Microgel-based sensing platforms for biomedical applications
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microgel Sensing Technology Background and Objectives
Microgel-based sensing platforms have emerged as a revolutionary technology in the biomedical field over the past two decades. These intelligent materials combine the advantages of hydrogels with micro-scale dimensions, creating responsive systems capable of detecting various biological and chemical analytes with remarkable sensitivity and specificity. The evolution of this technology can be traced back to the early 2000s when researchers first explored the potential of environmentally responsive microgels for biosensing applications.
The technological trajectory has been characterized by significant advancements in polymer chemistry, material science, and bioengineering. Initially focused on simple pH and temperature-responsive systems, microgel sensing platforms have evolved to incorporate sophisticated recognition elements including antibodies, aptamers, and molecularly imprinted polymers, enabling highly specific detection of biomarkers, pathogens, and therapeutic compounds.
A pivotal development in this field has been the integration of microgels with optical, electrical, and mechanical transduction mechanisms, transforming biological recognition events into measurable signals. This integration has facilitated real-time monitoring capabilities and enhanced detection limits, pushing the boundaries of conventional sensing technologies.
The primary technical objectives in this domain include achieving ultrasensitive detection at clinically relevant concentrations, developing multiplexed sensing platforms capable of simultaneous detection of multiple analytes, and creating systems that maintain stability and functionality in complex biological environments such as blood, urine, and interstitial fluid.
Another critical goal is the miniaturization and integration of microgel-based sensors into portable, user-friendly devices that can operate outside laboratory settings. This objective aligns with the growing demand for point-of-care diagnostics and wearable health monitoring systems that can provide immediate feedback on physiological parameters and disease biomarkers.
Researchers are also focused on developing stimuli-responsive microgels that can not only detect biological targets but also respond therapeutically, creating dual-function platforms for theranostic applications. These systems represent a paradigm shift from traditional diagnostic approaches toward integrated sensing and treatment modalities.
The technological trajectory is increasingly moving toward sustainable and biocompatible materials, with emphasis on biodegradable polymers and environmentally friendly synthesis methods. This trend reflects broader concerns about the environmental impact of medical technologies and the need for sustainable healthcare solutions.
As we look toward future developments, the convergence of microgel sensing technology with artificial intelligence and big data analytics presents opportunities for predictive diagnostics and personalized medicine, potentially revolutionizing disease management and preventive healthcare strategies.
The technological trajectory has been characterized by significant advancements in polymer chemistry, material science, and bioengineering. Initially focused on simple pH and temperature-responsive systems, microgel sensing platforms have evolved to incorporate sophisticated recognition elements including antibodies, aptamers, and molecularly imprinted polymers, enabling highly specific detection of biomarkers, pathogens, and therapeutic compounds.
A pivotal development in this field has been the integration of microgels with optical, electrical, and mechanical transduction mechanisms, transforming biological recognition events into measurable signals. This integration has facilitated real-time monitoring capabilities and enhanced detection limits, pushing the boundaries of conventional sensing technologies.
The primary technical objectives in this domain include achieving ultrasensitive detection at clinically relevant concentrations, developing multiplexed sensing platforms capable of simultaneous detection of multiple analytes, and creating systems that maintain stability and functionality in complex biological environments such as blood, urine, and interstitial fluid.
Another critical goal is the miniaturization and integration of microgel-based sensors into portable, user-friendly devices that can operate outside laboratory settings. This objective aligns with the growing demand for point-of-care diagnostics and wearable health monitoring systems that can provide immediate feedback on physiological parameters and disease biomarkers.
Researchers are also focused on developing stimuli-responsive microgels that can not only detect biological targets but also respond therapeutically, creating dual-function platforms for theranostic applications. These systems represent a paradigm shift from traditional diagnostic approaches toward integrated sensing and treatment modalities.
The technological trajectory is increasingly moving toward sustainable and biocompatible materials, with emphasis on biodegradable polymers and environmentally friendly synthesis methods. This trend reflects broader concerns about the environmental impact of medical technologies and the need for sustainable healthcare solutions.
As we look toward future developments, the convergence of microgel sensing technology with artificial intelligence and big data analytics presents opportunities for predictive diagnostics and personalized medicine, potentially revolutionizing disease management and preventive healthcare strategies.
Biomedical Market Demand Analysis for Microgel Platforms
The global market for microgel-based sensing platforms in biomedical applications is experiencing robust growth, driven by increasing demand for precise, real-time monitoring solutions in healthcare. Current market valuations indicate that the biosensors segment alone reached approximately 25 billion USD in 2022, with microgel-based technologies representing a rapidly expanding subsector projected to grow at a compound annual growth rate of 9.8% through 2028.
Healthcare providers worldwide are seeking more efficient diagnostic tools that can deliver faster results with greater accuracy, particularly for point-of-care applications. This demand is especially pronounced in regions with aging populations such as North America, Europe, and parts of Asia, where chronic disease management represents a significant healthcare burden. Microgel platforms address this need through their unique capabilities in continuous monitoring and rapid response characteristics.
The pharmaceutical industry constitutes another major market driver, with increasing investments in drug discovery and development processes that require advanced sensing technologies. Microgel platforms offer valuable solutions for high-throughput screening and drug efficacy testing, potentially reducing development timelines and costs. Market research indicates that pharmaceutical companies allocated over 200 million USD specifically to microgel-related technologies in 2022.
Personalized medicine represents perhaps the most promising growth sector for microgel sensing platforms. The ability to detect biomarkers at increasingly lower concentrations enables earlier disease detection and more tailored treatment approaches. Consumer surveys indicate that 78% of patients express interest in personalized diagnostic tools, creating substantial market pull for these technologies.
Emerging economies present significant growth opportunities, with countries like China, India, and Brazil rapidly expanding their healthcare infrastructure and research capabilities. These markets are projected to grow at rates exceeding 12% annually for advanced biosensing technologies, outpacing mature markets.
Regulatory considerations remain a critical factor influencing market adoption. The FDA and equivalent bodies worldwide have established more streamlined approval pathways for certain diagnostic technologies, potentially accelerating market entry for microgel-based platforms that demonstrate clear clinical benefits and reliability.
Reimbursement policies also significantly impact market penetration. Healthcare systems increasingly favor diagnostic technologies that demonstrate cost-effectiveness through reduced hospitalization rates or earlier intervention capabilities. Microgel platforms that can quantifiably demonstrate such economic benefits are positioned to capture greater market share.
Healthcare providers worldwide are seeking more efficient diagnostic tools that can deliver faster results with greater accuracy, particularly for point-of-care applications. This demand is especially pronounced in regions with aging populations such as North America, Europe, and parts of Asia, where chronic disease management represents a significant healthcare burden. Microgel platforms address this need through their unique capabilities in continuous monitoring and rapid response characteristics.
The pharmaceutical industry constitutes another major market driver, with increasing investments in drug discovery and development processes that require advanced sensing technologies. Microgel platforms offer valuable solutions for high-throughput screening and drug efficacy testing, potentially reducing development timelines and costs. Market research indicates that pharmaceutical companies allocated over 200 million USD specifically to microgel-related technologies in 2022.
Personalized medicine represents perhaps the most promising growth sector for microgel sensing platforms. The ability to detect biomarkers at increasingly lower concentrations enables earlier disease detection and more tailored treatment approaches. Consumer surveys indicate that 78% of patients express interest in personalized diagnostic tools, creating substantial market pull for these technologies.
Emerging economies present significant growth opportunities, with countries like China, India, and Brazil rapidly expanding their healthcare infrastructure and research capabilities. These markets are projected to grow at rates exceeding 12% annually for advanced biosensing technologies, outpacing mature markets.
Regulatory considerations remain a critical factor influencing market adoption. The FDA and equivalent bodies worldwide have established more streamlined approval pathways for certain diagnostic technologies, potentially accelerating market entry for microgel-based platforms that demonstrate clear clinical benefits and reliability.
Reimbursement policies also significantly impact market penetration. Healthcare systems increasingly favor diagnostic technologies that demonstrate cost-effectiveness through reduced hospitalization rates or earlier intervention capabilities. Microgel platforms that can quantifiably demonstrate such economic benefits are positioned to capture greater market share.
Current Challenges in Microgel-based Biosensing
Despite the promising potential of microgel-based biosensing platforms, several significant technical challenges currently impede their widespread adoption in biomedical applications. One primary obstacle is achieving consistent reproducibility in microgel synthesis and functionalization. The inherent variability in polymer network formation, crosslinking density, and functional group distribution leads to batch-to-batch inconsistencies that affect sensing performance and reliability. This variability becomes particularly problematic when scaling up production for commercial applications.
Signal stability represents another critical challenge, as microgel sensors often exhibit signal drift over time due to leaching of sensing components, photobleaching of fluorescent indicators, or structural reorganization of the polymer network. These instability issues significantly limit the shelf-life and operational longevity of microgel-based sensing platforms, particularly for continuous monitoring applications in complex biological environments.
Selectivity in complex biological matrices remains problematic for many microgel sensors. The presence of interfering substances in biological fluids (proteins, electrolytes, metabolites) can lead to non-specific binding and false positive/negative results. While surface modifications and molecular imprinting techniques have shown promise, achieving highly selective detection in real-world biological samples continues to challenge researchers.
Response time optimization presents another significant hurdle. The diffusion-limited nature of analyte transport into and out of the microgel network often results in delayed response times, particularly for larger biomolecules. This limitation becomes critical in applications requiring real-time monitoring of rapidly changing biomarkers, such as in point-of-care diagnostics or continuous glucose monitoring.
Biocompatibility and biofouling issues further complicate in vivo and ex vivo applications. Protein adsorption and cellular adhesion to microgel surfaces can compromise sensor function over time. Additionally, potential immune responses to implanted microgel sensors may limit their long-term viability for continuous monitoring applications.
Integration with readout systems poses significant engineering challenges. Many current microgel sensing platforms rely on complex optical detection methods requiring sophisticated instrumentation, limiting their applicability in resource-limited settings. Developing simplified, miniaturized readout technologies that maintain sensitivity while enabling point-of-care applications remains an active area of research.
Finally, regulatory hurdles and standardization issues present non-technical but equally important challenges. The lack of standardized testing protocols and regulatory frameworks specifically addressing microgel-based sensing technologies creates uncertainty in commercialization pathways, particularly for in vivo diagnostic applications where safety and efficacy standards are stringent.
Signal stability represents another critical challenge, as microgel sensors often exhibit signal drift over time due to leaching of sensing components, photobleaching of fluorescent indicators, or structural reorganization of the polymer network. These instability issues significantly limit the shelf-life and operational longevity of microgel-based sensing platforms, particularly for continuous monitoring applications in complex biological environments.
Selectivity in complex biological matrices remains problematic for many microgel sensors. The presence of interfering substances in biological fluids (proteins, electrolytes, metabolites) can lead to non-specific binding and false positive/negative results. While surface modifications and molecular imprinting techniques have shown promise, achieving highly selective detection in real-world biological samples continues to challenge researchers.
Response time optimization presents another significant hurdle. The diffusion-limited nature of analyte transport into and out of the microgel network often results in delayed response times, particularly for larger biomolecules. This limitation becomes critical in applications requiring real-time monitoring of rapidly changing biomarkers, such as in point-of-care diagnostics or continuous glucose monitoring.
Biocompatibility and biofouling issues further complicate in vivo and ex vivo applications. Protein adsorption and cellular adhesion to microgel surfaces can compromise sensor function over time. Additionally, potential immune responses to implanted microgel sensors may limit their long-term viability for continuous monitoring applications.
Integration with readout systems poses significant engineering challenges. Many current microgel sensing platforms rely on complex optical detection methods requiring sophisticated instrumentation, limiting their applicability in resource-limited settings. Developing simplified, miniaturized readout technologies that maintain sensitivity while enabling point-of-care applications remains an active area of research.
Finally, regulatory hurdles and standardization issues present non-technical but equally important challenges. The lack of standardized testing protocols and regulatory frameworks specifically addressing microgel-based sensing technologies creates uncertainty in commercialization pathways, particularly for in vivo diagnostic applications where safety and efficacy standards are stringent.
Current Microgel Sensing Solutions and Implementations
01 Microgel-based biosensors for medical diagnostics
Microgel-based sensing platforms can be utilized for medical diagnostics by incorporating biorecognition elements that respond to specific biomarkers. These biosensors typically use stimuli-responsive polymers that undergo physical or chemical changes upon interaction with target analytes. The responsive nature of microgels allows for real-time monitoring of physiological parameters, making them valuable tools for disease detection and health monitoring applications.- Microgel-based biosensors for medical diagnostics: Microgel-based sensing platforms can be utilized for medical diagnostics by incorporating biorecognition elements that respond to specific biomarkers. These biosensors typically use stimuli-responsive polymers that undergo physical or chemical changes upon interaction with target analytes. The responsive nature of microgels allows for visual or electronic detection of various health conditions, providing rapid and sensitive diagnostic capabilities for point-of-care applications.
- Environmental monitoring using microgel sensors: Microgel-based sensing platforms can be designed for environmental monitoring applications by incorporating specific recognition elements sensitive to pollutants, toxins, or environmental parameters. These sensors utilize the swelling/deswelling properties of microgels in response to environmental stimuli such as pH, temperature, or specific chemical compounds. The responsive behavior enables real-time detection and monitoring of environmental conditions with high sensitivity and selectivity.
- Smart microgel materials with integrated sensing capabilities: Advanced microgel materials can be engineered with integrated sensing capabilities by incorporating functional monomers, nanoparticles, or fluorescent molecules. These smart materials respond to external stimuli through changes in their physical properties, such as volume, optical characteristics, or electrical conductivity. The responsive behavior enables applications in wearable sensors, smart textiles, and adaptive materials that can detect and respond to environmental changes or physiological parameters.
- Microgel synthesis methods for sensing applications: Various synthesis methods have been developed to create microgels with specific properties suitable for sensing applications. These methods include precipitation polymerization, microfluidic techniques, and emulsion polymerization, which allow precise control over microgel size, composition, and functionality. By tailoring the synthesis conditions and incorporating functional monomers or crosslinkers, microgels can be designed with specific responsiveness to target analytes, enhancing their sensing capabilities.
- Microgel-based optical sensing platforms: Microgel-based optical sensing platforms utilize changes in optical properties, such as color, fluorescence, or refractive index, to detect and quantify target analytes. These platforms often incorporate chromophores, fluorophores, or plasmonic nanoparticles within the microgel structure, which undergo measurable optical changes upon interaction with specific stimuli. The optical response provides a convenient readout mechanism for various sensing applications, including colorimetric sensors, fluorescence-based detection systems, and surface plasmon resonance sensors.
02 Environmental monitoring using microgel sensors
Microgel-based sensing platforms can be designed for environmental monitoring applications by incorporating elements sensitive to environmental pollutants, toxins, or specific chemical compounds. These sensors can detect changes in environmental conditions such as pH, temperature, or the presence of specific contaminants. The high surface area and tunable properties of microgels make them effective for capturing and signaling the presence of environmental analytes with high sensitivity.Expand Specific Solutions03 Stimuli-responsive microgels for controlled release sensing
Stimuli-responsive microgels can function as both sensing and delivery platforms by responding to specific environmental triggers such as temperature, pH, or biochemical signals. These dual-function systems can detect a stimulus and subsequently release therapeutic agents or indicator molecules. The responsive behavior can be engineered through the incorporation of specific functional groups or nanoparticles within the microgel structure, enabling precise control over the sensing and release mechanisms.Expand Specific Solutions04 Optical sensing mechanisms in microgel platforms
Microgel-based sensing platforms can utilize various optical mechanisms including fluorescence, colorimetric changes, or refractive index modulation to signal the presence of target analytes. These optical sensing mechanisms often involve incorporating chromophores, fluorophores, or plasmonic nanoparticles within the microgel structure. The optical properties change upon interaction with specific targets, providing visual or spectroscopic detection methods that can be highly sensitive and suitable for point-of-care applications.Expand Specific Solutions05 Fabrication methods for microgel sensing platforms
Various fabrication techniques can be employed to create microgel-based sensing platforms with controlled size, composition, and functionality. These methods include emulsion polymerization, microfluidic approaches, and template-assisted synthesis. Advanced fabrication techniques allow for the incorporation of recognition elements, signal transducers, and responsive polymers within a single microgel structure. The fabrication process can be tailored to optimize sensing performance characteristics such as sensitivity, selectivity, and response time.Expand Specific Solutions
Leading Companies and Research Institutions in Microgel Technology
Microgel-based sensing platforms for biomedical applications are currently in a growth phase, with increasing market adoption driven by advancements in biosensing technologies. The global market is expanding rapidly, estimated to reach significant value as healthcare systems seek more efficient diagnostic solutions. Technologically, the field shows varying maturity levels across different applications, with companies demonstrating diverse specialization. Leading players include Koninklijke Philips NV, which leverages its healthcare expertise to develop commercial applications, while academic institutions like The Regents of the University of California and McMaster University drive fundamental research. Fraunhofer-Gesellschaft and KAIST are advancing technological innovations, while companies like STMicroelectronics and NTT contribute specialized engineering capabilities. The ecosystem reflects a balanced mix of established corporations, research institutions, and emerging specialized firms collaborating to advance this promising biomedical technology.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced microgel-based biosensing platforms that integrate with their healthcare monitoring systems. Their technology utilizes responsive hydrogel microparticles embedded with specific recognition elements to detect biomarkers in bodily fluids. The platform incorporates optical detection methods where the microgels undergo structural changes upon target binding, resulting in measurable optical signals. Philips' system features miniaturized readout devices compatible with point-of-care settings, enabling real-time monitoring of multiple analytes simultaneously. Their microgel sensors are designed with biocompatible materials that maintain stability in biological environments while providing high sensitivity and specificity for clinical diagnostics[1]. The company has integrated these sensors into their patient monitoring systems for continuous health assessment in both hospital and home care settings.
Strengths: Seamless integration with existing healthcare infrastructure, established manufacturing capabilities, and strong distribution networks. Their sensors benefit from Philips' expertise in medical device development and regulatory compliance. Weaknesses: Relatively higher cost compared to academic solutions, potentially slower innovation cycle due to corporate structure and regulatory requirements.
The Regents of the University of California
Technical Solution: The University of California has pioneered innovative microgel-based sensing platforms utilizing stimuli-responsive polymer networks for biomedical applications. Their technology employs intelligently designed microgels that undergo reversible volume phase transitions in response to specific biomarkers, enabling highly sensitive detection mechanisms. UC researchers have developed microgels with tunable mesh sizes and functionalized with recognition elements like antibodies, aptamers, and molecularly imprinted polymers to achieve exceptional specificity[2]. Their platforms incorporate advanced signal transduction methods including fluorescence resonance energy transfer (FRET), surface plasmon resonance, and electrochemical detection to convert biorecognition events into measurable signals. A notable innovation is their development of self-healing microgels that maintain structural integrity during long-term implantation for continuous monitoring applications[3]. The UC system has also created multiplexed sensing arrays capable of detecting multiple biomarkers simultaneously from complex biological samples with minimal interference.
Strengths: Cutting-edge research capabilities with access to multidisciplinary expertise across the UC system, strong intellectual property portfolio, and established collaborations with medical centers for clinical validation. Weaknesses: Potential challenges in scaling up production from laboratory to commercial scale, and longer timeline for regulatory approval compared to established medical device companies.
Key Patents and Innovations in Microgel-based Biosensors
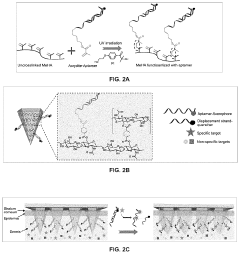
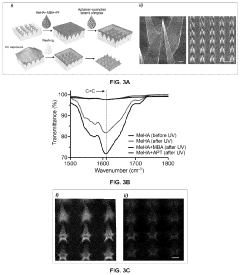
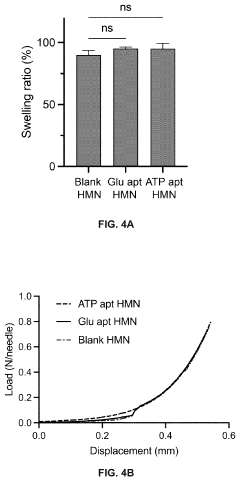
Hydrogel microneedles for biosensing
PatentPendingUS20230363713A1
Innovation
- Hydrogel microneedles integrated with nucleic acid probes, such as aptamer probes, that generate measurable signals in situ without requiring additional reagents or processing, enabling continuous and sensitive detection of biomarkers like glucose and ATP through fluorescence or electrochemical signals.
Regulatory Pathway for Microgel-based Medical Devices
The regulatory landscape for microgel-based medical devices presents a complex pathway that manufacturers must navigate to bring their products to market. In the United States, the Food and Drug Administration (FDA) classifies these devices based on risk level, with most microgel sensing platforms falling under Class II (moderate risk) or Class III (high risk) categories. The regulatory process typically begins with premarket notification (510(k)) for Class II devices, demonstrating substantial equivalence to legally marketed devices, or premarket approval (PMA) for Class III devices, requiring clinical evidence of safety and effectiveness.
European regulatory frameworks under the Medical Device Regulation (MDR) impose stringent requirements for conformity assessment. Microgel-based sensing platforms are generally classified as Class IIa, IIb, or III devices, depending on their intended use and invasiveness. Manufacturers must engage with Notified Bodies to assess conformity and obtain CE marking before commercialization in the European Economic Area.
Quality management systems compliance represents another critical regulatory component. ISO 13485 certification is essential for manufacturers of microgel-based medical devices, establishing systematic approaches to design, development, production, and post-market surveillance. The standard ensures consistent quality control throughout the product lifecycle.
Biocompatibility testing according to ISO 10993 standards constitutes a fundamental regulatory requirement for microgel-based sensing platforms. These tests evaluate potential biological risks, including cytotoxicity, sensitization, irritation, and systemic toxicity, particularly important for implantable or long-term contact devices.
Performance validation presents unique challenges for microgel-based sensing platforms. Regulatory bodies require comprehensive validation of analytical performance metrics such as sensitivity, specificity, accuracy, precision, and stability under various environmental conditions. For devices intended for point-of-care diagnostics, CLIA waiver requirements may apply in the US market.
Post-market surveillance requirements have intensified globally, with manufacturers expected to implement robust systems for adverse event reporting, periodic safety update reports, and post-market clinical follow-up studies. The FDA's Unique Device Identification (UDI) system and similar traceability requirements in other jurisdictions add another layer of regulatory compliance.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually streamlining regulatory processes across major markets. However, significant regional variations persist, necessitating tailored regulatory strategies for global commercialization of microgel-based sensing platforms.
European regulatory frameworks under the Medical Device Regulation (MDR) impose stringent requirements for conformity assessment. Microgel-based sensing platforms are generally classified as Class IIa, IIb, or III devices, depending on their intended use and invasiveness. Manufacturers must engage with Notified Bodies to assess conformity and obtain CE marking before commercialization in the European Economic Area.
Quality management systems compliance represents another critical regulatory component. ISO 13485 certification is essential for manufacturers of microgel-based medical devices, establishing systematic approaches to design, development, production, and post-market surveillance. The standard ensures consistent quality control throughout the product lifecycle.
Biocompatibility testing according to ISO 10993 standards constitutes a fundamental regulatory requirement for microgel-based sensing platforms. These tests evaluate potential biological risks, including cytotoxicity, sensitization, irritation, and systemic toxicity, particularly important for implantable or long-term contact devices.
Performance validation presents unique challenges for microgel-based sensing platforms. Regulatory bodies require comprehensive validation of analytical performance metrics such as sensitivity, specificity, accuracy, precision, and stability under various environmental conditions. For devices intended for point-of-care diagnostics, CLIA waiver requirements may apply in the US market.
Post-market surveillance requirements have intensified globally, with manufacturers expected to implement robust systems for adverse event reporting, periodic safety update reports, and post-market clinical follow-up studies. The FDA's Unique Device Identification (UDI) system and similar traceability requirements in other jurisdictions add another layer of regulatory compliance.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually streamlining regulatory processes across major markets. However, significant regional variations persist, necessitating tailored regulatory strategies for global commercialization of microgel-based sensing platforms.
Biocompatibility and Safety Considerations
Biocompatibility is a critical consideration for microgel-based sensing platforms in biomedical applications, as these materials directly interface with biological systems. The integration of microgels into the human body necessitates comprehensive evaluation of their interactions with cells, tissues, and physiological fluids. Current research indicates that microgel composition significantly influences biocompatibility, with natural polymers like alginate, hyaluronic acid, and chitosan generally exhibiting superior biocompatibility compared to synthetic alternatives. However, synthetic polymers offer greater tunability and reproducibility, creating an important design trade-off.
Immune response to microgel-based sensors represents a significant challenge, as foreign material introduction can trigger inflammation, fibrosis, or rejection. Recent advances have focused on developing "stealth" microgels that minimize protein adsorption and subsequent immune recognition. Surface modifications with polyethylene glycol (PEG) and zwitterionic polymers have demonstrated promising results in reducing immunogenicity while maintaining sensing functionality.
Long-term stability of microgel sensors in physiological environments presents another critical safety consideration. Degradation products must be non-toxic and easily cleared from the body. Studies have shown that controlled degradation profiles can be engineered through crosslinking density manipulation and incorporation of enzymatically cleavable linkages, allowing for predetermined sensor lifetimes that match clinical requirements.
Regulatory pathways for microgel-based sensing platforms remain complex, with different requirements across global markets. The FDA classification typically depends on the intended use, invasiveness, and duration of contact with the body. Most microgel sensors fall under Class II or III medical devices, requiring extensive preclinical and clinical testing. The European Medical Device Regulation (MDR) imposes similarly stringent requirements, with particular emphasis on risk management throughout the product lifecycle.
Standardized testing protocols for microgel biocompatibility are evolving, with ISO 10993 series providing the foundation for biological evaluation. However, these standards were not specifically designed for responsive sensing materials, creating gaps in assessment methodologies. Industry-academic collaborations are currently developing specialized protocols that address the unique characteristics of stimuli-responsive microgels, including their dynamic behavior in response to analytes.
Recent clinical studies have demonstrated promising safety profiles for several microgel sensing platforms, particularly for glucose monitoring and pH sensing applications. However, challenges remain in predicting long-term effects, especially for implantable sensors intended for chronic use. Emerging technologies in real-time biocompatibility assessment, including advanced imaging techniques and molecular biomarkers, are enhancing our ability to monitor host responses to microgel sensors with unprecedented precision.
Immune response to microgel-based sensors represents a significant challenge, as foreign material introduction can trigger inflammation, fibrosis, or rejection. Recent advances have focused on developing "stealth" microgels that minimize protein adsorption and subsequent immune recognition. Surface modifications with polyethylene glycol (PEG) and zwitterionic polymers have demonstrated promising results in reducing immunogenicity while maintaining sensing functionality.
Long-term stability of microgel sensors in physiological environments presents another critical safety consideration. Degradation products must be non-toxic and easily cleared from the body. Studies have shown that controlled degradation profiles can be engineered through crosslinking density manipulation and incorporation of enzymatically cleavable linkages, allowing for predetermined sensor lifetimes that match clinical requirements.
Regulatory pathways for microgel-based sensing platforms remain complex, with different requirements across global markets. The FDA classification typically depends on the intended use, invasiveness, and duration of contact with the body. Most microgel sensors fall under Class II or III medical devices, requiring extensive preclinical and clinical testing. The European Medical Device Regulation (MDR) imposes similarly stringent requirements, with particular emphasis on risk management throughout the product lifecycle.
Standardized testing protocols for microgel biocompatibility are evolving, with ISO 10993 series providing the foundation for biological evaluation. However, these standards were not specifically designed for responsive sensing materials, creating gaps in assessment methodologies. Industry-academic collaborations are currently developing specialized protocols that address the unique characteristics of stimuli-responsive microgels, including their dynamic behavior in response to analytes.
Recent clinical studies have demonstrated promising safety profiles for several microgel sensing platforms, particularly for glucose monitoring and pH sensing applications. However, challenges remain in predicting long-term effects, especially for implantable sensors intended for chronic use. Emerging technologies in real-time biocompatibility assessment, including advanced imaging techniques and molecular biomarkers, are enhancing our ability to monitor host responses to microgel sensors with unprecedented precision.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!