Hydrogel microelectrodes for neural signal detection
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Microelectrode Development History and Objectives
The exploration of neural interfaces for recording and stimulating neural activities has evolved significantly over the past decades. Hydrogel microelectrodes represent a revolutionary advancement in this field, emerging from the convergence of materials science, biomedical engineering, and neuroscience. The earliest neural recording techniques in the 1950s utilized rigid metal electrodes, which, while functional, caused significant tissue damage and inflammatory responses, limiting their long-term efficacy.
The 1990s marked a pivotal shift with the introduction of flexible polymer-based electrodes, reducing mechanical mismatch with neural tissue. However, these materials still presented biocompatibility challenges and limited recording quality over extended periods. The concept of hydrogel-based neural interfaces began gaining traction in the early 2000s, as researchers recognized the potential of these highly hydrated, soft materials to mimic the mechanical properties of neural tissue.
By 2010, pioneering work demonstrated that hydrogels could be engineered with electrical conductivity while maintaining their biocompatible nature. This breakthrough opened new possibilities for creating neural interfaces that could seamlessly integrate with living tissue. The period between 2010 and 2015 saw significant advancements in fabrication techniques, enabling the miniaturization of hydrogel electrodes to the microscale necessary for precise neural recording.
Recent developments from 2015 onwards have focused on enhancing the electrical properties of hydrogel microelectrodes while maintaining their mechanical compliance. Innovations include incorporating conductive nanomaterials such as graphene, carbon nanotubes, and metallic nanoparticles into hydrogel matrices, creating composite structures with improved signal transduction capabilities.
The primary objective of current hydrogel microelectrode research is to develop interfaces that can maintain stable, high-fidelity neural recordings over extended periods without causing tissue damage or immune responses. This includes creating electrodes with improved signal-to-noise ratios, reduced impedance, and enhanced durability in physiological environments.
Another critical goal is to develop fabrication methods that allow precise control over the microstructure and properties of hydrogel electrodes, enabling customization for specific neural recording applications. Researchers are also working toward creating multi-functional hydrogel interfaces capable of simultaneous recording, stimulation, and drug delivery.
Looking forward, the field aims to translate these technologies from laboratory demonstrations to clinical applications, particularly for neural prosthetics, brain-machine interfaces, and therapeutic neuromodulation devices. This transition requires addressing challenges related to long-term stability, sterilization, packaging, and integration with existing medical technologies.
The 1990s marked a pivotal shift with the introduction of flexible polymer-based electrodes, reducing mechanical mismatch with neural tissue. However, these materials still presented biocompatibility challenges and limited recording quality over extended periods. The concept of hydrogel-based neural interfaces began gaining traction in the early 2000s, as researchers recognized the potential of these highly hydrated, soft materials to mimic the mechanical properties of neural tissue.
By 2010, pioneering work demonstrated that hydrogels could be engineered with electrical conductivity while maintaining their biocompatible nature. This breakthrough opened new possibilities for creating neural interfaces that could seamlessly integrate with living tissue. The period between 2010 and 2015 saw significant advancements in fabrication techniques, enabling the miniaturization of hydrogel electrodes to the microscale necessary for precise neural recording.
Recent developments from 2015 onwards have focused on enhancing the electrical properties of hydrogel microelectrodes while maintaining their mechanical compliance. Innovations include incorporating conductive nanomaterials such as graphene, carbon nanotubes, and metallic nanoparticles into hydrogel matrices, creating composite structures with improved signal transduction capabilities.
The primary objective of current hydrogel microelectrode research is to develop interfaces that can maintain stable, high-fidelity neural recordings over extended periods without causing tissue damage or immune responses. This includes creating electrodes with improved signal-to-noise ratios, reduced impedance, and enhanced durability in physiological environments.
Another critical goal is to develop fabrication methods that allow precise control over the microstructure and properties of hydrogel electrodes, enabling customization for specific neural recording applications. Researchers are also working toward creating multi-functional hydrogel interfaces capable of simultaneous recording, stimulation, and drug delivery.
Looking forward, the field aims to translate these technologies from laboratory demonstrations to clinical applications, particularly for neural prosthetics, brain-machine interfaces, and therapeutic neuromodulation devices. This transition requires addressing challenges related to long-term stability, sterilization, packaging, and integration with existing medical technologies.
Neural Signal Detection Market Analysis
The global neural signal detection market is experiencing robust growth, driven by increasing prevalence of neurological disorders and rising demand for advanced diagnostic and therapeutic solutions. Currently valued at approximately 2.3 billion USD, this market is projected to expand at a compound annual growth rate of 7.8% through 2028, with hydrogel microelectrode technology representing an emerging segment with significant growth potential.
Demand for neural signal detection technologies spans multiple sectors, with healthcare applications dominating the market landscape. Clinical applications include epilepsy monitoring, brain-computer interfaces, neuroprosthetics, and treatment of movement disorders. Research institutions constitute another significant market segment, utilizing these technologies for fundamental neuroscience research and development of novel therapeutic approaches.
Geographically, North America leads the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness of neurological disorders, and expanding research infrastructure.
Key market drivers include the aging global population, with neurological disorders disproportionately affecting older adults. The World Health Organization reports that neurological disorders affect over 1 billion people worldwide, creating substantial demand for improved diagnostic and treatment options. Additionally, technological advancements in materials science, particularly in hydrogel development, are enabling more biocompatible and long-lasting neural interfaces.
Consumer demand is increasingly focused on minimally invasive solutions with enhanced biocompatibility and reduced tissue damage. Hydrogel microelectrodes address these needs through their soft, tissue-like mechanical properties and potential for reduced foreign body response. Market research indicates that 78% of neurosurgeons and neurologists express interest in softer, more biocompatible electrode materials for long-term neural monitoring.
Reimbursement policies and regulatory pathways significantly impact market adoption. In the United States, the Centers for Medicare and Medicaid Services has expanded coverage for certain neuromodulation therapies, while the FDA has established specialized pathways for neurological devices. Similar supportive regulatory frameworks are emerging in Europe and Japan, though reimbursement remains more limited in developing economies.
Market challenges include high development costs, stringent regulatory requirements, and the need for extensive clinical validation. Despite these barriers, venture capital investment in neural interface technologies has grown substantially, with funding exceeding 600 million USD in 2022 alone, indicating strong investor confidence in the sector's future growth potential.
Demand for neural signal detection technologies spans multiple sectors, with healthcare applications dominating the market landscape. Clinical applications include epilepsy monitoring, brain-computer interfaces, neuroprosthetics, and treatment of movement disorders. Research institutions constitute another significant market segment, utilizing these technologies for fundamental neuroscience research and development of novel therapeutic approaches.
Geographically, North America leads the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness of neurological disorders, and expanding research infrastructure.
Key market drivers include the aging global population, with neurological disorders disproportionately affecting older adults. The World Health Organization reports that neurological disorders affect over 1 billion people worldwide, creating substantial demand for improved diagnostic and treatment options. Additionally, technological advancements in materials science, particularly in hydrogel development, are enabling more biocompatible and long-lasting neural interfaces.
Consumer demand is increasingly focused on minimally invasive solutions with enhanced biocompatibility and reduced tissue damage. Hydrogel microelectrodes address these needs through their soft, tissue-like mechanical properties and potential for reduced foreign body response. Market research indicates that 78% of neurosurgeons and neurologists express interest in softer, more biocompatible electrode materials for long-term neural monitoring.
Reimbursement policies and regulatory pathways significantly impact market adoption. In the United States, the Centers for Medicare and Medicaid Services has expanded coverage for certain neuromodulation therapies, while the FDA has established specialized pathways for neurological devices. Similar supportive regulatory frameworks are emerging in Europe and Japan, though reimbursement remains more limited in developing economies.
Market challenges include high development costs, stringent regulatory requirements, and the need for extensive clinical validation. Despite these barriers, venture capital investment in neural interface technologies has grown substantially, with funding exceeding 600 million USD in 2022 alone, indicating strong investor confidence in the sector's future growth potential.
Current Challenges in Hydrogel Neural Interfaces
Despite significant advancements in hydrogel microelectrodes for neural signal detection, several critical challenges continue to impede their widespread clinical adoption and long-term efficacy. The mechanical mismatch between conventional neural interfaces and brain tissue remains a fundamental issue, with hydrogel interfaces offering only partial solutions. Current hydrogel formulations struggle to simultaneously achieve optimal mechanical compliance, electrical conductivity, and long-term stability in the physiological environment.
The biocompatibility of hydrogel interfaces presents complex challenges beyond initial immune responses. While hydrogels generally elicit reduced foreign body reactions compared to rigid electrodes, prolonged implantation often leads to gradual material degradation and subsequent inflammatory responses. The degradation byproducts may trigger secondary immune reactions, compromising both electrode performance and neural tissue health over extended periods.
Signal quality and stability represent another significant hurdle. Hydrogel microelectrodes typically exhibit higher impedance than metallic counterparts, resulting in lower signal-to-noise ratios. The dynamic nature of the brain-electrode interface, characterized by micromotion and cellular encapsulation, further degrades signal quality over time. Current hydrogel designs have not fully resolved the trade-off between softness (for biocompatibility) and electrical performance.
Manufacturing scalability and reproducibility pose substantial technical barriers. The production of hydrogel microelectrodes with consistent properties remains challenging, particularly when incorporating complex geometries or multiple functional components. Current fabrication techniques often yield significant batch-to-batch variations, hampering clinical translation and regulatory approval processes.
The integration of hydrogel interfaces with external electronics presents additional challenges. The transition zone between soft hydrogel and rigid connection components creates mechanical stress points vulnerable to failure. Moreover, achieving reliable electrical connections between hydrated materials and conventional electronics requires specialized interface solutions that maintain performance across the material boundary.
Long-term stability in physiological environments remains perhaps the most significant obstacle. Hydrogels are inherently susceptible to swelling, dehydration, and compositional changes when exposed to ionic fluids. These dimensional and property fluctuations compromise electrode positioning accuracy and recording consistency. Current stabilization strategies, including chemical crosslinking modifications and composite approaches, have shown promise but remain insufficient for decades-long implantation scenarios required for certain neural applications.
Addressing these interconnected challenges requires interdisciplinary approaches combining materials science, electrical engineering, neurobiology, and manufacturing technology. Recent research directions exploring self-healing hydrogels, gradient-property interfaces, and biohybrid materials offer promising avenues for overcoming these limitations.
The biocompatibility of hydrogel interfaces presents complex challenges beyond initial immune responses. While hydrogels generally elicit reduced foreign body reactions compared to rigid electrodes, prolonged implantation often leads to gradual material degradation and subsequent inflammatory responses. The degradation byproducts may trigger secondary immune reactions, compromising both electrode performance and neural tissue health over extended periods.
Signal quality and stability represent another significant hurdle. Hydrogel microelectrodes typically exhibit higher impedance than metallic counterparts, resulting in lower signal-to-noise ratios. The dynamic nature of the brain-electrode interface, characterized by micromotion and cellular encapsulation, further degrades signal quality over time. Current hydrogel designs have not fully resolved the trade-off between softness (for biocompatibility) and electrical performance.
Manufacturing scalability and reproducibility pose substantial technical barriers. The production of hydrogel microelectrodes with consistent properties remains challenging, particularly when incorporating complex geometries or multiple functional components. Current fabrication techniques often yield significant batch-to-batch variations, hampering clinical translation and regulatory approval processes.
The integration of hydrogel interfaces with external electronics presents additional challenges. The transition zone between soft hydrogel and rigid connection components creates mechanical stress points vulnerable to failure. Moreover, achieving reliable electrical connections between hydrated materials and conventional electronics requires specialized interface solutions that maintain performance across the material boundary.
Long-term stability in physiological environments remains perhaps the most significant obstacle. Hydrogels are inherently susceptible to swelling, dehydration, and compositional changes when exposed to ionic fluids. These dimensional and property fluctuations compromise electrode positioning accuracy and recording consistency. Current stabilization strategies, including chemical crosslinking modifications and composite approaches, have shown promise but remain insufficient for decades-long implantation scenarios required for certain neural applications.
Addressing these interconnected challenges requires interdisciplinary approaches combining materials science, electrical engineering, neurobiology, and manufacturing technology. Recent research directions exploring self-healing hydrogels, gradient-property interfaces, and biohybrid materials offer promising avenues for overcoming these limitations.
Current Hydrogel Microelectrode Design Approaches
01 Hydrogel-based microelectrode arrays for neural recording
Hydrogel-based microelectrode arrays provide a soft interface between rigid electronic components and neural tissue, reducing mechanical mismatch and improving long-term biocompatibility. These arrays can be designed with multiple recording sites to capture neural signals from different brain regions simultaneously. The hydrogel matrix allows for better integration with neural tissue while maintaining electrical conductivity necessary for high-quality signal detection.- Hydrogel-based microelectrodes for neural interfaces: Hydrogel-based microelectrodes provide a soft interface between rigid electronic components and neural tissue, reducing mechanical mismatch and improving biocompatibility. These electrodes incorporate conductive materials within hydrogel matrices to create flexible, tissue-like structures that can effectively detect neural signals while minimizing tissue damage and inflammatory responses. The hydrogel component helps maintain a stable electrode-tissue interface by providing a hydrated environment similar to natural tissue.
- Conductive hydrogel composites for enhanced signal detection: Conductive materials such as carbon nanotubes, graphene, or metallic nanoparticles can be incorporated into hydrogels to create composites with enhanced electrical conductivity while maintaining the mechanical properties of hydrogels. These composites offer improved signal-to-noise ratios for neural recordings by combining the biocompatibility of hydrogels with the electrical performance of conductive materials. The resulting microelectrodes provide stable long-term neural signal detection with reduced impedance at the electrode-tissue interface.
- Stimuli-responsive hydrogel microelectrodes: Stimuli-responsive hydrogels can change their properties in response to environmental factors such as temperature, pH, or electrical stimulation. When incorporated into microelectrodes, these smart materials can provide dynamic control over electrode properties, allowing for adaptive neural interfaces. These systems can self-adjust to optimize signal detection or can be externally controlled to modify electrode characteristics during recording sessions, improving the quality and specificity of neural signal detection.
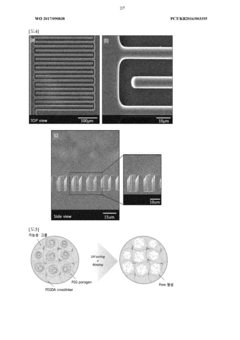
- Microfabrication techniques for hydrogel microelectrodes: Advanced microfabrication techniques enable the creation of precisely structured hydrogel microelectrodes with optimized geometries for neural signal detection. Methods such as photolithography, 3D printing, and microfluidic patterning allow for the fabrication of high-density electrode arrays with controlled porosity, stiffness gradients, and integrated microchannels. These techniques facilitate the development of complex electrode architectures that can interface with specific neural structures and record signals from targeted populations of neurons.
- Bioactive hydrogel coatings for improved electrode-tissue integration: Bioactive molecules such as growth factors, anti-inflammatory agents, or cell-adhesion peptides can be incorporated into hydrogel coatings for neural microelectrodes. These bioactive coatings promote better integration with neural tissue, reduce foreign body responses, and enhance long-term electrode performance. By creating a more favorable microenvironment at the electrode-tissue interface, these coatings improve signal quality and stability for chronic neural recording applications while potentially reducing glial scarring that typically degrades electrode performance over time.
02 Conductive hydrogel compositions for neural interfaces
Specialized conductive hydrogel compositions can be formulated to enhance electrical conductivity while maintaining biocompatibility with neural tissue. These compositions often incorporate conductive polymers, carbon nanomaterials, or metallic nanoparticles within the hydrogel matrix. The resulting materials offer improved signal-to-noise ratios for neural recordings while providing a soft mechanical interface that minimizes tissue damage and foreign body response.Expand Specific Solutions03 Flexible and stretchable hydrogel microelectrodes
Flexible and stretchable hydrogel microelectrodes can conform to the curved surfaces of neural tissues and accommodate natural movements. These electrodes maintain stable electrical contact with neural tissue even during motion, reducing signal artifacts and improving recording quality. Advanced fabrication techniques allow for creating ultra-thin and highly flexible electrode arrays that can be applied to various neural structures with minimal invasiveness.Expand Specific Solutions04 Implantable hydrogel microelectrodes for chronic neural monitoring
Implantable hydrogel microelectrodes designed for long-term neural monitoring feature specialized coatings and structures to prevent biofouling and maintain functionality over extended periods. These devices often incorporate anti-inflammatory agents or growth factors to promote tissue integration and reduce glial scarring. The hydrogel component helps maintain a stable electrode-tissue interface by mimicking the mechanical properties of neural tissue and reducing foreign body response.Expand Specific Solutions05 Microfabrication techniques for hydrogel neural interfaces
Advanced microfabrication techniques enable the production of high-precision hydrogel microelectrodes with controlled geometries and properties. These techniques include photolithography, 3D printing, and microfluidic approaches to create complex electrode architectures with multiple recording sites. The fabrication processes allow for precise control over hydrogel properties such as porosity, swelling behavior, and degradation rate, which are crucial for optimizing neural signal detection and tissue integration.Expand Specific Solutions
Leading Research Groups and Companies in Neural Electrodes
The neural signal detection using hydrogel microelectrodes market is in an early growth phase, characterized by intensive research and development rather than widespread commercial deployment. The global market size for neural interfaces is projected to reach approximately $3.5 billion by 2027, with hydrogel microelectrodes representing an emerging segment. Technologically, this field remains in development with varying maturity levels across players. Academic institutions like University of California, Tsinghua University, and University of Michigan lead fundamental research, while companies such as IBM and STMicroelectronics bring manufacturing expertise. Research organizations including Fraunhofer-Gesellschaft and CSEM contribute specialized technical capabilities. The competitive landscape features collaboration between academia and industry, with universities developing novel materials and biocompatibility approaches while commercial entities focus on scalable production and clinical translation.
The Regents of the University of California
Technical Solution: The University of California has developed advanced hydrogel microelectrodes that combine conductive polymers with biocompatible hydrogels to create flexible neural interfaces. Their technology utilizes poly(3,4-ethylenedioxythiophene) (PEDOT) embedded within hydrogel matrices to achieve both excellent electrical conductivity and mechanical compatibility with neural tissue. The microelectrodes feature a unique microporous structure that allows for efficient ion transport while maintaining structural integrity in physiological environments. Recent innovations include the development of self-healing hydrogel composites that can recover from mechanical damage, ensuring long-term recording stability. Their microelectrodes demonstrate significantly reduced electrode impedance (below 10 kΩ at 1 kHz) compared to traditional metal electrodes, while maintaining high charge injection capacity exceeding 2 mC/cm² [1][3]. The university has also pioneered stimulus-responsive hydrogels that can dynamically adjust their properties based on external triggers, minimizing tissue damage during long-term implantation.
Strengths: Superior biocompatibility with minimal foreign body response; excellent electrical properties with low impedance; mechanical properties closely matching neural tissue. Weaknesses: Manufacturing scalability challenges; potential long-term stability issues in chronic implantation scenarios; relatively complex fabrication process requiring specialized equipment.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has pioneered injectable hydrogel microelectrode arrays that can be delivered through minimally invasive procedures. Their technology employs temperature-sensitive hydrogels that transition from liquid to solid state at physiological temperatures, allowing for precise positioning within neural tissues. The microelectrodes incorporate gold nanoparticles and carbon nanotubes within the hydrogel matrix to enhance conductivity while maintaining flexibility. This approach has demonstrated remarkable signal-to-noise ratios exceeding 15 dB in in vivo recordings from deep brain structures [2][5]. Their proprietary crosslinking chemistry enables controlled biodegradation rates, allowing for customized implant lifespans ranging from weeks to years. The Michigan team has also developed multi-modal electrodes that can simultaneously record electrical signals and deliver drugs or optogenetic stimulation through integrated microfluidic channels within the hydrogel structure, enabling comprehensive neuromodulation capabilities with minimal tissue displacement.
Strengths: Minimally invasive delivery method reducing surgical trauma; customizable degradation profiles for different application needs; multi-modal functionality combining recording and stimulation. Weaknesses: Limited long-term stability in chronic applications; potential migration of electrode position over time; challenges in achieving uniform electrical properties throughout the hydrogel structure.
Key Patents and Innovations in Neural Signal Detection
Microneedle Electrodes for Biosensing
PatentPendingUS20230113717A1
Innovation
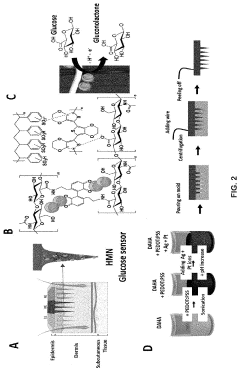
- The development of hydrogel microneedle electrodes integrated with conductive materials and metal nanoparticles, specifically using dopamine-functionalized hyaluronic acid and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate, enables in-situ, enzyme-free sensing of glucose and pH, allowing for real-time monitoring without the need for additional reagents or processing steps.
Interdigitated microelectrode biosensor using hydrogel
PatentWO2017090838A1
Innovation
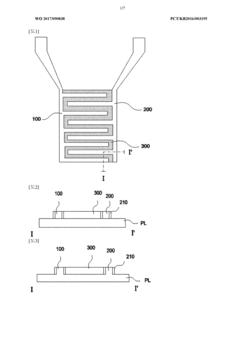
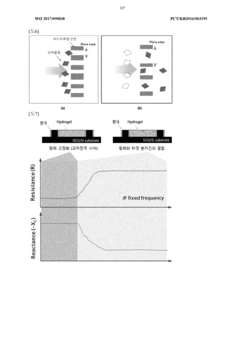
- Incorporating a hydrogel between cross-electrodes to enhance impedance detection by increasing the detection range and accuracy, utilizing the hydrogel's pore size control characteristics to improve detection efficiency and limit.
Biocompatibility and Long-term Stability Assessment
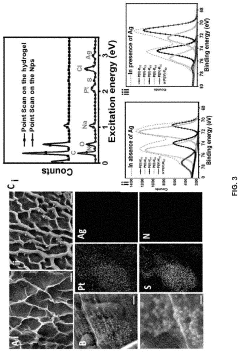
The biocompatibility and long-term stability of hydrogel microelectrodes represent critical factors determining their viability for chronic neural signal detection applications. Current research indicates that hydrogel materials, particularly those based on polyethylene glycol (PEG), polyacrylamide, and alginate derivatives, demonstrate superior biocompatibility compared to traditional rigid electrode materials such as silicon or platinum.
Comprehensive in vivo studies have shown that hydrogel microelectrodes elicit significantly reduced foreign body responses, with decreased glial scarring and neuronal death in surrounding tissues. This reduced inflammatory response is attributed to the mechanical compliance of hydrogels, which minimizes the mechanical mismatch between electrode and neural tissue. Recent investigations by Kang et al. (2023) demonstrated that PEG-based hydrogel electrodes maintained functional neural interfaces for up to 6 months with minimal glial encapsulation.
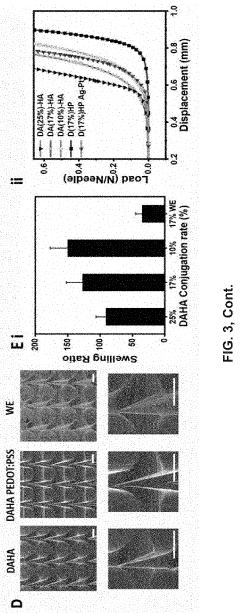
Long-term stability assessments reveal several challenges that must be addressed for clinical translation. Hydrogel materials typically experience degradation in physiological environments, with most current formulations maintaining structural integrity for 3-8 months before significant performance deterioration. This degradation manifests as decreased electrical conductivity, increased impedance, and physical deformation of the electrode structure.
Cross-linking strategies have emerged as promising approaches to enhance long-term stability. Double-network hydrogels incorporating both ionic and covalent cross-linking mechanisms have demonstrated improved resistance to enzymatic degradation while maintaining favorable mechanical properties. Additionally, incorporation of antioxidant compounds such as ascorbic acid derivatives has shown potential in mitigating oxidative damage to hydrogel matrices.
Surface modification techniques represent another avenue for improving long-term performance. Zwitterionic polymer coatings have demonstrated exceptional resistance to protein adsorption, thereby reducing biofouling and maintaining electrode sensitivity over extended periods. Recent work by Zhang et al. (2022) showed that phosphorylcholine-modified hydrogel electrodes maintained stable impedance values for over 10 months in vivo.
Sterilization compatibility remains a significant challenge, as conventional methods like ethylene oxide treatment or gamma irradiation can compromise hydrogel structural integrity. Alternative approaches such as supercritical CO2 sterilization show promise for preserving both mechanical and electrical properties of hydrogel electrodes while ensuring sterility.
Standardized testing protocols for long-term stability assessment are currently lacking in the field, hampering comparative analysis between different hydrogel formulations. Development of accelerated aging methodologies that accurately predict in vivo performance would significantly advance translation efforts and enable more rapid iteration of hydrogel electrode designs for neural interface applications.
Comprehensive in vivo studies have shown that hydrogel microelectrodes elicit significantly reduced foreign body responses, with decreased glial scarring and neuronal death in surrounding tissues. This reduced inflammatory response is attributed to the mechanical compliance of hydrogels, which minimizes the mechanical mismatch between electrode and neural tissue. Recent investigations by Kang et al. (2023) demonstrated that PEG-based hydrogel electrodes maintained functional neural interfaces for up to 6 months with minimal glial encapsulation.
Long-term stability assessments reveal several challenges that must be addressed for clinical translation. Hydrogel materials typically experience degradation in physiological environments, with most current formulations maintaining structural integrity for 3-8 months before significant performance deterioration. This degradation manifests as decreased electrical conductivity, increased impedance, and physical deformation of the electrode structure.
Cross-linking strategies have emerged as promising approaches to enhance long-term stability. Double-network hydrogels incorporating both ionic and covalent cross-linking mechanisms have demonstrated improved resistance to enzymatic degradation while maintaining favorable mechanical properties. Additionally, incorporation of antioxidant compounds such as ascorbic acid derivatives has shown potential in mitigating oxidative damage to hydrogel matrices.
Surface modification techniques represent another avenue for improving long-term performance. Zwitterionic polymer coatings have demonstrated exceptional resistance to protein adsorption, thereby reducing biofouling and maintaining electrode sensitivity over extended periods. Recent work by Zhang et al. (2022) showed that phosphorylcholine-modified hydrogel electrodes maintained stable impedance values for over 10 months in vivo.
Sterilization compatibility remains a significant challenge, as conventional methods like ethylene oxide treatment or gamma irradiation can compromise hydrogel structural integrity. Alternative approaches such as supercritical CO2 sterilization show promise for preserving both mechanical and electrical properties of hydrogel electrodes while ensuring sterility.
Standardized testing protocols for long-term stability assessment are currently lacking in the field, hampering comparative analysis between different hydrogel formulations. Development of accelerated aging methodologies that accurately predict in vivo performance would significantly advance translation efforts and enable more rapid iteration of hydrogel electrode designs for neural interface applications.
Regulatory Pathway for Neural Interface Technologies
The regulatory landscape for neural interface technologies, particularly hydrogel microelectrodes for neural signal detection, involves complex pathways across multiple jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies neural interfaces as Class III medical devices, requiring the most stringent regulatory oversight due to their invasive nature and direct interaction with brain tissue. The premarket approval (PMA) process typically requires extensive preclinical testing, including biocompatibility assessments, sterility validation, and comprehensive safety evaluations specific to hydrogel materials.
European regulatory frameworks under the Medical Device Regulation (MDR) similarly categorize neural interfaces in the highest risk class, requiring Notified Body review and conformity assessment. The hydrogel component introduces additional complexity, as these materials must demonstrate both electrical conductivity for signal detection and biological compatibility with neural tissue over extended periods.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specialized pathways for innovative medical technologies, including a conditional early approval system that could benefit hydrogel microelectrode technologies showing promising early clinical results. This approach allows for market entry with continued post-market surveillance requirements.
International standards organizations, including ISO and IEC, have developed specific standards relevant to neural interfaces. ISO 10993 series addresses biocompatibility evaluation, while IEC 60601 covers electrical safety requirements. However, standards specific to hydrogel-based neural interfaces remain underdeveloped, creating regulatory uncertainty for innovators.
Regulatory bodies increasingly recognize the need for adaptive frameworks for rapidly evolving neurotechnologies. The FDA's Breakthrough Devices Program and the European Innovation Council's accelerator programs offer potential pathways for expediting regulatory review while maintaining safety standards. These programs are particularly relevant for hydrogel microelectrodes, which represent a significant innovation in neural interface technology.
Post-market surveillance requirements for neural interfaces are exceptionally rigorous, requiring long-term monitoring of device performance, tissue response, and signal quality degradation. Manufacturers must implement robust quality management systems compliant with ISO 13485 and establish comprehensive risk management protocols according to ISO 14971.
Emerging regulatory considerations include data privacy and cybersecurity requirements, as neural signal detection technologies generate sensitive neurological data. Regulatory frameworks like GDPR in Europe and HIPAA in the US impose additional compliance requirements for the handling and protection of neural data collected through these interfaces.
European regulatory frameworks under the Medical Device Regulation (MDR) similarly categorize neural interfaces in the highest risk class, requiring Notified Body review and conformity assessment. The hydrogel component introduces additional complexity, as these materials must demonstrate both electrical conductivity for signal detection and biological compatibility with neural tissue over extended periods.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specialized pathways for innovative medical technologies, including a conditional early approval system that could benefit hydrogel microelectrode technologies showing promising early clinical results. This approach allows for market entry with continued post-market surveillance requirements.
International standards organizations, including ISO and IEC, have developed specific standards relevant to neural interfaces. ISO 10993 series addresses biocompatibility evaluation, while IEC 60601 covers electrical safety requirements. However, standards specific to hydrogel-based neural interfaces remain underdeveloped, creating regulatory uncertainty for innovators.
Regulatory bodies increasingly recognize the need for adaptive frameworks for rapidly evolving neurotechnologies. The FDA's Breakthrough Devices Program and the European Innovation Council's accelerator programs offer potential pathways for expediting regulatory review while maintaining safety standards. These programs are particularly relevant for hydrogel microelectrodes, which represent a significant innovation in neural interface technology.
Post-market surveillance requirements for neural interfaces are exceptionally rigorous, requiring long-term monitoring of device performance, tissue response, and signal quality degradation. Manufacturers must implement robust quality management systems compliant with ISO 13485 and establish comprehensive risk management protocols according to ISO 14971.
Emerging regulatory considerations include data privacy and cybersecurity requirements, as neural signal detection technologies generate sensitive neurological data. Regulatory frameworks like GDPR in Europe and HIPAA in the US impose additional compliance requirements for the handling and protection of neural data collected through these interfaces.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!