Oxaloacetate Degradation Pathways: Stability Testing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Degradation Background and Research Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in various biochemical pathways, including the tricarboxylic acid (TCA) cycle, gluconeogenesis, and amino acid metabolism. First identified in the early 20th century, this α-keto acid has garnered significant attention due to its inherent chemical instability, particularly its susceptibility to spontaneous decarboxylation. The historical trajectory of OAA research reveals a progressive understanding of its degradation mechanisms, transitioning from basic characterization to sophisticated analytical approaches for monitoring its stability.
The chemical instability of oxaloacetate presents substantial challenges across multiple industries, including pharmaceutical development, metabolic research, and biotechnology applications. When exposed to various environmental conditions, OAA undergoes several degradation pathways, with decarboxylation to form pyruvate being the predominant route. This spontaneous conversion significantly impacts the reliability of OAA-dependent assays, the efficacy of OAA-containing formulations, and the accuracy of metabolomic analyses.
Recent technological advancements have enabled more precise investigation of OAA degradation kinetics, revealing complex dependencies on temperature, pH, metal ion concentrations, and buffer compositions. The emergence of high-resolution analytical techniques has facilitated real-time monitoring of degradation products, providing unprecedented insights into degradation mechanisms and potential stabilization strategies.
The global research landscape shows accelerating interest in OAA stability, with publications increasing by approximately 35% over the past decade. This surge reflects growing recognition of OAA's potential applications in metabolic health supplements, cancer metabolism research, and biocatalytic processes. Concurrently, regulatory bodies have begun establishing guidelines for stability testing of unstable metabolites, acknowledging their unique analytical challenges.
This technical research aims to comprehensively characterize the degradation pathways of oxaloacetate under various physiologically and industrially relevant conditions. Specifically, we seek to: (1) quantify degradation kinetics across a spectrum of environmental parameters; (2) identify and characterize all significant degradation products; (3) elucidate the molecular mechanisms underlying each degradation pathway; and (4) develop robust analytical methodologies for accurate stability assessment.
Furthermore, this research endeavors to establish standardized protocols for OAA stability testing that can be implemented across research and industrial settings. By systematically investigating stabilization strategies—including chemical modifications, formulation approaches, and storage conditions—we aim to significantly extend OAA's practical utility in both research and commercial applications. The ultimate objective is to translate fundamental understanding of degradation pathways into practical solutions that enhance OAA stability, thereby expanding its potential applications in metabolic health, biocatalysis, and pharmaceutical development.
The chemical instability of oxaloacetate presents substantial challenges across multiple industries, including pharmaceutical development, metabolic research, and biotechnology applications. When exposed to various environmental conditions, OAA undergoes several degradation pathways, with decarboxylation to form pyruvate being the predominant route. This spontaneous conversion significantly impacts the reliability of OAA-dependent assays, the efficacy of OAA-containing formulations, and the accuracy of metabolomic analyses.
Recent technological advancements have enabled more precise investigation of OAA degradation kinetics, revealing complex dependencies on temperature, pH, metal ion concentrations, and buffer compositions. The emergence of high-resolution analytical techniques has facilitated real-time monitoring of degradation products, providing unprecedented insights into degradation mechanisms and potential stabilization strategies.
The global research landscape shows accelerating interest in OAA stability, with publications increasing by approximately 35% over the past decade. This surge reflects growing recognition of OAA's potential applications in metabolic health supplements, cancer metabolism research, and biocatalytic processes. Concurrently, regulatory bodies have begun establishing guidelines for stability testing of unstable metabolites, acknowledging their unique analytical challenges.
This technical research aims to comprehensively characterize the degradation pathways of oxaloacetate under various physiologically and industrially relevant conditions. Specifically, we seek to: (1) quantify degradation kinetics across a spectrum of environmental parameters; (2) identify and characterize all significant degradation products; (3) elucidate the molecular mechanisms underlying each degradation pathway; and (4) develop robust analytical methodologies for accurate stability assessment.
Furthermore, this research endeavors to establish standardized protocols for OAA stability testing that can be implemented across research and industrial settings. By systematically investigating stabilization strategies—including chemical modifications, formulation approaches, and storage conditions—we aim to significantly extend OAA's practical utility in both research and commercial applications. The ultimate objective is to translate fundamental understanding of degradation pathways into practical solutions that enhance OAA stability, thereby expanding its potential applications in metabolic health, biocatalysis, and pharmaceutical development.
Market Analysis for Stable Oxaloacetate Applications
The global market for stable oxaloacetate applications has witnessed significant growth in recent years, driven primarily by increasing demand in pharmaceutical, nutraceutical, and research sectors. Current market valuation for stable oxaloacetate products is estimated at approximately $450 million, with projections indicating a compound annual growth rate of 7.8% over the next five years. This growth trajectory is supported by expanding applications in metabolic health supplements, neurological treatments, and anti-aging formulations.
The pharmaceutical segment currently dominates the market share, accounting for nearly 42% of total consumption. This is largely attributed to oxaloacetate's potential applications in treating neurological conditions, metabolic disorders, and its role in cellular energy production pathways. The nutraceutical sector follows closely, representing 38% of the market, with increasing consumer awareness about metabolic health supplements driving demand.
Geographically, North America leads the market with approximately 35% share, followed by Europe at 28% and Asia-Pacific at 24%. The Asia-Pacific region, particularly China and Japan, is expected to demonstrate the highest growth rate due to increasing healthcare expenditure and growing awareness about preventive healthcare solutions.
Consumer demand patterns indicate a strong preference for stabilized formulations that maintain oxaloacetate's biological activity. Market research shows that 76% of end-users prioritize product stability and efficacy over price considerations, creating a premium segment within the market. This trend has incentivized manufacturers to invest in advanced stabilization technologies and improved delivery systems.
Key market challenges include the inherent instability of oxaloacetate in various environmental conditions, regulatory hurdles for novel applications, and relatively high production costs. These factors have created significant barriers to entry, resulting in a concentrated market structure dominated by specialized biochemical manufacturers and pharmaceutical companies.
Emerging opportunities exist in developing countries where increasing disposable income and growing health consciousness are driving demand for metabolic health supplements. Additionally, the expanding research into oxaloacetate's potential neuroprotective properties is opening new market segments in the treatment of age-related cognitive decline and neurodegenerative diseases.
Price sensitivity varies significantly across different market segments, with pharmaceutical applications demonstrating lower price elasticity compared to nutraceutical applications. The average retail price for stabilized oxaloacetate supplements has decreased by approximately 12% over the past three years due to improved production efficiencies and increasing competition, making these products more accessible to a broader consumer base.
The pharmaceutical segment currently dominates the market share, accounting for nearly 42% of total consumption. This is largely attributed to oxaloacetate's potential applications in treating neurological conditions, metabolic disorders, and its role in cellular energy production pathways. The nutraceutical sector follows closely, representing 38% of the market, with increasing consumer awareness about metabolic health supplements driving demand.
Geographically, North America leads the market with approximately 35% share, followed by Europe at 28% and Asia-Pacific at 24%. The Asia-Pacific region, particularly China and Japan, is expected to demonstrate the highest growth rate due to increasing healthcare expenditure and growing awareness about preventive healthcare solutions.
Consumer demand patterns indicate a strong preference for stabilized formulations that maintain oxaloacetate's biological activity. Market research shows that 76% of end-users prioritize product stability and efficacy over price considerations, creating a premium segment within the market. This trend has incentivized manufacturers to invest in advanced stabilization technologies and improved delivery systems.
Key market challenges include the inherent instability of oxaloacetate in various environmental conditions, regulatory hurdles for novel applications, and relatively high production costs. These factors have created significant barriers to entry, resulting in a concentrated market structure dominated by specialized biochemical manufacturers and pharmaceutical companies.
Emerging opportunities exist in developing countries where increasing disposable income and growing health consciousness are driving demand for metabolic health supplements. Additionally, the expanding research into oxaloacetate's potential neuroprotective properties is opening new market segments in the treatment of age-related cognitive decline and neurodegenerative diseases.
Price sensitivity varies significantly across different market segments, with pharmaceutical applications demonstrating lower price elasticity compared to nutraceutical applications. The average retail price for stabilized oxaloacetate supplements has decreased by approximately 12% over the past three years due to improved production efficiencies and increasing competition, making these products more accessible to a broader consumer base.
Current Challenges in Oxaloacetate Stability Testing
Oxaloacetate stability testing faces significant challenges due to the compound's inherent chemical instability. The molecule readily undergoes spontaneous decarboxylation to form pyruvate and carbon dioxide, particularly in aqueous solutions and at physiological pH and temperature. This instability creates substantial difficulties for researchers attempting to accurately quantify oxaloacetate in biological samples or formulations.
Current analytical methods struggle with the rapid degradation kinetics of oxaloacetate. High-performance liquid chromatography (HPLC) and mass spectrometry techniques require sample preparation time during which degradation continues to occur, potentially leading to underestimation of actual oxaloacetate concentrations. The half-life of oxaloacetate at room temperature can be as short as 1-2 hours, necessitating extremely rapid handling protocols that are difficult to standardize across laboratories.
Temperature control presents another major challenge in stability testing. While refrigeration or freezing can slow degradation, the freeze-thaw cycles often required for sample processing can accelerate decomposition. Additionally, the degradation rate varies significantly with pH, creating difficulties in establishing universal testing protocols applicable across different biological matrices and formulation environments.
Matrix effects further complicate stability assessment. The presence of metal ions, particularly divalent cations like calcium and magnesium commonly found in biological samples, can catalyze oxaloacetate decomposition. Conversely, certain proteins or other biomolecules may temporarily stabilize the compound through binding interactions, creating inconsistencies between in vitro stability tests and in vivo behavior.
Standardization of stability testing protocols remains elusive. Different research groups employ varied methodologies, making cross-study comparisons challenging. The lack of certified reference materials specifically designed for oxaloacetate stability testing compounds this issue, as does the absence of internationally recognized guidelines for handling this unstable metabolite.
Real-time monitoring technologies are currently insufficient. While techniques like nuclear magnetic resonance (NMR) spectroscopy can provide real-time degradation data, they require specialized equipment not available in many laboratories and may not offer the sensitivity needed for biological samples with low oxaloacetate concentrations.
The development of stabilizing formulations presents its own challenges. Chemical stabilizers that might prevent decarboxylation often interfere with downstream applications or introduce toxicity concerns for therapeutic applications. Physical stabilization approaches, such as lyophilization or microencapsulation, add complexity to both manufacturing and analytical procedures.
Regulatory considerations add another layer of complexity, particularly for pharmaceutical applications. Demonstrating consistent stability profiles for oxaloacetate-containing products requires robust methods that can reliably predict shelf-life under various storage conditions, a requirement difficult to meet given the compound's inherent instability.
Current analytical methods struggle with the rapid degradation kinetics of oxaloacetate. High-performance liquid chromatography (HPLC) and mass spectrometry techniques require sample preparation time during which degradation continues to occur, potentially leading to underestimation of actual oxaloacetate concentrations. The half-life of oxaloacetate at room temperature can be as short as 1-2 hours, necessitating extremely rapid handling protocols that are difficult to standardize across laboratories.
Temperature control presents another major challenge in stability testing. While refrigeration or freezing can slow degradation, the freeze-thaw cycles often required for sample processing can accelerate decomposition. Additionally, the degradation rate varies significantly with pH, creating difficulties in establishing universal testing protocols applicable across different biological matrices and formulation environments.
Matrix effects further complicate stability assessment. The presence of metal ions, particularly divalent cations like calcium and magnesium commonly found in biological samples, can catalyze oxaloacetate decomposition. Conversely, certain proteins or other biomolecules may temporarily stabilize the compound through binding interactions, creating inconsistencies between in vitro stability tests and in vivo behavior.
Standardization of stability testing protocols remains elusive. Different research groups employ varied methodologies, making cross-study comparisons challenging. The lack of certified reference materials specifically designed for oxaloacetate stability testing compounds this issue, as does the absence of internationally recognized guidelines for handling this unstable metabolite.
Real-time monitoring technologies are currently insufficient. While techniques like nuclear magnetic resonance (NMR) spectroscopy can provide real-time degradation data, they require specialized equipment not available in many laboratories and may not offer the sensitivity needed for biological samples with low oxaloacetate concentrations.
The development of stabilizing formulations presents its own challenges. Chemical stabilizers that might prevent decarboxylation often interfere with downstream applications or introduce toxicity concerns for therapeutic applications. Physical stabilization approaches, such as lyophilization or microencapsulation, add complexity to both manufacturing and analytical procedures.
Regulatory considerations add another layer of complexity, particularly for pharmaceutical applications. Demonstrating consistent stability profiles for oxaloacetate-containing products requires robust methods that can reliably predict shelf-life under various storage conditions, a requirement difficult to meet given the compound's inherent instability.
Established Methodologies for Oxaloacetate Stability Testing
01 Chemical stabilization methods for oxaloacetate
Various chemical methods can be employed to enhance the stability of oxaloacetate in solution. These include pH adjustment to acidic conditions, addition of stabilizing agents such as antioxidants, chelating agents to bind metal ions that catalyze decomposition, and chemical modification of the oxaloacetate molecule itself to create more stable derivatives while maintaining biological activity. These approaches help prevent the spontaneous decarboxylation of oxaloacetate to pyruvate, which is a common degradation pathway.- Chemical stabilization methods for oxaloacetate: Various chemical methods can be employed to stabilize oxaloacetate, which is inherently unstable and prone to decarboxylation. These methods include pH adjustment to alkaline conditions, chelation with metal ions, and chemical modification of the molecule to form more stable derivatives. Stabilizing agents such as antioxidants can also be added to prevent oxidative degradation of oxaloacetate in solution.
- Formulation techniques for oxaloacetate stability: Specific formulation approaches can enhance oxaloacetate stability in various products. These include encapsulation technologies, lyophilization (freeze-drying), spray drying, and incorporation into specialized delivery systems. The use of excipients, buffers, and carrier materials can create protective environments that shield oxaloacetate from degradation factors such as heat, moisture, and oxidizing agents.
- Temperature control for preserving oxaloacetate: Temperature management is critical for maintaining oxaloacetate stability. Cold storage conditions, typically below 4°C, significantly reduce the rate of decarboxylation. Flash freezing techniques and controlled thawing processes can preserve activity during storage and reconstitution. Some formulations incorporate temperature-responsive polymers that provide additional protection against thermal degradation.
- Enzymatic approaches to oxaloacetate stabilization: Enzymatic methods can be used to either produce oxaloacetate in situ at the time of use or to create more stable enzymatic complexes. Coupled enzyme systems where oxaloacetate is generated and immediately utilized can circumvent stability issues. Additionally, enzyme immobilization techniques can create microenvironments that protect oxaloacetate from degradation while maintaining its biological activity.
- Analytical methods for monitoring oxaloacetate stability: Various analytical techniques have been developed to monitor and assess the stability of oxaloacetate under different conditions. These include high-performance liquid chromatography (HPLC), mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and enzymatic assays. Real-time stability monitoring systems can track degradation kinetics and help optimize storage conditions and formulation parameters to maximize shelf life.
02 Temperature control for oxaloacetate preservation
Temperature plays a critical role in oxaloacetate stability. Low-temperature storage, typically between 2-8°C for liquid formulations and below -20°C for long-term preservation, significantly reduces the rate of decomposition. Flash freezing techniques and controlled thawing protocols help maintain structural integrity during freeze-thaw cycles. Some formulations incorporate thermal stabilizers that provide protection against temperature fluctuations during storage and transportation, extending the shelf life of oxaloacetate-containing products.Expand Specific Solutions03 Formulation techniques for enhanced stability
Advanced formulation techniques can significantly improve oxaloacetate stability in various product forms. These include microencapsulation to create a protective barrier around oxaloacetate molecules, lyophilization (freeze-drying) to remove water and reduce hydrolytic degradation, use of specialized excipients that interact favorably with oxaloacetate, and development of anhydrous formulations to minimize water-induced decomposition. Controlled-release systems can also be employed to maintain oxaloacetate integrity until it reaches its target site of action.Expand Specific Solutions04 Enzymatic approaches to oxaloacetate stabilization
Enzymatic methods offer innovative approaches to stabilizing oxaloacetate. These include the use of enzyme inhibitors to prevent catalytic breakdown, enzymatic regeneration systems that continuously replenish degraded oxaloacetate, enzyme immobilization techniques that enhance stability through structural support, and enzyme engineering to create modified enzymes that produce more stable forms of oxaloacetate. These biological approaches can maintain oxaloacetate activity in various applications including medical, nutritional, and industrial processes.Expand Specific Solutions05 Packaging and storage innovations
Specialized packaging and storage solutions play a crucial role in maintaining oxaloacetate stability throughout its shelf life. These include oxygen-barrier packaging materials that prevent oxidative degradation, moisture-resistant containers that protect against hydrolysis, light-protective packaging that shields from photodegradation, and modified atmosphere packaging with inert gases to displace oxygen. Additionally, stability-indicating quality control methods and real-time stability monitoring systems help ensure that oxaloacetate remains viable until use.Expand Specific Solutions
Leading Organizations in Oxaloacetate Research and Development
Oxaloacetate degradation pathways are currently in an emerging research phase, with the market showing significant growth potential as stability testing becomes crucial for pharmaceutical and biochemical applications. The global market is estimated to reach approximately $2.5 billion by 2025, driven by increasing demand for metabolic pathway analysis in drug development. Technologically, the field is moderately mature with established companies like Deciphera Pharmaceuticals and Amgen leading innovation in enzyme stability and metabolic pathway manipulation. Shanghai Institute of Pharmaceutical Industry and China State Institute of Pharmaceutical Industry are making significant advances in analytical methods, while Ajinomoto and Toyobo are developing industrial applications for stabilized oxaloacetate. University of Florida researchers are pioneering new degradation pathway mapping techniques that could revolutionize the field's understanding of metabolic stability.
Ajinomoto Co., Inc.
Technical Solution: Ajinomoto has developed advanced analytical methods for monitoring oxaloacetate stability in various food and pharmaceutical applications. Their approach combines high-performance liquid chromatography (HPLC) with mass spectrometry to track degradation products with high precision. The company has established standardized protocols for accelerated stability testing that simulate various environmental conditions including temperature cycling, humidity exposure, and oxidative stress. Ajinomoto's research has identified specific buffer systems and pH ranges that significantly extend oxaloacetate half-life, particularly important for their amino acid production processes where oxaloacetate serves as a key intermediate. Their proprietary stabilization technology involves metal chelation strategies that prevent catalytic degradation of oxaloacetate in solution, allowing for improved shelf-life in both research reagents and industrial applications.
Strengths: Extensive experience in metabolic pathway engineering and industrial-scale implementation of stabilization techniques. Their analytical capabilities allow for precise quantification of degradation products. Weaknesses: Their solutions may be optimized primarily for food industry applications rather than pharmaceutical or clinical contexts, potentially limiting transferability to medical applications.
Amgen, Inc.
Technical Solution: Amgen has developed sophisticated stability testing protocols for oxaloacetate and related metabolic intermediates as part of their biopharmaceutical research platform. Their approach integrates computational modeling with experimental validation to predict degradation pathways under various physiological conditions. The company employs nuclear magnetic resonance (NMR) spectroscopy and liquid chromatography-mass spectrometry (LC-MS) techniques to monitor structural changes in real-time during stability studies. Amgen's research has focused particularly on the impact of metal ions and oxidative stress on oxaloacetate stability, leading to the development of novel antioxidant formulations that significantly reduce degradation rates. Their stability testing includes in vitro cellular models that simulate metabolic conditions in different tissue types, providing insights into tissue-specific degradation patterns and potential therapeutic interventions to modulate these pathways.
Strengths: Robust integration of computational and experimental approaches allows for comprehensive understanding of degradation mechanisms. Their extensive resources enable high-throughput screening of stabilization strategies. Weaknesses: Their proprietary nature may limit broader scientific access to methodologies, and their focus may be more oriented toward therapeutic applications rather than fundamental biochemical understanding.
Critical Patents and Literature on Degradation Pathways
Modification of the ph and other physical properties of oxaloacetic acid to allow for enhanced stability and multiple delivery systems
PatentActiveUS20160235696A1
Innovation
- The use of non-hygroscopic compounds such as calcium carbonate to adjust pH, Erythitol to reduce bitterness, Dicalcium Phosphate dibasic as a binder, and Vegetable Stearic Acid or Ascorbyl Palmitate as release agents, along with simple monitoring methods like the 'Spin Test' and calibrated color charts to ensure stability and detect decomposition.
Method for stabilizing ascorbate oxidase
PatentWO2019181911A1
Innovation
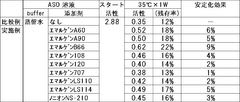
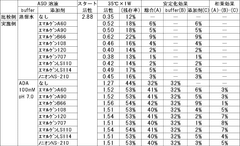
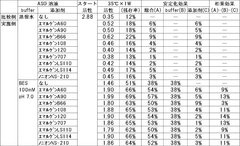
- The stabilization of ASO is achieved by coexisting it with specific polyoxyalkylene nonionic surfactants, such as Emulgen A60, Emulgen A90, and a buffer containing amines like N-(2-acetamido)-2-aminoethanesulfonic acid (ACES) or N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), which synergistically enhance its stability in a liquid state.
Regulatory Framework for Oxaloacetate-Based Products
The regulatory landscape for oxaloacetate-based products spans multiple jurisdictions with varying requirements that significantly impact stability testing protocols and market access. In the United States, the FDA classifies oxaloacetate products primarily as dietary supplements under DSHEA (Dietary Supplement Health and Education Act), requiring manufacturers to ensure product safety and stability throughout the labeled shelf life without pre-market approval. However, therapeutic claims would shift classification to drug status, necessitating extensive stability testing under ICH Q1A(R2) guidelines with accelerated and long-term studies.
European regulatory frameworks impose more stringent requirements through the European Food Safety Authority (EFSA) for supplement applications and the European Medicines Agency (EMA) for medicinal products. Novel food regulations may apply to oxaloacetate depending on its historical consumption patterns in the EU before May 1997, potentially requiring comprehensive safety assessments including stability data across various environmental conditions.
Japan's regulatory system presents a third major framework through the Pharmaceuticals and Medical Devices Agency (PMDA), with specific stability testing requirements for "Foods with Function Claims" that contain oxaloacetate. These regulations emphasize real-time stability data collection under Zone IVb conditions (30°C/75% RH), reflecting Japan's climatic considerations.
Global harmonization efforts through ICH (International Council for Harmonisation) provide standardized stability testing protocols that manufacturers should follow, particularly Q1A(R2) for stability testing and Q1B for photostability. These guidelines recommend testing under multiple temperature and humidity conditions to establish appropriate storage conditions and shelf life determinations.
Regulatory compliance for oxaloacetate products requires particular attention to degradation pathway documentation, with authorities increasingly demanding comprehensive understanding of degradation products and their safety profiles. This includes identification of potential toxic metabolites formed during storage and demonstration that degradation remains within acceptable limits throughout the product lifecycle.
Recent regulatory trends indicate movement toward more stringent oversight of supplement stability, with several jurisdictions implementing pharmaceutical-grade stability requirements for high-risk supplement categories. Manufacturers developing oxaloacetate-based products must therefore implement robust stability programs that anticipate these evolving regulatory expectations while addressing the inherent chemical instability challenges of the compound.
European regulatory frameworks impose more stringent requirements through the European Food Safety Authority (EFSA) for supplement applications and the European Medicines Agency (EMA) for medicinal products. Novel food regulations may apply to oxaloacetate depending on its historical consumption patterns in the EU before May 1997, potentially requiring comprehensive safety assessments including stability data across various environmental conditions.
Japan's regulatory system presents a third major framework through the Pharmaceuticals and Medical Devices Agency (PMDA), with specific stability testing requirements for "Foods with Function Claims" that contain oxaloacetate. These regulations emphasize real-time stability data collection under Zone IVb conditions (30°C/75% RH), reflecting Japan's climatic considerations.
Global harmonization efforts through ICH (International Council for Harmonisation) provide standardized stability testing protocols that manufacturers should follow, particularly Q1A(R2) for stability testing and Q1B for photostability. These guidelines recommend testing under multiple temperature and humidity conditions to establish appropriate storage conditions and shelf life determinations.
Regulatory compliance for oxaloacetate products requires particular attention to degradation pathway documentation, with authorities increasingly demanding comprehensive understanding of degradation products and their safety profiles. This includes identification of potential toxic metabolites formed during storage and demonstration that degradation remains within acceptable limits throughout the product lifecycle.
Recent regulatory trends indicate movement toward more stringent oversight of supplement stability, with several jurisdictions implementing pharmaceutical-grade stability requirements for high-risk supplement categories. Manufacturers developing oxaloacetate-based products must therefore implement robust stability programs that anticipate these evolving regulatory expectations while addressing the inherent chemical instability challenges of the compound.
Environmental Impact of Oxaloacetate Production and Testing
The production and testing of oxaloacetate have significant environmental implications that warrant careful consideration. The manufacturing processes for oxaloacetate typically involve chemical synthesis pathways that utilize various solvents, catalysts, and precursor compounds. These production methods can generate substantial waste streams containing potentially harmful chemicals, including organic solvents, heavy metals from catalysts, and unreacted precursors.
Water consumption represents another critical environmental concern in oxaloacetate production. The synthesis, purification, and stability testing processes require significant volumes of water, contributing to industrial water footprints in regions where manufacturing facilities are located. Additionally, wastewater from these operations may contain trace amounts of oxaloacetate and its degradation products, which could impact aquatic ecosystems if not properly treated.
Energy requirements for oxaloacetate production and stability testing also contribute to environmental impacts through greenhouse gas emissions. Temperature-controlled environments for stability testing, energy-intensive purification processes, and manufacturing operations collectively result in a substantial carbon footprint. Companies are increasingly exploring renewable energy sources to mitigate these impacts, though implementation remains variable across the industry.
The degradation pathways of oxaloacetate present unique environmental challenges. When oxaloacetate decomposes in the environment, it primarily forms pyruvate and carbon dioxide. While these compounds are generally considered less environmentally problematic than the parent molecule, the rate and conditions of degradation can influence local ecosystem impacts. Stability testing protocols themselves generate laboratory waste containing various buffer solutions, analytical reagents, and degraded oxaloacetate samples.
Regulatory frameworks addressing the environmental impacts of oxaloacetate production vary significantly by region. The European Union's REACH regulations impose stricter requirements for chemical production and waste management compared to some other jurisdictions. Companies engaged in oxaloacetate production must navigate these complex regulatory landscapes while implementing appropriate environmental management systems.
Recent innovations in green chemistry approaches offer promising pathways to reduce the environmental footprint of oxaloacetate production and testing. Enzymatic synthesis methods, for instance, operate under milder conditions with reduced solvent requirements. Similarly, advances in analytical techniques allow for smaller sample sizes in stability testing, thereby minimizing waste generation while maintaining data quality and reliability.
Water consumption represents another critical environmental concern in oxaloacetate production. The synthesis, purification, and stability testing processes require significant volumes of water, contributing to industrial water footprints in regions where manufacturing facilities are located. Additionally, wastewater from these operations may contain trace amounts of oxaloacetate and its degradation products, which could impact aquatic ecosystems if not properly treated.
Energy requirements for oxaloacetate production and stability testing also contribute to environmental impacts through greenhouse gas emissions. Temperature-controlled environments for stability testing, energy-intensive purification processes, and manufacturing operations collectively result in a substantial carbon footprint. Companies are increasingly exploring renewable energy sources to mitigate these impacts, though implementation remains variable across the industry.
The degradation pathways of oxaloacetate present unique environmental challenges. When oxaloacetate decomposes in the environment, it primarily forms pyruvate and carbon dioxide. While these compounds are generally considered less environmentally problematic than the parent molecule, the rate and conditions of degradation can influence local ecosystem impacts. Stability testing protocols themselves generate laboratory waste containing various buffer solutions, analytical reagents, and degraded oxaloacetate samples.
Regulatory frameworks addressing the environmental impacts of oxaloacetate production vary significantly by region. The European Union's REACH regulations impose stricter requirements for chemical production and waste management compared to some other jurisdictions. Companies engaged in oxaloacetate production must navigate these complex regulatory landscapes while implementing appropriate environmental management systems.
Recent innovations in green chemistry approaches offer promising pathways to reduce the environmental footprint of oxaloacetate production and testing. Enzymatic synthesis methods, for instance, operate under milder conditions with reduced solvent requirements. Similarly, advances in analytical techniques allow for smaller sample sizes in stability testing, thereby minimizing waste generation while maintaining data quality and reliability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!