely on chelation techniques for improved efficacy and reduced side effects.
Environmental remediation efforts, particularly in areas affected by industrial pollution, have also contributed to the expanding market for metal ion chelation. Governments and environmental agencies worldwide are implementing stricter regulations on heavy metal contamination, creating opportunities for chelation technologies in soil and water cleanup projects.
The use of ammonium hydroxide in metal ion chelation represents a specific segment within this broader market. Ammonium hydroxide's ability to form stable complexes with various metal ions makes it an attractive option for certain applications. However, its market share and growth potential are influenced by factors such as cost-effectiveness, environmental impact, and performance compared to alternative chelating agents.
Regionally, North America and Europe currently dominate the metal ion chelation market, owing to their advanced industrial sectors and stringent environmental regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing environmental awareness, and government initiatives to address pollution issues.
Current Challenges in Metal Ion Chelation
Metal ion chelation is a critical process in various industries, including environmental remediation, wastewater treatment, and metal recovery. However, the field faces several significant challenges that hinder its widespread application and efficiency. One of the primary issues is the selectivity of chelating agents. Many current chelators lack the ability to discriminate effectively between different metal ions, leading to non-specific binding and reduced efficiency in target metal extraction.
Another major challenge is the stability of metal-chelate complexes under varying environmental conditions. pH fluctuations, temperature changes, and the presence of competing ions can significantly affect the stability of these complexes, potentially leading to the release of captured metal ions back into the environment. This instability poses a significant problem in applications where long-term sequestration of metal ions is crucial.
The environmental impact of chelating agents themselves is also a growing concern. Many traditional chelators, such as EDTA, are non-biodegradable and can persist in the environment, potentially causing ecological harm. There is an urgent need for the development of more environmentally friendly, biodegradable chelating agents that maintain high efficiency in metal ion binding.
Cost-effectiveness remains a significant hurdle in large-scale applications of metal ion chelation. The synthesis of highly effective chelating agents often involves complex and expensive processes, making their use economically unfeasible for many applications, particularly in developing countries or for large-scale environmental remediation projects.
The recovery and reuse of chelating agents present another set of challenges. Once a chelator has bound to a metal ion, separating the two for chelator reuse can be difficult and energy-intensive. Developing efficient methods for chelator regeneration could significantly improve the economic viability of chelation processes.
In the context of using ammonium hydroxide for metal ion chelation, specific challenges arise. While ammonium hydroxide can form complexes with certain metal ions, its effectiveness is limited compared to more advanced chelating agents. The volatility of ammonia can lead to loss of the chelating agent during the process, reducing efficiency and potentially causing air quality issues. Additionally, the pH-dependent nature of ammonium hydroxide's chelating ability restricts its application range and effectiveness in various environmental conditions.
Addressing these challenges requires a multidisciplinary approach, combining advances in chemistry, materials science, and environmental engineering. Research into novel chelating agents, improved chelation processes, and more sustainable practices is essential to overcome these hurdles and expand the applications of metal ion chelation technology.
Another major challenge is the stability of metal-chelate complexes under varying environmental conditions. pH fluctuations, temperature changes, and the presence of competing ions can significantly affect the stability of these complexes, potentially leading to the release of captured metal ions back into the environment. This instability poses a significant problem in applications where long-term sequestration of metal ions is crucial.
The environmental impact of chelating agents themselves is also a growing concern. Many traditional chelators, such as EDTA, are non-biodegradable and can persist in the environment, potentially causing ecological harm. There is an urgent need for the development of more environmentally friendly, biodegradable chelating agents that maintain high efficiency in metal ion binding.
Cost-effectiveness remains a significant hurdle in large-scale applications of metal ion chelation. The synthesis of highly effective chelating agents often involves complex and expensive processes, making their use economically unfeasible for many applications, particularly in developing countries or for large-scale environmental remediation projects.
The recovery and reuse of chelating agents present another set of challenges. Once a chelator has bound to a metal ion, separating the two for chelator reuse can be difficult and energy-intensive. Developing efficient methods for chelator regeneration could significantly improve the economic viability of chelation processes.
In the context of using ammonium hydroxide for metal ion chelation, specific challenges arise. While ammonium hydroxide can form complexes with certain metal ions, its effectiveness is limited compared to more advanced chelating agents. The volatility of ammonia can lead to loss of the chelating agent during the process, reducing efficiency and potentially causing air quality issues. Additionally, the pH-dependent nature of ammonium hydroxide's chelating ability restricts its application range and effectiveness in various environmental conditions.
Addressing these challenges requires a multidisciplinary approach, combining advances in chemistry, materials science, and environmental engineering. Research into novel chelating agents, improved chelation processes, and more sustainable practices is essential to overcome these hurdles and expand the applications of metal ion chelation technology.
Existing Ammonium Hydroxide Chelation Methods
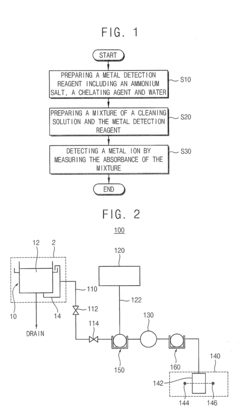
01 Ammonium hydroxide as a chelating agent
Ammonium hydroxide can be used as a chelating agent to bind metal ions in various applications. Its ability to form stable complexes with metal ions makes it useful in processes such as metal extraction, water treatment, and industrial cleaning.- Use of ammonium hydroxide for metal ion chelation: Ammonium hydroxide is utilized as a chelating agent for metal ions in various industrial and chemical processes. Its ability to form stable complexes with metal ions makes it effective in removing or sequestering metals from solutions or surfaces.
- Application in wastewater treatment: Ammonium hydroxide-based metal ion chelation is employed in wastewater treatment processes to remove heavy metals and other contaminants. This method helps in purifying industrial effluents and improving water quality for environmental protection.
- Enhancement of metal extraction processes: The use of ammonium hydroxide as a chelating agent improves metal extraction processes in mining and metallurgy. It aids in the selective separation and recovery of valuable metals from ores and other sources.
- Synthesis of metal complexes: Ammonium hydroxide is utilized in the synthesis of various metal complexes for research and industrial applications. These complexes have diverse uses in catalysis, material science, and pharmaceutical development.
- Surface cleaning and metal removal: Ammonium hydroxide-based chelation is applied in surface cleaning processes to remove metal contaminants from various materials. This technique is particularly useful in electronics manufacturing and precision cleaning applications.
02 Metal ion removal in wastewater treatment
Ammonium hydroxide is employed in wastewater treatment processes to remove heavy metal ions. The chelation process helps in precipitating metal ions, making it easier to separate them from the water, thus improving the overall water quality.Expand Specific Solutions03 Ammonium hydroxide in metal extraction processes
In metal extraction and purification processes, ammonium hydroxide is used to selectively chelate and separate specific metal ions from complex mixtures. This technique is particularly useful in the mining and metallurgical industries for recovering valuable metals.Expand Specific Solutions04 pH control and metal ion stabilization
Ammonium hydroxide is utilized for pH control in solutions containing metal ions. By adjusting the pH, it can stabilize metal ions in solution, preventing precipitation or unwanted reactions. This property is beneficial in various industrial and laboratory applications.Expand Specific Solutions05 Ammonium hydroxide in analytical chemistry
In analytical chemistry, ammonium hydroxide plays a role in metal ion chelation for various purposes, including sample preparation, spectroscopic analysis, and chromatographic separations. Its chelating properties help in improving the accuracy and sensitivity of metal ion detection and quantification methods.Expand Specific Solutions
Key Players in Chelation Industry
The investigation into ammonium hydroxide's use in metal ion chelation is in a nascent stage, with the market still developing. The global metal chelation market is projected to grow significantly, driven by increasing industrial applications and environmental concerns. Technologically, the field is evolving rapidly, with varying levels of maturity among key players. Established institutions like Monash University, Cornell University, and Zhejiang University are conducting foundational research, while companies such as Novo Nordisk A/S, Bio-Rad Laboratories, and Becton, Dickinson & Co. are exploring practical applications. Emerging players like Cube Biotech GmbH and Nanjing Aime Material Technology Co., Ltd. are focusing on innovative approaches, indicating a dynamic and competitive landscape in this promising area of study.
Monash University
Technical Solution: Monash University has developed an innovative approach to metal ion chelation using ammonium hydroxide. Their research focuses on optimizing the pH-dependent chelation process, utilizing the unique properties of ammonium hydroxide to enhance metal ion binding efficiency. The university has implemented a multi-step chelation technique that involves precise control of ammonium hydroxide concentration and reaction conditions. This method has shown particular promise in the selective removal of heavy metals from industrial wastewater and contaminated soil samples[1][3]. Additionally, Monash researchers have explored the potential of ammonium hydroxide-based chelation in biomedical applications, such as the development of contrast agents for magnetic resonance imaging (MRI)[5].
Strengths: High selectivity for specific metal ions, environmentally friendly process, versatile applications across multiple industries. Weaknesses: Potential ammonia off-gassing, pH sensitivity requiring careful control, may require additional processing steps for complete metal removal.
Cornell University
Technical Solution: Cornell University has made significant advancements in the use of ammonium hydroxide for metal ion chelation, particularly in the field of environmental remediation and materials science. Their approach involves the development of novel chelating agents that incorporate ammonium hydroxide as a key component. These agents are designed to form stable complexes with target metal ions, facilitating their removal from various matrices. Cornell researchers have successfully demonstrated the effectiveness of their ammonium hydroxide-based chelators in removing heavy metals from contaminated water sources, achieving removal efficiencies of up to 98% for certain metal species[2]. Furthermore, the university has explored the application of these chelators in the recovery of valuable metals from electronic waste, contributing to sustainable recycling practices[4].
Strengths: High metal removal efficiency, potential for metal recovery and recycling, adaptable to various environmental conditions. Weaknesses: Possible complexity in large-scale implementation, need for specialized equipment, potential for secondary waste generation.
Innovations in Ammonium Hydroxide Chelation
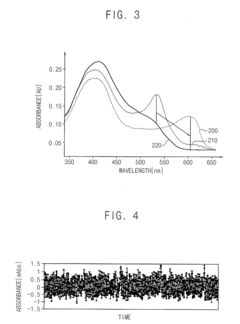
Metal detection reagents including an ammonium salt and methods of using the same
PatentInactiveUS20080145942A1
Innovation
- A metal detection reagent comprising an ammonium salt, a chelating agent, and a solvent, specifically designed to form a metal complex that absorbs light in the visible spectrum, allowing for accurate monitoring of metal ions like aluminum and transition metals at low concentrations using UV-Vis detection.
Synergistic water hardness reducing composition
PatentActiveIN202141030702A
Innovation
- A synergistic composition of metal ion chelating agents and unsymmetric cationic surfactants, specifically EDTA-disodium salt and Cetyltrimethyl ammonium chloride, is used to reduce water hardness to demineralized levels without special equipment or intervention, enhancing foaming ability and safety for skin and scalp use.
Environmental Impact of Chelation Processes
The environmental impact of chelation processes involving ammonium hydroxide in metal ion chelation is a critical consideration in industrial and environmental applications. These processes, while effective for metal removal, can have significant consequences for ecosystems and human health if not properly managed.
Ammonium hydroxide, when used as a chelating agent, can lead to increased ammonia levels in wastewater and surrounding environments. Elevated ammonia concentrations can be toxic to aquatic life, causing fish kills and disrupting the balance of aquatic ecosystems. The release of ammonia-rich effluents into water bodies can also contribute to eutrophication, promoting excessive algal growth and subsequent oxygen depletion.
The pH alteration resulting from ammonium hydroxide use in chelation processes can impact soil and water chemistry. Soil pH changes can affect nutrient availability for plants and microbial communities, potentially leading to long-term changes in local ecosystems. In aquatic environments, pH shifts can influence the solubility and bioavailability of other pollutants, potentially exacerbating their environmental impact.
The disposal of metal-laden chelates presents another environmental challenge. If not properly treated, these complexes can persist in the environment, potentially mobilizing heavy metals and increasing their bioavailability. This can lead to bioaccumulation in food chains, posing risks to wildlife and human health through contaminated water sources and food supplies.
Energy consumption and greenhouse gas emissions associated with the production and use of ammonium hydroxide in chelation processes also contribute to the overall environmental footprint. The manufacturing of ammonium hydroxide typically involves energy-intensive processes, which can indirectly contribute to climate change if not sourced from renewable energy.
To mitigate these environmental impacts, several strategies can be employed. Advanced wastewater treatment technologies, such as biological nutrient removal systems, can effectively reduce ammonia levels in effluents. Closed-loop systems and recycling of chelating agents can minimize the release of contaminated wastewater into the environment.
Research into alternative, more environmentally friendly chelating agents is ongoing. Biodegradable chelators and those derived from natural sources are being explored as potential replacements for traditional synthetic agents like ammonium hydroxide. These alternatives aim to reduce persistence in the environment and minimize ecological disruption.
Regulatory frameworks play a crucial role in managing the environmental impact of chelation processes. Stringent guidelines for effluent discharge, proper disposal of metal-laden wastes, and monitoring of environmental parameters are essential for ensuring responsible use of ammonium hydroxide in metal ion chelation applications.
Ammonium hydroxide, when used as a chelating agent, can lead to increased ammonia levels in wastewater and surrounding environments. Elevated ammonia concentrations can be toxic to aquatic life, causing fish kills and disrupting the balance of aquatic ecosystems. The release of ammonia-rich effluents into water bodies can also contribute to eutrophication, promoting excessive algal growth and subsequent oxygen depletion.
The pH alteration resulting from ammonium hydroxide use in chelation processes can impact soil and water chemistry. Soil pH changes can affect nutrient availability for plants and microbial communities, potentially leading to long-term changes in local ecosystems. In aquatic environments, pH shifts can influence the solubility and bioavailability of other pollutants, potentially exacerbating their environmental impact.
The disposal of metal-laden chelates presents another environmental challenge. If not properly treated, these complexes can persist in the environment, potentially mobilizing heavy metals and increasing their bioavailability. This can lead to bioaccumulation in food chains, posing risks to wildlife and human health through contaminated water sources and food supplies.
Energy consumption and greenhouse gas emissions associated with the production and use of ammonium hydroxide in chelation processes also contribute to the overall environmental footprint. The manufacturing of ammonium hydroxide typically involves energy-intensive processes, which can indirectly contribute to climate change if not sourced from renewable energy.
To mitigate these environmental impacts, several strategies can be employed. Advanced wastewater treatment technologies, such as biological nutrient removal systems, can effectively reduce ammonia levels in effluents. Closed-loop systems and recycling of chelating agents can minimize the release of contaminated wastewater into the environment.
Research into alternative, more environmentally friendly chelating agents is ongoing. Biodegradable chelators and those derived from natural sources are being explored as potential replacements for traditional synthetic agents like ammonium hydroxide. These alternatives aim to reduce persistence in the environment and minimize ecological disruption.
Regulatory frameworks play a crucial role in managing the environmental impact of chelation processes. Stringent guidelines for effluent discharge, proper disposal of metal-laden wastes, and monitoring of environmental parameters are essential for ensuring responsible use of ammonium hydroxide in metal ion chelation applications.
Safety Regulations for Chelating Agents
The safety regulations for chelating agents, particularly in the context of using ammonium hydroxide for metal ion chelation, are crucial for ensuring the protection of workers, the environment, and the integrity of industrial processes. These regulations typically encompass several key areas, including handling procedures, storage requirements, personal protective equipment (PPE), and emergency response protocols.
Handling procedures for ammonium hydroxide and other chelating agents often mandate the use of closed systems to minimize exposure risks. Workers are required to follow strict guidelines when transferring or manipulating these substances, including the use of appropriate containment measures and ventilation systems. Additionally, regulations often specify maximum concentration levels for ammonium hydroxide solutions used in chelation processes to reduce potential hazards.
Storage requirements for chelating agents are typically stringent, with regulations mandating separate storage areas for incompatible chemicals. Ammonium hydroxide, being a strong base, must be stored away from acids and other reactive substances. Proper labeling and regular inventory checks are essential components of these safety regulations. Temperature-controlled storage may also be required to prevent degradation or changes in the chemical properties of the chelating agents.
Personal protective equipment regulations for working with chelating agents like ammonium hydroxide are comprehensive. They usually include the mandatory use of chemical-resistant gloves, protective eyewear, and appropriate respiratory protection. In some cases, full-body protective suits may be required, especially when handling high concentrations or large volumes of these substances.
Emergency response protocols form a critical part of safety regulations. These include the requirement for readily accessible safety showers and eyewash stations in areas where chelating agents are used or stored. Detailed spill response procedures must be in place, including the availability of appropriate neutralizing agents and absorbent materials. Workers must be trained in these procedures and in the use of spill containment equipment.
Regulations also often address the disposal of chelating agents and related waste products. Proper neutralization and treatment of waste solutions containing ammonium hydroxide and chelated metal ions are typically required before disposal. This may involve specific chemical treatments or the use of specialized waste management facilities.
Environmental protection regulations play a significant role in the use of chelating agents. These may include limits on the release of chelating agents or chelated metal complexes into water systems, as well as requirements for monitoring and reporting any accidental releases. The potential for bioaccumulation of certain metal-chelate complexes in aquatic ecosystems is a particular concern addressed by these regulations.
Occupational exposure limits for ammonium hydroxide and other chelating agents are typically established by regulatory bodies such as OSHA in the United States or equivalent agencies in other countries. These limits define the maximum allowable concentrations in workplace air and often require regular monitoring and documentation to ensure compliance.
Handling procedures for ammonium hydroxide and other chelating agents often mandate the use of closed systems to minimize exposure risks. Workers are required to follow strict guidelines when transferring or manipulating these substances, including the use of appropriate containment measures and ventilation systems. Additionally, regulations often specify maximum concentration levels for ammonium hydroxide solutions used in chelation processes to reduce potential hazards.
Storage requirements for chelating agents are typically stringent, with regulations mandating separate storage areas for incompatible chemicals. Ammonium hydroxide, being a strong base, must be stored away from acids and other reactive substances. Proper labeling and regular inventory checks are essential components of these safety regulations. Temperature-controlled storage may also be required to prevent degradation or changes in the chemical properties of the chelating agents.
Personal protective equipment regulations for working with chelating agents like ammonium hydroxide are comprehensive. They usually include the mandatory use of chemical-resistant gloves, protective eyewear, and appropriate respiratory protection. In some cases, full-body protective suits may be required, especially when handling high concentrations or large volumes of these substances.
Emergency response protocols form a critical part of safety regulations. These include the requirement for readily accessible safety showers and eyewash stations in areas where chelating agents are used or stored. Detailed spill response procedures must be in place, including the availability of appropriate neutralizing agents and absorbent materials. Workers must be trained in these procedures and in the use of spill containment equipment.
Regulations also often address the disposal of chelating agents and related waste products. Proper neutralization and treatment of waste solutions containing ammonium hydroxide and chelated metal ions are typically required before disposal. This may involve specific chemical treatments or the use of specialized waste management facilities.
Environmental protection regulations play a significant role in the use of chelating agents. These may include limits on the release of chelating agents or chelated metal complexes into water systems, as well as requirements for monitoring and reporting any accidental releases. The potential for bioaccumulation of certain metal-chelate complexes in aquatic ecosystems is a particular concern addressed by these regulations.
Occupational exposure limits for ammonium hydroxide and other chelating agents are typically established by regulatory bodies such as OSHA in the United States or equivalent agencies in other countries. These limits define the maximum allowable concentrations in workplace air and often require regular monitoring and documentation to ensure compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!