Operando Characterization Methods For Electride Catalysts
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electride Catalysts Background and Research Objectives
Electride catalysts represent a revolutionary class of materials in heterogeneous catalysis, characterized by their unique electronic structure where electrons serve as anions. First discovered in the 1980s, these materials remained largely academic curiosities until the past decade, when their exceptional catalytic properties began attracting significant research attention. The defining feature of electrides is their loosely bound electrons localized in structural cavities, creating an unprecedented electronic environment that facilitates numerous catalytic reactions, particularly those involving electron transfer processes.
The evolution of electride catalysis has accelerated dramatically since 2012, when mayenite-based electrides (C12A7:e-) demonstrated remarkable activity for ammonia synthesis under mild conditions. This breakthrough sparked intensive research into various electride systems, including 2D electrides and intermetallic electrides, each offering unique catalytic properties. The field has expanded from primarily nitrogen activation to encompass hydrogen evolution, CO2 reduction, and various hydrogenation reactions.
Current research objectives in electride catalysis focus on several critical areas. First, understanding the fundamental relationship between the electride's electronic structure and its catalytic performance remains paramount. This includes elucidating how the spatial distribution and energetics of anionic electrons influence adsorption energetics and reaction pathways. Second, developing stable electride catalysts capable of withstanding harsh reaction conditions presents a significant challenge, as many electrides exhibit sensitivity to air, moisture, and elevated temperatures.
Another key objective involves expanding the synthetic diversity of electride materials. While C12A7:e- and Ca2N represent well-studied systems, exploring new compositional spaces could yield electrides with enhanced stability and selectivity. Additionally, researchers aim to bridge the gap between theoretical predictions and experimental observations through advanced characterization techniques that can probe the dynamic behavior of anionic electrons during catalytic processes.
The ultimate goal of electride catalyst research is to develop industrially viable catalytic systems that can address global challenges in energy conversion and chemical production. This includes creating electride-based catalysts for ammonia synthesis that operate at near-ambient conditions, thereby potentially revolutionizing fertilizer production. Similarly, electrides show promise for sustainable hydrogen production and carbon dioxide utilization, aligning with broader objectives in green chemistry and renewable energy technologies.
The evolution of electride catalysis has accelerated dramatically since 2012, when mayenite-based electrides (C12A7:e-) demonstrated remarkable activity for ammonia synthesis under mild conditions. This breakthrough sparked intensive research into various electride systems, including 2D electrides and intermetallic electrides, each offering unique catalytic properties. The field has expanded from primarily nitrogen activation to encompass hydrogen evolution, CO2 reduction, and various hydrogenation reactions.
Current research objectives in electride catalysis focus on several critical areas. First, understanding the fundamental relationship between the electride's electronic structure and its catalytic performance remains paramount. This includes elucidating how the spatial distribution and energetics of anionic electrons influence adsorption energetics and reaction pathways. Second, developing stable electride catalysts capable of withstanding harsh reaction conditions presents a significant challenge, as many electrides exhibit sensitivity to air, moisture, and elevated temperatures.
Another key objective involves expanding the synthetic diversity of electride materials. While C12A7:e- and Ca2N represent well-studied systems, exploring new compositional spaces could yield electrides with enhanced stability and selectivity. Additionally, researchers aim to bridge the gap between theoretical predictions and experimental observations through advanced characterization techniques that can probe the dynamic behavior of anionic electrons during catalytic processes.
The ultimate goal of electride catalyst research is to develop industrially viable catalytic systems that can address global challenges in energy conversion and chemical production. This includes creating electride-based catalysts for ammonia synthesis that operate at near-ambient conditions, thereby potentially revolutionizing fertilizer production. Similarly, electrides show promise for sustainable hydrogen production and carbon dioxide utilization, aligning with broader objectives in green chemistry and renewable energy technologies.
Market Applications and Demand Analysis for Electride Catalysts
The global market for electride catalysts is experiencing significant growth driven by increasing demand for sustainable and energy-efficient chemical processes. These novel materials, characterized by their unique electron-donating properties, are positioned to revolutionize several key industrial sectors including ammonia synthesis, hydrogen production, and carbon dioxide conversion. Current market estimates suggest that the catalyst market relevant to these applications exceeds $25 billion annually, with electride catalysts poised to capture an expanding share.
In the ammonia synthesis sector, electride catalysts offer a compelling alternative to traditional Haber-Bosch processes, potentially reducing energy requirements by up to 40%. This represents a substantial opportunity given that ammonia production consumes approximately 1-2% of global energy and serves as the foundation for fertilizer manufacturing. The agricultural sector's growing need for more sustainable fertilizer production methods is creating strong pull for these advanced catalytic technologies.
Hydrogen economy initiatives worldwide are further driving demand for electride catalysts. With global hydrogen production projected to increase tenfold by 2050 according to the Hydrogen Council, efficient catalytic materials for water splitting and hydrogen evolution reactions are becoming increasingly valuable. Electride catalysts demonstrate superior performance in these applications, achieving higher conversion rates at lower energy inputs compared to conventional alternatives.
The petrochemical industry represents another significant market, where electride catalysts show promise for more efficient hydrogenation reactions and C-H bond activations. This sector's continuous pursuit of process intensification and reduced energy consumption aligns perfectly with the capabilities of these advanced materials.
Environmental applications constitute a rapidly expanding market segment, particularly in carbon dioxide conversion and utilization technologies. As carbon pricing mechanisms become more widespread, the economic incentives for efficient CO2 conversion catalysts are strengthening. Electride catalysts' ability to activate stable molecules like CO2 under milder conditions presents a compelling value proposition in this emerging market.
Regional analysis indicates that Asia-Pacific currently leads in electride catalyst research and development, with Japan, China, and South Korea making substantial investments. However, North America and Europe are rapidly accelerating their activities in this space, driven by decarbonization policies and green chemistry initiatives.
Market forecasts suggest compound annual growth rates exceeding 15% for specialized catalysts in renewable energy applications, with electride catalysts positioned to capture significant market share due to their performance advantages. This growth trajectory is further supported by increasing industrial focus on circular economy principles and sustainable manufacturing processes.
In the ammonia synthesis sector, electride catalysts offer a compelling alternative to traditional Haber-Bosch processes, potentially reducing energy requirements by up to 40%. This represents a substantial opportunity given that ammonia production consumes approximately 1-2% of global energy and serves as the foundation for fertilizer manufacturing. The agricultural sector's growing need for more sustainable fertilizer production methods is creating strong pull for these advanced catalytic technologies.
Hydrogen economy initiatives worldwide are further driving demand for electride catalysts. With global hydrogen production projected to increase tenfold by 2050 according to the Hydrogen Council, efficient catalytic materials for water splitting and hydrogen evolution reactions are becoming increasingly valuable. Electride catalysts demonstrate superior performance in these applications, achieving higher conversion rates at lower energy inputs compared to conventional alternatives.
The petrochemical industry represents another significant market, where electride catalysts show promise for more efficient hydrogenation reactions and C-H bond activations. This sector's continuous pursuit of process intensification and reduced energy consumption aligns perfectly with the capabilities of these advanced materials.
Environmental applications constitute a rapidly expanding market segment, particularly in carbon dioxide conversion and utilization technologies. As carbon pricing mechanisms become more widespread, the economic incentives for efficient CO2 conversion catalysts are strengthening. Electride catalysts' ability to activate stable molecules like CO2 under milder conditions presents a compelling value proposition in this emerging market.
Regional analysis indicates that Asia-Pacific currently leads in electride catalyst research and development, with Japan, China, and South Korea making substantial investments. However, North America and Europe are rapidly accelerating their activities in this space, driven by decarbonization policies and green chemistry initiatives.
Market forecasts suggest compound annual growth rates exceeding 15% for specialized catalysts in renewable energy applications, with electride catalysts positioned to capture significant market share due to their performance advantages. This growth trajectory is further supported by increasing industrial focus on circular economy principles and sustainable manufacturing processes.
Current Operando Characterization Challenges and Limitations
Despite significant advancements in electride catalyst research, operando characterization methods face substantial technical challenges that limit comprehensive understanding of these materials under working conditions. The extreme sensitivity of electrides to air and moisture presents a fundamental obstacle, requiring sophisticated environmental control systems during measurements. Even brief exposure can irreversibly alter their electronic structure, compromising data integrity and reproducibility.
Spatial resolution limitations represent another critical barrier. Current techniques struggle to capture the heterogeneous nature of active sites on electride surfaces, particularly at the nanoscale where catalytic reactions primarily occur. This resolution gap prevents researchers from establishing clear structure-property relationships essential for rational catalyst design.
Temporal resolution constraints further complicate characterization efforts. Many catalytic processes on electride surfaces occur on microsecond to nanosecond timescales, while conventional spectroscopic methods often operate at significantly slower acquisition rates. This mismatch results in averaged data that obscures transient intermediate states crucial for mechanizing reaction pathways.
The complex electronic structure of electrides poses unique analytical challenges. Traditional characterization techniques were developed for conventional materials and often fail to accurately capture the distinctive electron localization patterns in electride cavities. This fundamental incompatibility necessitates new methodological approaches specifically tailored to these unconventional electronic configurations.
In-situ and operando measurements under realistic reaction conditions introduce additional complications. High temperatures, pressures, and reactive environments required for catalytic processes can interfere with measurement accuracy and equipment durability. The integration of spectroscopic techniques with reaction cells that maintain these conditions while allowing sufficient signal collection remains technically demanding.
Data interpretation presents perhaps the most significant intellectual challenge. The multidimensional datasets generated from operando measurements require sophisticated computational approaches for meaningful analysis. Current analytical frameworks often struggle to deconvolute overlapping spectral features and correlate them with specific catalytic mechanisms.
Standardization across the field is notably lacking, with different research groups employing varied experimental setups and protocols. This inconsistency complicates direct comparison between studies and hinders collective progress toward unified mechanistic understanding. Establishing community-wide best practices for operando characterization of electride catalysts represents an urgent need for advancing this promising research direction.
Spatial resolution limitations represent another critical barrier. Current techniques struggle to capture the heterogeneous nature of active sites on electride surfaces, particularly at the nanoscale where catalytic reactions primarily occur. This resolution gap prevents researchers from establishing clear structure-property relationships essential for rational catalyst design.
Temporal resolution constraints further complicate characterization efforts. Many catalytic processes on electride surfaces occur on microsecond to nanosecond timescales, while conventional spectroscopic methods often operate at significantly slower acquisition rates. This mismatch results in averaged data that obscures transient intermediate states crucial for mechanizing reaction pathways.
The complex electronic structure of electrides poses unique analytical challenges. Traditional characterization techniques were developed for conventional materials and often fail to accurately capture the distinctive electron localization patterns in electride cavities. This fundamental incompatibility necessitates new methodological approaches specifically tailored to these unconventional electronic configurations.
In-situ and operando measurements under realistic reaction conditions introduce additional complications. High temperatures, pressures, and reactive environments required for catalytic processes can interfere with measurement accuracy and equipment durability. The integration of spectroscopic techniques with reaction cells that maintain these conditions while allowing sufficient signal collection remains technically demanding.
Data interpretation presents perhaps the most significant intellectual challenge. The multidimensional datasets generated from operando measurements require sophisticated computational approaches for meaningful analysis. Current analytical frameworks often struggle to deconvolute overlapping spectral features and correlate them with specific catalytic mechanisms.
Standardization across the field is notably lacking, with different research groups employing varied experimental setups and protocols. This inconsistency complicates direct comparison between studies and hinders collective progress toward unified mechanistic understanding. Establishing community-wide best practices for operando characterization of electride catalysts represents an urgent need for advancing this promising research direction.
State-of-the-Art Operando Characterization Methods
01 Spectroscopic techniques for electride catalyst characterization
Various spectroscopic methods are employed to characterize the electronic structure and properties of electride catalysts. These include X-ray photoelectron spectroscopy (XPS) to analyze surface composition and oxidation states, UV-visible spectroscopy to study optical properties and band gaps, and electron spin resonance (ESR) to detect unpaired electrons. These techniques provide crucial information about the electronic configuration of electrides, which is essential for understanding their catalytic mechanisms.- Spectroscopic techniques for electride catalyst characterization: Various spectroscopic methods are employed to characterize the electronic structure and surface properties of electride catalysts. These include X-ray photoelectron spectroscopy (XPS) to analyze electron binding energies and oxidation states, UV-visible spectroscopy to study optical properties and band gaps, and infrared spectroscopy to identify functional groups and surface species. These techniques provide crucial information about the electron localization and chemical environment in electride materials, which directly correlates with their catalytic performance.
- Electron microscopy and structural analysis methods: Electron microscopy techniques are essential for characterizing the morphology and structure of electride catalysts at nanoscale resolution. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) reveal particle size, shape, and distribution, while high-resolution TEM can visualize crystal lattice structures. Energy-dispersive X-ray spectroscopy (EDX) coupled with electron microscopy provides elemental mapping and composition analysis. These structural characterization methods help establish relationships between the catalyst's physical structure and its catalytic activity.
- Electrochemical characterization techniques: Electrochemical methods are crucial for evaluating the performance and properties of electride catalysts. Cyclic voltammetry measures redox properties and electron transfer kinetics, while electrochemical impedance spectroscopy analyzes interface properties and charge transfer resistance. Chronoamperometry and chronopotentiometry assess stability and durability under operating conditions. These techniques provide insights into the catalytic activity, reaction mechanisms, and electron donation capabilities of electride materials, which are fundamental to their application in various catalytic processes.
- Surface area and porosity measurement methods: Characterization of surface area and porosity is essential for understanding electride catalyst performance, as these properties directly influence catalytic activity through accessible active sites. Brunauer-Emmett-Teller (BET) analysis quantifies specific surface area, while Barrett-Joyner-Halenda (BJH) method determines pore size distribution. Temperature-programmed techniques such as desorption, reduction, and oxidation provide information about surface reactivity and adsorption properties. These methods help optimize catalyst design by establishing correlations between surface characteristics and catalytic efficiency.
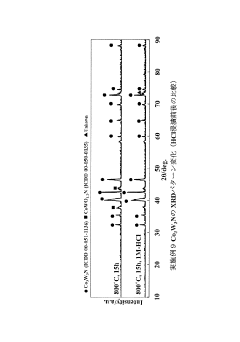
- Computational and advanced analytical methods: Advanced computational and analytical techniques provide deeper insights into electride catalyst properties and mechanisms. Density functional theory (DFT) calculations model electronic structures and predict catalytic behavior. Synchrotron-based X-ray techniques, including X-ray absorption spectroscopy (XAS) and X-ray diffraction (XRD), offer high-resolution structural and electronic information. In-situ and operando characterization methods allow real-time observation of catalysts under reaction conditions, revealing dynamic changes during catalytic processes. These sophisticated approaches enable rational design and optimization of electride catalysts for specific applications.
02 Microscopy and imaging methods for electride structure analysis
Advanced microscopy techniques are used to visualize and analyze the structural properties of electride catalysts at various scales. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) reveal surface morphology and particle size distribution, while atomic force microscopy (AFM) provides information about surface topography at the nanoscale. These imaging methods help researchers understand the relationship between the structural features of electrides and their catalytic performance.Expand Specific Solutions03 Electrochemical characterization methods for electride catalysts
Electrochemical techniques are essential for evaluating the performance and properties of electride catalysts. Cyclic voltammetry measures redox properties and catalytic activity, while impedance spectroscopy provides insights into charge transfer processes and interface properties. Chronoamperometry and chronopotentiometry are used to assess stability and durability under operating conditions. These methods help optimize electride catalysts for specific applications by understanding their electron transfer capabilities.Expand Specific Solutions04 Computational and modeling approaches for electride characterization
Computational methods complement experimental techniques in characterizing electride catalysts. Density functional theory (DFT) calculations predict electronic structures and catalytic activity, while molecular dynamics simulations model behavior under reaction conditions. Machine learning algorithms analyze complex datasets to identify structure-property relationships. These computational approaches accelerate the development of new electride catalysts by providing insights that may be difficult to obtain through experiments alone.Expand Specific Solutions05 In-situ and operando characterization techniques for electride catalysts
In-situ and operando characterization methods allow for real-time monitoring of electride catalysts under working conditions. Techniques such as in-situ X-ray diffraction (XRD) track structural changes during catalysis, while operando infrared spectroscopy identifies reaction intermediates. Environmental transmission electron microscopy (ETEM) observes morphological changes under reaction conditions. These advanced methods provide valuable insights into the dynamic behavior of electride catalysts, helping to bridge the gap between fundamental understanding and practical applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
The operando characterization of electride catalysts is currently in an early development stage, with significant research momentum building across academic and industrial sectors. The market is expanding rapidly due to increasing demand for advanced catalytic materials in energy conversion and chemical synthesis, though still relatively niche compared to established catalyst technologies. Leading research institutions like Dalian Institute of Chemical Physics, Centre National de la Recherche Scientifique, and Tsinghua University are advancing fundamental understanding, while industrial players including Toyota, Asahi Kasei, and Sumitomo Chemical are focusing on commercial applications. The technology maturity varies significantly, with academic research demonstrating promising proof-of-concept results, but industrial implementation remains limited by challenges in operando characterization techniques and scale-up processes.
Dalian Institute of Chemical Physics Chinese Academy of Sci
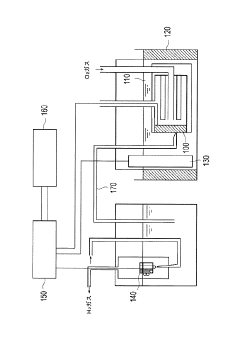
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced operando characterization methods for electride catalysts focusing on in-situ X-ray absorption spectroscopy (XAS) and environmental transmission electron microscopy (ETEM). Their approach combines time-resolved XAS with differential electrochemical mass spectrometry to monitor electronic structure changes and reaction intermediates during catalytic processes. DICP has pioneered the use of synchrotron radiation facilities to perform operando XANES and EXAFS measurements under realistic reaction conditions, allowing for direct observation of electron transfer between electride materials and reactant molecules[1]. Their specialized sample cells maintain ultra-high vacuum conditions while enabling precise control of temperature and gas environment, critical for studying the unique electron donation properties of electride catalysts in ammonia synthesis and CO2 reduction reactions[3].
Strengths: Superior integration of multiple characterization techniques allowing for comprehensive understanding of reaction mechanisms; access to advanced synchrotron facilities enabling high-resolution structural analysis. Weakness: Highly specialized equipment requirements limit widespread application; complex data interpretation necessitates advanced computational modeling support.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei has developed proprietary operando characterization methods for electride catalysts centered on their C12A7 (12CaO·7Al2O3) electride materials. Their approach combines in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) with simultaneous mass spectrometry and electrical conductivity measurements in custom-designed reaction chambers. This integrated system allows real-time monitoring of surface species, product formation, and electronic property changes during catalytic reactions. Asahi Kasei's methodology incorporates specialized sample holders that maintain precise temperature control while allowing for controlled exposure to reactant gases and application of electrical potentials[2]. Their characterization platform includes capabilities for operando Raman spectroscopy with spatial mapping to identify active sites and track the migration of anionic electrons within the electride structure during catalytic processes, particularly for ammonia synthesis reactions where their C12A7 electrides have shown exceptional performance[4].
Strengths: Highly specialized expertise with C12A7 electride materials; integrated multi-technique approach provides comprehensive mechanistic insights. Weaknesses: Methodology optimized primarily for their proprietary materials; limited applicability to broader electride catalyst families; requires significant expertise in multiple analytical techniques.
Key Technical Innovations in Electride Catalyst Analysis
Electrode catalyst and manufacturing method thereof
PatentActiveJP2016062826A
Innovation
- Employing metal nitrides containing molybdenum (Mo) or tungsten (W) as the catalyst, which are chemically stable and exhibit excellent catalytic activity in acidic to alkaline electrolytes, produced via a precursor oxide nitridation process.
Instrumentation Requirements and Development Roadmap
Effective operando characterization of electride catalysts requires sophisticated instrumentation that can monitor catalytic processes in real-time under reaction conditions. Current instrumentation setups typically combine spectroscopic techniques with electrochemical cells, but significant limitations exist in terms of spatial resolution, temporal sensitivity, and compatibility with harsh reaction environments.
The immediate instrumentation needs include advanced in-situ X-ray absorption spectroscopy (XAS) systems with improved energy resolution below 0.1 eV to better capture electronic structure changes during catalysis. Synchrotron-based facilities currently offer the best capabilities, but development of laboratory-scale XAS systems with comparable resolution would dramatically increase accessibility for routine catalyst development.
Environmental transmission electron microscopy (E-TEM) systems require enhancement to maintain atomic resolution while operating at higher pressures (>1 atm) and temperatures relevant to industrial catalytic processes. Current E-TEM systems struggle to maintain resolution above 100 mbar, limiting their applicability for realistic reaction conditions.
For surface-sensitive characterization, ambient pressure X-ray photoelectron spectroscopy (AP-XPS) systems need further development to operate at higher pressures while maintaining energy resolution. The roadmap should prioritize increasing pressure capabilities from current ~30 mbar to >100 mbar within five years.
Integration of multiple characterization techniques into single platforms represents a critical development path. Combined spectroscopic-microscopic systems that can simultaneously capture structural and electronic information would provide unprecedented insights into electride catalyst function. Initial prototypes combining Raman spectroscopy with scanning probe microscopy show promise but require further refinement.
Data acquisition and processing infrastructure must evolve alongside hardware improvements. Machine learning algorithms specifically trained for operando data interpretation could significantly accelerate analysis of the complex, multimodal datasets generated during characterization. Development of standardized data formats and automated analysis pipelines should be prioritized within the next three years.
The instrumentation development roadmap should follow a phased approach: near-term (1-2 years) focusing on improving existing technologies; mid-term (3-5 years) developing integrated characterization platforms; and long-term (5-10 years) creating next-generation systems capable of atomic-scale spatial resolution under true operating conditions with millisecond temporal resolution.
The immediate instrumentation needs include advanced in-situ X-ray absorption spectroscopy (XAS) systems with improved energy resolution below 0.1 eV to better capture electronic structure changes during catalysis. Synchrotron-based facilities currently offer the best capabilities, but development of laboratory-scale XAS systems with comparable resolution would dramatically increase accessibility for routine catalyst development.
Environmental transmission electron microscopy (E-TEM) systems require enhancement to maintain atomic resolution while operating at higher pressures (>1 atm) and temperatures relevant to industrial catalytic processes. Current E-TEM systems struggle to maintain resolution above 100 mbar, limiting their applicability for realistic reaction conditions.
For surface-sensitive characterization, ambient pressure X-ray photoelectron spectroscopy (AP-XPS) systems need further development to operate at higher pressures while maintaining energy resolution. The roadmap should prioritize increasing pressure capabilities from current ~30 mbar to >100 mbar within five years.
Integration of multiple characterization techniques into single platforms represents a critical development path. Combined spectroscopic-microscopic systems that can simultaneously capture structural and electronic information would provide unprecedented insights into electride catalyst function. Initial prototypes combining Raman spectroscopy with scanning probe microscopy show promise but require further refinement.
Data acquisition and processing infrastructure must evolve alongside hardware improvements. Machine learning algorithms specifically trained for operando data interpretation could significantly accelerate analysis of the complex, multimodal datasets generated during characterization. Development of standardized data formats and automated analysis pipelines should be prioritized within the next three years.
The instrumentation development roadmap should follow a phased approach: near-term (1-2 years) focusing on improving existing technologies; mid-term (3-5 years) developing integrated characterization platforms; and long-term (5-10 years) creating next-generation systems capable of atomic-scale spatial resolution under true operating conditions with millisecond temporal resolution.
Environmental Impact and Sustainability Considerations
The development and implementation of electride catalysts present significant environmental implications that must be thoroughly evaluated within the broader context of sustainable chemical processes. Electride catalysts offer promising pathways for more energy-efficient chemical transformations, potentially reducing the carbon footprint of various industrial processes compared to conventional catalytic systems. Their unique electron-donating properties enable reactions to proceed under milder conditions, thereby decreasing energy requirements and associated greenhouse gas emissions.
When assessing the environmental impact of operando characterization methods for electride catalysts, it is essential to consider the entire lifecycle of these materials. The synthesis of electride catalysts often involves rare earth elements or transition metals, whose extraction and processing can lead to substantial environmental degradation, including habitat destruction, water pollution, and high energy consumption. Sustainable sourcing strategies and recycling protocols must be developed to mitigate these concerns.
The operando characterization techniques themselves vary considerably in their environmental footprints. Advanced synchrotron-based methods require large-scale facilities with significant energy demands, while laboratory-scale spectroscopic approaches may have lower immediate impacts but limited analytical capabilities. Balancing analytical power with environmental considerations represents a critical challenge for researchers in this field.
Water consumption during catalyst preparation and characterization also warrants attention, particularly as many electride materials are moisture-sensitive. Closed-loop systems for water recycling and treatment of chemical waste streams from characterization processes should be implemented to minimize environmental burden. Additionally, the potential release of nanoparticulate matter during handling and characterization poses risks that require appropriate containment and filtration systems.
From a sustainability perspective, electride catalysts offer promising advantages through their potential to enable chemical transformations with atom economy and reduced waste generation. Their application in renewable energy technologies, such as water splitting for hydrogen production and CO2 reduction, aligns with global sustainability goals. However, the stability and longevity of these catalysts under operating conditions directly impact their sustainability profile, as frequent replacement would diminish their environmental benefits.
Future research directions should prioritize the development of operando characterization methods that not only provide mechanistic insights but also operate with minimal environmental impact. This includes designing more energy-efficient instrumentation, utilizing renewable energy sources for power-intensive techniques, and exploring greener alternatives to traditional characterization reagents and solvents. Comprehensive life cycle assessments should become standard practice when evaluating new electride catalyst systems and their characterization methodologies.
When assessing the environmental impact of operando characterization methods for electride catalysts, it is essential to consider the entire lifecycle of these materials. The synthesis of electride catalysts often involves rare earth elements or transition metals, whose extraction and processing can lead to substantial environmental degradation, including habitat destruction, water pollution, and high energy consumption. Sustainable sourcing strategies and recycling protocols must be developed to mitigate these concerns.
The operando characterization techniques themselves vary considerably in their environmental footprints. Advanced synchrotron-based methods require large-scale facilities with significant energy demands, while laboratory-scale spectroscopic approaches may have lower immediate impacts but limited analytical capabilities. Balancing analytical power with environmental considerations represents a critical challenge for researchers in this field.
Water consumption during catalyst preparation and characterization also warrants attention, particularly as many electride materials are moisture-sensitive. Closed-loop systems for water recycling and treatment of chemical waste streams from characterization processes should be implemented to minimize environmental burden. Additionally, the potential release of nanoparticulate matter during handling and characterization poses risks that require appropriate containment and filtration systems.
From a sustainability perspective, electride catalysts offer promising advantages through their potential to enable chemical transformations with atom economy and reduced waste generation. Their application in renewable energy technologies, such as water splitting for hydrogen production and CO2 reduction, aligns with global sustainability goals. However, the stability and longevity of these catalysts under operating conditions directly impact their sustainability profile, as frequent replacement would diminish their environmental benefits.
Future research directions should prioritize the development of operando characterization methods that not only provide mechanistic insights but also operate with minimal environmental impact. This includes designing more energy-efficient instrumentation, utilizing renewable energy sources for power-intensive techniques, and exploring greener alternatives to traditional characterization reagents and solvents. Comprehensive life cycle assessments should become standard practice when evaluating new electride catalyst systems and their characterization methodologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!