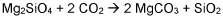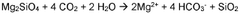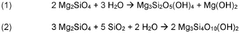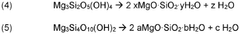Magnesium Carbonate’s Potential in CO2 Sequestration Strategies
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Sequestration Background and Objectives
Carbon dioxide (CO2) sequestration has emerged as a critical strategy in the global effort to mitigate climate change. As atmospheric CO2 levels continue to rise, the need for effective and sustainable methods to capture and store this greenhouse gas has become increasingly urgent. The concept of CO2 sequestration involves the long-term storage of carbon dioxide to prevent its release into the atmosphere, thereby reducing its impact on global warming.
The primary objective of CO2 sequestration is to significantly reduce the amount of anthropogenic CO2 emissions entering the atmosphere. This goal aligns with international agreements and national policies aimed at limiting global temperature increase to well below 2°C above pre-industrial levels. To achieve this, various sequestration strategies have been proposed and implemented, ranging from geological storage to enhanced natural processes.
Geological sequestration, which involves injecting CO2 into deep underground formations, has been a prominent focus of research and implementation. However, the search for alternative methods that offer improved efficiency, cost-effectiveness, and environmental safety has led to the exploration of mineral carbonation processes. Among these, magnesium carbonate has shown promising potential as a CO2 sequestration medium.
Magnesium carbonate (MgCO3) formation through the reaction of CO2 with magnesium-rich minerals presents a unique opportunity for permanent carbon storage. This process mimics natural weathering reactions but accelerates them to achieve meaningful CO2 reduction on human timescales. The abundance of magnesium-rich minerals in the Earth's crust makes this approach particularly attractive for large-scale implementation.
The technical evolution of CO2 sequestration strategies has seen a shift from purely geological approaches to more diverse and innovative methods. This progression reflects the growing understanding of the complexities involved in carbon capture and storage, as well as the need for solutions that can be tailored to different geographical and industrial contexts.
As research in this field advances, the objectives of CO2 sequestration technologies have expanded beyond mere storage. Current goals include developing processes that are energy-efficient, economically viable, and capable of integration with existing industrial operations. Additionally, there is an increasing focus on creating value-added products from sequestered CO2, turning a waste product into a potential resource.
The exploration of magnesium carbonate's potential in CO2 sequestration strategies represents a convergence of these evolving objectives. It offers the promise of a stable, long-term storage solution while potentially providing useful byproducts. As such, it exemplifies the current direction of CO2 sequestration research: seeking multifaceted solutions that address both environmental imperatives and economic considerations.
The primary objective of CO2 sequestration is to significantly reduce the amount of anthropogenic CO2 emissions entering the atmosphere. This goal aligns with international agreements and national policies aimed at limiting global temperature increase to well below 2°C above pre-industrial levels. To achieve this, various sequestration strategies have been proposed and implemented, ranging from geological storage to enhanced natural processes.
Geological sequestration, which involves injecting CO2 into deep underground formations, has been a prominent focus of research and implementation. However, the search for alternative methods that offer improved efficiency, cost-effectiveness, and environmental safety has led to the exploration of mineral carbonation processes. Among these, magnesium carbonate has shown promising potential as a CO2 sequestration medium.
Magnesium carbonate (MgCO3) formation through the reaction of CO2 with magnesium-rich minerals presents a unique opportunity for permanent carbon storage. This process mimics natural weathering reactions but accelerates them to achieve meaningful CO2 reduction on human timescales. The abundance of magnesium-rich minerals in the Earth's crust makes this approach particularly attractive for large-scale implementation.
The technical evolution of CO2 sequestration strategies has seen a shift from purely geological approaches to more diverse and innovative methods. This progression reflects the growing understanding of the complexities involved in carbon capture and storage, as well as the need for solutions that can be tailored to different geographical and industrial contexts.
As research in this field advances, the objectives of CO2 sequestration technologies have expanded beyond mere storage. Current goals include developing processes that are energy-efficient, economically viable, and capable of integration with existing industrial operations. Additionally, there is an increasing focus on creating value-added products from sequestered CO2, turning a waste product into a potential resource.
The exploration of magnesium carbonate's potential in CO2 sequestration strategies represents a convergence of these evolving objectives. It offers the promise of a stable, long-term storage solution while potentially providing useful byproducts. As such, it exemplifies the current direction of CO2 sequestration research: seeking multifaceted solutions that address both environmental imperatives and economic considerations.
Market Analysis for Carbon Capture Technologies
The carbon capture and storage (CCS) market has been experiencing significant growth in recent years, driven by increasing global efforts to mitigate climate change and reduce greenhouse gas emissions. The market for carbon capture technologies is expected to expand rapidly in the coming decades, with projections indicating a compound annual growth rate (CAGR) of over 20% through 2030.
Key factors contributing to this market growth include stringent government regulations on carbon emissions, rising corporate commitments to achieve net-zero targets, and growing public awareness of climate change impacts. The Paris Agreement and subsequent national policies have created a favorable environment for CCS technologies, incentivizing both public and private investments in this sector.
The global CCS market can be segmented based on technology type, application, and end-use industry. Post-combustion capture, pre-combustion capture, and oxy-fuel combustion are the primary technology types, with post-combustion capture currently dominating the market due to its compatibility with existing infrastructure. Major applications include enhanced oil recovery (EOR) and dedicated geological storage, while key end-use industries comprise power generation, oil and gas, cement, and chemical manufacturing.
Geographically, North America leads the CCS market, followed by Europe and Asia-Pacific. The United States, in particular, has shown strong commitment to CCS development through supportive policies and substantial investments. However, emerging economies in Asia, such as China and India, are expected to witness the fastest growth in CCS adoption due to their rapidly expanding industrial sectors and increasing focus on emissions reduction.
The market landscape for carbon capture technologies is characterized by a mix of established players and innovative startups. Major companies in this space include Aker Solutions, Fluor Corporation, Mitsubishi Heavy Industries, and Schlumberger. These firms are continuously investing in research and development to improve capture efficiency and reduce costs, which remain significant barriers to widespread CCS adoption.
Magnesium carbonate's potential in CO2 sequestration strategies represents an emerging niche within the broader CCS market. While traditional CCS methods focus on geological storage or EOR, mineral carbonation using magnesium-rich materials offers a promising alternative. This approach could potentially address some of the limitations of conventional CCS, such as long-term storage stability and scalability.
As the CCS market continues to evolve, factors such as technological advancements, cost reductions, and supportive policy frameworks will play crucial roles in shaping its trajectory. The integration of novel materials and processes, including magnesium carbonate-based solutions, could significantly impact market dynamics and create new opportunities for innovation and growth in the carbon capture and storage sector.
Key factors contributing to this market growth include stringent government regulations on carbon emissions, rising corporate commitments to achieve net-zero targets, and growing public awareness of climate change impacts. The Paris Agreement and subsequent national policies have created a favorable environment for CCS technologies, incentivizing both public and private investments in this sector.
The global CCS market can be segmented based on technology type, application, and end-use industry. Post-combustion capture, pre-combustion capture, and oxy-fuel combustion are the primary technology types, with post-combustion capture currently dominating the market due to its compatibility with existing infrastructure. Major applications include enhanced oil recovery (EOR) and dedicated geological storage, while key end-use industries comprise power generation, oil and gas, cement, and chemical manufacturing.
Geographically, North America leads the CCS market, followed by Europe and Asia-Pacific. The United States, in particular, has shown strong commitment to CCS development through supportive policies and substantial investments. However, emerging economies in Asia, such as China and India, are expected to witness the fastest growth in CCS adoption due to their rapidly expanding industrial sectors and increasing focus on emissions reduction.
The market landscape for carbon capture technologies is characterized by a mix of established players and innovative startups. Major companies in this space include Aker Solutions, Fluor Corporation, Mitsubishi Heavy Industries, and Schlumberger. These firms are continuously investing in research and development to improve capture efficiency and reduce costs, which remain significant barriers to widespread CCS adoption.
Magnesium carbonate's potential in CO2 sequestration strategies represents an emerging niche within the broader CCS market. While traditional CCS methods focus on geological storage or EOR, mineral carbonation using magnesium-rich materials offers a promising alternative. This approach could potentially address some of the limitations of conventional CCS, such as long-term storage stability and scalability.
As the CCS market continues to evolve, factors such as technological advancements, cost reductions, and supportive policy frameworks will play crucial roles in shaping its trajectory. The integration of novel materials and processes, including magnesium carbonate-based solutions, could significantly impact market dynamics and create new opportunities for innovation and growth in the carbon capture and storage sector.
Current State of Magnesium Carbonate in CO2 Sequestration
Magnesium carbonate has emerged as a promising candidate for CO2 sequestration strategies, offering significant potential in mitigating greenhouse gas emissions. Currently, the use of magnesium carbonate in carbon capture and storage (CCS) technologies is in various stages of research and development across the globe.
The primary focus of magnesium carbonate in CO2 sequestration lies in its ability to form stable carbonate minerals through the process of mineral carbonation. This process involves the reaction of CO2 with magnesium-rich minerals or industrial byproducts to form magnesium carbonate, effectively locking away the carbon dioxide in a solid, stable form.
Several pilot projects and laboratory studies have demonstrated the feasibility of using magnesium-rich materials for CO2 sequestration. Notable among these are the use of serpentine and olivine, naturally occurring magnesium silicate minerals, which can be processed to enhance their CO2 absorption capacity.
Industrial applications of magnesium carbonate in CO2 sequestration are still limited, primarily due to the energy-intensive nature of the mineral carbonation process. However, recent advancements in process optimization and the utilization of waste heat from industrial processes have shown promise in improving the economic viability of this approach.
One of the key advantages of magnesium carbonate-based sequestration is the permanence of CO2 storage. Unlike some other CCS methods, the formation of stable carbonate minerals ensures that the captured CO2 remains locked away for geological timescales, minimizing the risk of future release.
Challenges in the widespread adoption of magnesium carbonate for CO2 sequestration include the need for large quantities of magnesium-rich materials, the energy requirements for mineral processing, and the relatively slow kinetics of the carbonation reaction. Ongoing research is focused on addressing these challenges through innovative approaches such as enhanced weathering techniques and the development of more efficient catalysts.
Geographically, research and development efforts in magnesium carbonate-based CO2 sequestration are concentrated in regions with abundant magnesium-rich mineral deposits or industrial waste streams. Countries like the United States, Canada, and several European nations are at the forefront of this technology, with significant projects underway to scale up and commercialize the process.
The current state of magnesium carbonate in CO2 sequestration reflects a technology with immense potential but still facing hurdles in large-scale implementation. As global efforts to combat climate change intensify, the role of magnesium carbonate in carbon capture strategies is likely to grow, driven by ongoing technological advancements and increasing environmental imperatives.
The primary focus of magnesium carbonate in CO2 sequestration lies in its ability to form stable carbonate minerals through the process of mineral carbonation. This process involves the reaction of CO2 with magnesium-rich minerals or industrial byproducts to form magnesium carbonate, effectively locking away the carbon dioxide in a solid, stable form.
Several pilot projects and laboratory studies have demonstrated the feasibility of using magnesium-rich materials for CO2 sequestration. Notable among these are the use of serpentine and olivine, naturally occurring magnesium silicate minerals, which can be processed to enhance their CO2 absorption capacity.
Industrial applications of magnesium carbonate in CO2 sequestration are still limited, primarily due to the energy-intensive nature of the mineral carbonation process. However, recent advancements in process optimization and the utilization of waste heat from industrial processes have shown promise in improving the economic viability of this approach.
One of the key advantages of magnesium carbonate-based sequestration is the permanence of CO2 storage. Unlike some other CCS methods, the formation of stable carbonate minerals ensures that the captured CO2 remains locked away for geological timescales, minimizing the risk of future release.
Challenges in the widespread adoption of magnesium carbonate for CO2 sequestration include the need for large quantities of magnesium-rich materials, the energy requirements for mineral processing, and the relatively slow kinetics of the carbonation reaction. Ongoing research is focused on addressing these challenges through innovative approaches such as enhanced weathering techniques and the development of more efficient catalysts.
Geographically, research and development efforts in magnesium carbonate-based CO2 sequestration are concentrated in regions with abundant magnesium-rich mineral deposits or industrial waste streams. Countries like the United States, Canada, and several European nations are at the forefront of this technology, with significant projects underway to scale up and commercialize the process.
The current state of magnesium carbonate in CO2 sequestration reflects a technology with immense potential but still facing hurdles in large-scale implementation. As global efforts to combat climate change intensify, the role of magnesium carbonate in carbon capture strategies is likely to grow, driven by ongoing technological advancements and increasing environmental imperatives.
Existing Magnesium Carbonate-based CO2 Capture Solutions
01 Direct air capture of CO2 using magnesium carbonate
This method involves using magnesium carbonate to directly capture CO2 from the atmosphere. The process typically includes exposing magnesium carbonate to air, allowing it to absorb CO2, and then regenerating the material for reuse. This technique is considered efficient for large-scale carbon sequestration and can be implemented in various environmental conditions.- Direct air capture of CO2 using magnesium carbonate: This method involves using magnesium carbonate to directly capture CO2 from the atmosphere. The process typically includes exposing magnesium carbonate to air, allowing it to absorb CO2, and then regenerating the material for reuse. This technique is considered efficient for large-scale carbon sequestration and can be implemented in various environmental conditions.
- Enhanced weathering of magnesium-rich minerals for CO2 sequestration: This approach accelerates the natural weathering process of magnesium-rich minerals to capture and store CO2. It involves crushing and spreading these minerals over large areas, increasing their surface area and reaction rate with atmospheric CO2. The process can be applied in various settings, including agricultural lands, enhancing both carbon sequestration and soil quality.
- Magnesium carbonate-based CO2 capture in industrial processes: This method focuses on integrating magnesium carbonate-based CO2 capture systems into industrial processes, particularly in high-emission sectors. The technique involves using magnesium carbonate to absorb CO2 from flue gases or process streams, effectively reducing industrial carbon emissions while potentially producing valuable by-products.
- Aqueous mineral carbonation for CO2 sequestration: This process involves the reaction of CO2 with magnesium-rich minerals in an aqueous medium to form stable magnesium carbonate. The method can be applied to various magnesium-containing materials, including industrial by-products and naturally occurring minerals, offering a versatile approach to carbon sequestration with potential for large-scale implementation.
- Biologically-mediated magnesium carbonate formation for CO2 sequestration: This innovative approach utilizes microorganisms to facilitate the formation of magnesium carbonate from CO2 and magnesium-rich materials. The biological processes can enhance the rate and efficiency of carbonate formation, potentially offering a more sustainable and energy-efficient method for carbon sequestration compared to traditional chemical processes.
02 Mineral carbonation for CO2 sequestration
Mineral carbonation involves the reaction of CO2 with magnesium-rich minerals to form stable carbonate compounds. This process mimics natural weathering but at an accelerated rate. The technique can be applied to various magnesium-rich materials, including serpentine and olivine, and offers a permanent storage solution for captured CO2.Expand Specific Solutions03 Enhanced weathering techniques for CO2 sequestration
Enhanced weathering accelerates the natural process of rock weathering to capture and store CO2. This method often involves spreading finely ground magnesium-rich rocks over large areas, such as agricultural lands or coastal regions. As these materials weather, they react with atmospheric CO2, forming stable carbonate minerals and effectively sequestering carbon.Expand Specific Solutions04 Utilization of industrial by-products for CO2 sequestration
This approach focuses on using magnesium-rich industrial by-products, such as steel slag or fly ash, for CO2 sequestration. These materials are often rich in reactive magnesium compounds and can be processed to enhance their CO2 absorption capacity. This method not only sequesters carbon but also provides a sustainable solution for industrial waste management.Expand Specific Solutions05 Aqueous carbonation processes for CO2 sequestration
Aqueous carbonation involves the reaction of CO2 with magnesium-rich materials in an aqueous medium. This process can be enhanced through various methods such as increasing pressure, temperature, or using catalysts. The resulting magnesium carbonate can be used in various applications or stored safely, providing an effective means of carbon sequestration.Expand Specific Solutions
Key Players in CO2 Sequestration Industry
The CO2 sequestration market using magnesium carbonate is in its early development stage, with growing interest due to climate change concerns. The market size is expanding, driven by increasing environmental regulations and corporate sustainability goals. Technologically, it's still evolving, with companies like UT-Battelle LLC, Carbonfree Chemicals Holdings LLC, and Cambridge Carbon Capture Ltd leading research and development efforts. Academic institutions such as Sichuan University and Chongqing University are also contributing to advancements. While promising, the technology's commercial viability and scalability are still being explored, indicating a competitive landscape focused on innovation and efficiency improvements.
Carbonfree Chemicals Holdings LLC
Technical Solution: Carbonfree Chemicals has developed a proprietary technology called SkyMine® for CO2 sequestration using magnesium carbonate. The process involves capturing CO2 from industrial flue gases and reacting it with naturally occurring minerals to form stable carbonate compounds. Their approach utilizes a series of chemical reactions to convert CO2 into bicarbonate solutions, which are then mineralized with magnesium-rich materials to produce magnesium carbonate[1]. This method not only sequesters CO2 but also generates valuable by-products such as hydrochloric acid and sodium bicarbonate, making the process economically viable[2]. The company has demonstrated the technology at a commercial scale, processing up to 75,000 metric tons of CO2 annually[3].
Strengths: Proven commercial-scale implementation, production of valuable by-products, permanent CO2 storage. Weaknesses: High energy requirements, limited by availability of suitable mineral feedstocks, potential environmental impacts of large-scale mineral extraction.
Cambridge Carbon Capture Ltd.
Technical Solution: Cambridge Carbon Capture (CCC) has developed an innovative process called CO2LOC for carbon dioxide sequestration using magnesium-rich minerals. Their technology focuses on enhancing the natural weathering process of silicate rocks, particularly those rich in magnesium, to capture and store CO2. The process involves grinding these minerals to increase their surface area and reactivity, then exposing them to CO2-rich environments. CCC's method accelerates the carbonation reaction, forming stable magnesium carbonate compounds[1]. The company has reported achieving carbonation rates up to 100 times faster than natural processes[2]. Additionally, CCC's technology can be integrated with existing industrial processes, potentially capturing up to 30% of CO2 emissions from cement and steel production[3].
Strengths: Accelerated carbonation rates, integration with existing industrial processes, utilization of abundant silicate minerals. Weaknesses: Energy-intensive mineral grinding process, potential scalability challenges, environmental concerns related to large-scale mineral extraction.
Innovative Magnesium Carbonate CO2 Sequestration Techniques
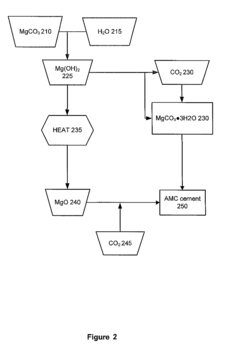
Sequestration of co2
PatentWO2023134849A1
Innovation
- A method involving the use of ultramafic rocks, weathering products, or industrial waste with high MgO content, treated hydrothermally to convert them into magnesium hydroxide and magnesium silicate hydrate, followed by dehydration and reaction with CO2 to form magnesium carbonate, utilizing a BET surface area of 0.1 m^2/g or finer to enhance binding efficiency.
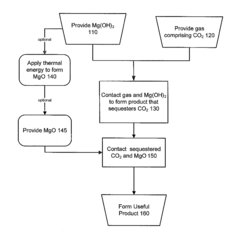
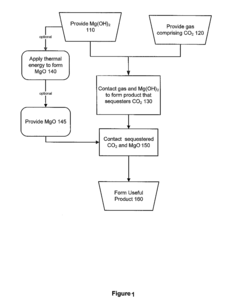
Methods and products utilizing magnesium oxide for carbon dioxide sequestration
PatentInactiveUS20120291675A1
Innovation
- The method involves contacting carbon dioxide with Mg(OH)2 to form magnesium carbonate, which is then converted into cementitious products using MgO, either from thermal energy or industrial waste, allowing for the production of stable storage forms for carbon dioxide, including nesquehonite or amorphous magnesium carbonate, suitable for building materials or nuclear waste storage.
Environmental Impact Assessment
The environmental impact assessment of magnesium carbonate's potential in CO2 sequestration strategies reveals both positive and negative effects on ecosystems and natural resources. On the positive side, the process of carbon sequestration using magnesium carbonate can significantly reduce atmospheric CO2 levels, mitigating the effects of climate change. This reduction in greenhouse gases can lead to a decrease in global temperatures, potentially slowing the rate of sea-level rise and reducing the frequency and intensity of extreme weather events.
Furthermore, the use of magnesium carbonate in CO2 sequestration can help to stabilize soil pH levels in areas affected by acid rain or industrial pollution. This soil improvement can promote healthier plant growth and increase biodiversity in affected regions. Additionally, the process may contribute to the formation of new mineral deposits, which could have beneficial effects on local geology and potentially create new habitats for various species.
However, there are also potential negative environmental impacts to consider. The extraction of magnesium-rich minerals for use in CO2 sequestration may lead to habitat destruction and landscape alteration in mining areas. This could result in the loss of biodiversity and disruption of local ecosystems. The mining process itself may also contribute to air and water pollution if not properly managed, potentially affecting both terrestrial and aquatic environments.
The large-scale implementation of magnesium carbonate-based CO2 sequestration strategies could also have implications for water resources. The process may require significant amounts of water, potentially leading to water scarcity issues in regions where water resources are already strained. Additionally, there is a risk of groundwater contamination if the sequestration process is not carefully monitored and controlled.
Another consideration is the potential for unintended consequences on local and regional climate patterns. While the overall goal is to reduce global CO2 levels, large-scale carbon sequestration projects could potentially alter local weather patterns, affecting precipitation and temperature regimes in ways that may impact ecosystems and agriculture.
Lastly, the long-term stability and safety of stored CO2 must be carefully assessed. There is a risk of CO2 leakage from storage sites, which could have detrimental effects on local flora and fauna, as well as potentially pose risks to human health. Ongoing monitoring and management of sequestration sites would be necessary to ensure environmental safety and prevent any sudden release of stored CO2.
Furthermore, the use of magnesium carbonate in CO2 sequestration can help to stabilize soil pH levels in areas affected by acid rain or industrial pollution. This soil improvement can promote healthier plant growth and increase biodiversity in affected regions. Additionally, the process may contribute to the formation of new mineral deposits, which could have beneficial effects on local geology and potentially create new habitats for various species.
However, there are also potential negative environmental impacts to consider. The extraction of magnesium-rich minerals for use in CO2 sequestration may lead to habitat destruction and landscape alteration in mining areas. This could result in the loss of biodiversity and disruption of local ecosystems. The mining process itself may also contribute to air and water pollution if not properly managed, potentially affecting both terrestrial and aquatic environments.
The large-scale implementation of magnesium carbonate-based CO2 sequestration strategies could also have implications for water resources. The process may require significant amounts of water, potentially leading to water scarcity issues in regions where water resources are already strained. Additionally, there is a risk of groundwater contamination if the sequestration process is not carefully monitored and controlled.
Another consideration is the potential for unintended consequences on local and regional climate patterns. While the overall goal is to reduce global CO2 levels, large-scale carbon sequestration projects could potentially alter local weather patterns, affecting precipitation and temperature regimes in ways that may impact ecosystems and agriculture.
Lastly, the long-term stability and safety of stored CO2 must be carefully assessed. There is a risk of CO2 leakage from storage sites, which could have detrimental effects on local flora and fauna, as well as potentially pose risks to human health. Ongoing monitoring and management of sequestration sites would be necessary to ensure environmental safety and prevent any sudden release of stored CO2.
Economic Viability Analysis
The economic viability of magnesium carbonate in CO2 sequestration strategies is a critical factor in determining its potential for large-scale implementation. Initial cost-benefit analyses suggest that while the technology shows promise, several economic challenges need to be addressed for widespread adoption.
One of the primary economic advantages of magnesium carbonate-based CO2 sequestration is the abundance of raw materials. Magnesium-rich minerals, such as olivine and serpentine, are widely available and relatively inexpensive. This abundance could potentially lead to lower operational costs compared to other carbon capture and storage (CCS) methods.
However, the process of mineral carbonation, which involves the reaction of CO2 with magnesium-rich minerals to form stable carbonate compounds, is energy-intensive. The high energy requirements for grinding, heating, and pressurizing the minerals significantly impact the overall cost-effectiveness of the process. Current estimates suggest that the energy penalty could be as high as 30-50% of the power output from a typical coal-fired power plant.
The capital expenditure (CAPEX) for implementing magnesium carbonate-based CO2 sequestration systems is another significant economic consideration. The construction of mineral carbonation plants and the necessary infrastructure for CO2 capture and transportation require substantial upfront investments. These costs can be a deterrent for many industries, particularly in the absence of strong carbon pricing mechanisms or government incentives.
On the operational expenditure (OPEX) side, the costs associated with mineral extraction, transportation, and processing are key factors. The location of magnesium-rich mineral deposits relative to CO2 emission sources can greatly influence the economic viability of the process. Long-distance transportation of either the minerals or the captured CO2 can significantly increase operational costs.
Despite these challenges, there are potential economic benefits that could improve the viability of magnesium carbonate-based CO2 sequestration. The production of valuable by-products, such as high-purity silica or metal oxides, could offset some of the operational costs. Additionally, the development of more efficient mineral carbonation processes, such as those utilizing industrial wastes or by-products as feedstock, could reduce both CAPEX and OPEX.
The economic landscape for CO2 sequestration technologies is also influenced by evolving carbon markets and regulatory frameworks. As carbon pricing mechanisms become more prevalent and stringent, the economic case for magnesium carbonate-based sequestration may improve. Government incentives, tax credits, and research funding could further enhance the economic viability of this technology.
In conclusion, while magnesium carbonate shows potential for CO2 sequestration, its economic viability currently faces significant challenges. Continued research and development efforts, coupled with supportive policy frameworks, will be crucial in improving the cost-effectiveness of this technology and positioning it as a viable solution in the fight against climate change.
One of the primary economic advantages of magnesium carbonate-based CO2 sequestration is the abundance of raw materials. Magnesium-rich minerals, such as olivine and serpentine, are widely available and relatively inexpensive. This abundance could potentially lead to lower operational costs compared to other carbon capture and storage (CCS) methods.
However, the process of mineral carbonation, which involves the reaction of CO2 with magnesium-rich minerals to form stable carbonate compounds, is energy-intensive. The high energy requirements for grinding, heating, and pressurizing the minerals significantly impact the overall cost-effectiveness of the process. Current estimates suggest that the energy penalty could be as high as 30-50% of the power output from a typical coal-fired power plant.
The capital expenditure (CAPEX) for implementing magnesium carbonate-based CO2 sequestration systems is another significant economic consideration. The construction of mineral carbonation plants and the necessary infrastructure for CO2 capture and transportation require substantial upfront investments. These costs can be a deterrent for many industries, particularly in the absence of strong carbon pricing mechanisms or government incentives.
On the operational expenditure (OPEX) side, the costs associated with mineral extraction, transportation, and processing are key factors. The location of magnesium-rich mineral deposits relative to CO2 emission sources can greatly influence the economic viability of the process. Long-distance transportation of either the minerals or the captured CO2 can significantly increase operational costs.
Despite these challenges, there are potential economic benefits that could improve the viability of magnesium carbonate-based CO2 sequestration. The production of valuable by-products, such as high-purity silica or metal oxides, could offset some of the operational costs. Additionally, the development of more efficient mineral carbonation processes, such as those utilizing industrial wastes or by-products as feedstock, could reduce both CAPEX and OPEX.
The economic landscape for CO2 sequestration technologies is also influenced by evolving carbon markets and regulatory frameworks. As carbon pricing mechanisms become more prevalent and stringent, the economic case for magnesium carbonate-based sequestration may improve. Government incentives, tax credits, and research funding could further enhance the economic viability of this technology.
In conclusion, while magnesium carbonate shows potential for CO2 sequestration, its economic viability currently faces significant challenges. Continued research and development efforts, coupled with supportive policy frameworks, will be crucial in improving the cost-effectiveness of this technology and positioning it as a viable solution in the fight against climate change.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!