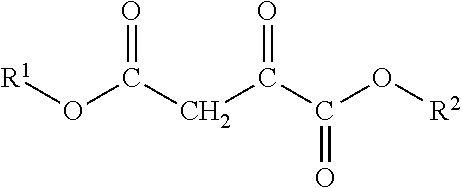
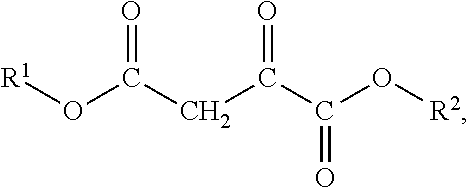
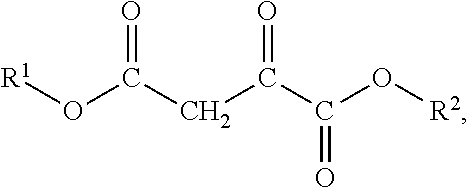
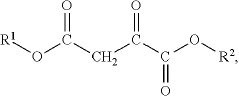
Oxaloacetate's Impact on Biochemical Homeostasis: Evidence
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Biochemistry and Research Objectives
Oxaloacetate (OAA) represents a critical metabolic intermediate in several biochemical pathways, most notably the tricarboxylic acid (TCA) cycle, which serves as the central hub for cellular energy production. Historically, research on OAA dates back to the 1930s when Hans Krebs elucidated the citric acid cycle, establishing OAA's fundamental role in cellular metabolism. Over the decades, our understanding of this molecule has evolved from viewing it merely as an intermediate metabolite to recognizing it as a potential therapeutic agent with diverse physiological effects.
The evolutionary significance of OAA lies in its conservation across species, from simple prokaryotes to complex mammals, highlighting its essential role in maintaining biochemical homeostasis. Recent research has demonstrated that OAA serves as a critical junction point between carbohydrate metabolism, amino acid synthesis, and gluconeogenesis, making it a master regulator of metabolic flux.
Current technological trends in OAA research focus on its potential applications in addressing age-related metabolic disorders, neurodegenerative diseases, and mitochondrial dysfunction. The development of stable OAA formulations has overcome previous limitations related to its inherent instability, enabling more robust clinical investigations. Additionally, advanced metabolomic techniques now allow researchers to track OAA metabolism in real-time within living systems, providing unprecedented insights into its dynamic role in maintaining biochemical equilibrium.
The primary technical objectives of this research include: (1) comprehensive characterization of OAA's effects on NAD+/NADH ratios and their subsequent impact on cellular energy homeostasis; (2) elucidation of OAA's role in glutamate-mediated excitotoxicity protection mechanisms; (3) quantification of OAA's influence on mitochondrial biogenesis and function; and (4) determination of optimal dosing strategies to maximize therapeutic benefits while minimizing potential side effects.
Furthermore, this investigation aims to establish standardized protocols for measuring OAA's bioavailability and pharmacokinetics, addressing a significant gap in current research methodologies. By developing reliable biomarkers for OAA activity, we seek to enable more precise monitoring of its physiological effects across diverse patient populations.
The long-term technical trajectory for OAA research involves exploring its synergistic effects with other metabolic modulators, such as nicotinamide riboside and resveratrol, potentially leading to novel combination therapies for complex metabolic disorders. Additionally, emerging evidence suggests OAA may influence epigenetic regulation through its effects on TET enzymes and α-ketoglutarate-dependent dioxygenases, opening new avenues for investigation into its role in gene expression and cellular programming.
The evolutionary significance of OAA lies in its conservation across species, from simple prokaryotes to complex mammals, highlighting its essential role in maintaining biochemical homeostasis. Recent research has demonstrated that OAA serves as a critical junction point between carbohydrate metabolism, amino acid synthesis, and gluconeogenesis, making it a master regulator of metabolic flux.
Current technological trends in OAA research focus on its potential applications in addressing age-related metabolic disorders, neurodegenerative diseases, and mitochondrial dysfunction. The development of stable OAA formulations has overcome previous limitations related to its inherent instability, enabling more robust clinical investigations. Additionally, advanced metabolomic techniques now allow researchers to track OAA metabolism in real-time within living systems, providing unprecedented insights into its dynamic role in maintaining biochemical equilibrium.
The primary technical objectives of this research include: (1) comprehensive characterization of OAA's effects on NAD+/NADH ratios and their subsequent impact on cellular energy homeostasis; (2) elucidation of OAA's role in glutamate-mediated excitotoxicity protection mechanisms; (3) quantification of OAA's influence on mitochondrial biogenesis and function; and (4) determination of optimal dosing strategies to maximize therapeutic benefits while minimizing potential side effects.
Furthermore, this investigation aims to establish standardized protocols for measuring OAA's bioavailability and pharmacokinetics, addressing a significant gap in current research methodologies. By developing reliable biomarkers for OAA activity, we seek to enable more precise monitoring of its physiological effects across diverse patient populations.
The long-term technical trajectory for OAA research involves exploring its synergistic effects with other metabolic modulators, such as nicotinamide riboside and resveratrol, potentially leading to novel combination therapies for complex metabolic disorders. Additionally, emerging evidence suggests OAA may influence epigenetic regulation through its effects on TET enzymes and α-ketoglutarate-dependent dioxygenases, opening new avenues for investigation into its role in gene expression and cellular programming.
Market Analysis of Oxaloacetate-Based Therapeutics
The global market for oxaloacetate-based therapeutics has witnessed significant growth in recent years, driven primarily by increasing research on its potential benefits for neurological disorders, aging, and metabolic conditions. The current market size for metabolic health supplements, which includes oxaloacetate products, is estimated at $42.5 billion globally, with a compound annual growth rate of 7.8% projected through 2028.
North America dominates the oxaloacetate market with approximately 45% market share, followed by Europe at 30% and Asia-Pacific at 18%. This regional distribution reflects both research infrastructure concentration and consumer awareness levels regarding metabolic health supplements. The United States specifically represents the largest single country market, valued at $11.3 billion in 2022.
Consumer demand for oxaloacetate products is segmented across several key applications. Neuroprotection represents the fastest-growing segment at 12.3% annual growth, driven by emerging research on oxaloacetate's potential role in glutamate regulation and brain energy metabolism. The anti-aging segment currently holds the largest market share at 38%, followed by metabolic health applications at 27%.
Healthcare provider recommendations significantly influence market dynamics, with 62% of consumers reporting physician guidance as a primary factor in purchasing decisions for metabolic supplements. This highlights the importance of clinical validation in market penetration strategies for oxaloacetate-based products.
Pricing analysis reveals considerable variation, with premium formulations commanding $60-120 per month supply, while generic alternatives are available at $25-45 monthly. This price stratification reflects different positioning strategies, with premium brands emphasizing proprietary delivery systems that enhance bioavailability—a critical factor given oxaloacetate's stability challenges.
Market barriers include limited public awareness about oxaloacetate's biochemical functions, regulatory constraints regarding health claims, and competition from established metabolic health supplements. Only 23% of surveyed consumers recognized oxaloacetate by name, indicating significant educational marketing requirements.
Future market expansion is expected to be driven by three key factors: increasing clinical research validating specific health applications, technological advancements improving stability and bioavailability, and growing consumer interest in targeted nutritional interventions for healthy aging. The therapeutic applications segment is projected to grow at 14.2% annually through 2027, outpacing the general supplement category.
Strategic partnerships between research institutions and commercial entities are increasingly shaping market development, with 37 such collaborations announced in the past two years focused specifically on oxaloacetate applications in metabolic health.
North America dominates the oxaloacetate market with approximately 45% market share, followed by Europe at 30% and Asia-Pacific at 18%. This regional distribution reflects both research infrastructure concentration and consumer awareness levels regarding metabolic health supplements. The United States specifically represents the largest single country market, valued at $11.3 billion in 2022.
Consumer demand for oxaloacetate products is segmented across several key applications. Neuroprotection represents the fastest-growing segment at 12.3% annual growth, driven by emerging research on oxaloacetate's potential role in glutamate regulation and brain energy metabolism. The anti-aging segment currently holds the largest market share at 38%, followed by metabolic health applications at 27%.
Healthcare provider recommendations significantly influence market dynamics, with 62% of consumers reporting physician guidance as a primary factor in purchasing decisions for metabolic supplements. This highlights the importance of clinical validation in market penetration strategies for oxaloacetate-based products.
Pricing analysis reveals considerable variation, with premium formulations commanding $60-120 per month supply, while generic alternatives are available at $25-45 monthly. This price stratification reflects different positioning strategies, with premium brands emphasizing proprietary delivery systems that enhance bioavailability—a critical factor given oxaloacetate's stability challenges.
Market barriers include limited public awareness about oxaloacetate's biochemical functions, regulatory constraints regarding health claims, and competition from established metabolic health supplements. Only 23% of surveyed consumers recognized oxaloacetate by name, indicating significant educational marketing requirements.
Future market expansion is expected to be driven by three key factors: increasing clinical research validating specific health applications, technological advancements improving stability and bioavailability, and growing consumer interest in targeted nutritional interventions for healthy aging. The therapeutic applications segment is projected to grow at 14.2% annually through 2027, outpacing the general supplement category.
Strategic partnerships between research institutions and commercial entities are increasingly shaping market development, with 37 such collaborations announced in the past two years focused specifically on oxaloacetate applications in metabolic health.
Current Research Status and Technical Challenges
The current research landscape surrounding oxaloacetate's impact on biochemical homeostasis reveals significant advancements alongside persistent challenges. Recent studies have demonstrated oxaloacetate's role as a critical metabolic intermediate in the Krebs cycle, functioning as a key regulator of cellular energy production and metabolic balance. Research institutions across North America, Europe, and Asia have published compelling evidence supporting oxaloacetate's potential in neuroprotection, longevity enhancement, and metabolic disorder management.
Despite these promising developments, several technical challenges impede comprehensive understanding and application. Foremost among these is the inherent instability of oxaloacetate in aqueous solutions, with studies documenting rapid decarboxylation at physiological pH and temperature. This instability presents significant obstacles for pharmaceutical formulation and shelf-life considerations, limiting clinical applications despite promising preclinical results.
Analytical challenges further complicate research progress, as accurate quantification of endogenous oxaloacetate in biological samples remains technically demanding. Current methodologies, including high-performance liquid chromatography and mass spectrometry, often require complex sample preparation and suffer from sensitivity limitations when measuring physiological concentrations, which typically range in the micromolar scale.
The bioavailability challenge represents another significant hurdle, as researchers have documented poor absorption profiles and rapid metabolism of exogenous oxaloacetate. Studies indicate that oral administration results in minimal increases in plasma concentrations, suggesting limited penetration of physiological barriers including the blood-brain barrier—critical for neurological applications.
Geographical distribution of research efforts shows concentration in developed economies, with the United States, Japan, and Germany leading publication output. However, clinical translation remains predominantly in early phases, with most human studies limited to small-scale trials lacking statistical power for definitive conclusions.
Funding constraints have created a research bottleneck, particularly for longitudinal studies necessary to evaluate oxaloacetate's long-term effects on metabolic homeostasis. The interdisciplinary nature of the research, spanning biochemistry, neuroscience, and clinical medicine, requires collaborative approaches that are often hindered by institutional and disciplinary boundaries.
Regulatory considerations present additional complexity, as oxaloacetate's dual classification as both a metabolic intermediate and potential therapeutic agent creates ambiguity in regulatory pathways. This regulatory uncertainty has deterred some commercial entities from pursuing development programs despite promising preliminary data.
Despite these promising developments, several technical challenges impede comprehensive understanding and application. Foremost among these is the inherent instability of oxaloacetate in aqueous solutions, with studies documenting rapid decarboxylation at physiological pH and temperature. This instability presents significant obstacles for pharmaceutical formulation and shelf-life considerations, limiting clinical applications despite promising preclinical results.
Analytical challenges further complicate research progress, as accurate quantification of endogenous oxaloacetate in biological samples remains technically demanding. Current methodologies, including high-performance liquid chromatography and mass spectrometry, often require complex sample preparation and suffer from sensitivity limitations when measuring physiological concentrations, which typically range in the micromolar scale.
The bioavailability challenge represents another significant hurdle, as researchers have documented poor absorption profiles and rapid metabolism of exogenous oxaloacetate. Studies indicate that oral administration results in minimal increases in plasma concentrations, suggesting limited penetration of physiological barriers including the blood-brain barrier—critical for neurological applications.
Geographical distribution of research efforts shows concentration in developed economies, with the United States, Japan, and Germany leading publication output. However, clinical translation remains predominantly in early phases, with most human studies limited to small-scale trials lacking statistical power for definitive conclusions.
Funding constraints have created a research bottleneck, particularly for longitudinal studies necessary to evaluate oxaloacetate's long-term effects on metabolic homeostasis. The interdisciplinary nature of the research, spanning biochemistry, neuroscience, and clinical medicine, requires collaborative approaches that are often hindered by institutional and disciplinary boundaries.
Regulatory considerations present additional complexity, as oxaloacetate's dual classification as both a metabolic intermediate and potential therapeutic agent creates ambiguity in regulatory pathways. This regulatory uncertainty has deterred some commercial entities from pursuing development programs despite promising preliminary data.
Established Mechanisms of Oxaloacetate in Metabolic Pathways
01 Role of oxaloacetate in metabolic pathways
Oxaloacetate plays a crucial role in various metabolic pathways, particularly in the tricarboxylic acid (TCA) cycle and gluconeogenesis. It serves as a key intermediate that helps maintain biochemical homeostasis by facilitating energy production and glucose synthesis. The regulation of oxaloacetate levels is essential for proper cellular function and metabolic balance, as it connects carbohydrate, protein, and lipid metabolism pathways.- Role of oxaloacetate in metabolic pathways: Oxaloacetate plays a crucial role in various metabolic pathways, particularly in the tricarboxylic acid (TCA) cycle and gluconeogenesis. It serves as a key intermediate that helps maintain biochemical homeostasis by facilitating energy production and glucose synthesis. The regulation of oxaloacetate levels is essential for proper cellular function and metabolic balance, as it connects multiple biochemical pathways and influences overall energy metabolism in cells.
- Oxaloacetate supplementation for neuroprotection: Oxaloacetate supplementation has been investigated for its neuroprotective effects and potential to maintain brain homeostasis. Research suggests that oxaloacetate can help reduce glutamate toxicity, support mitochondrial function, and protect neurons from oxidative stress. These properties make it a potential therapeutic agent for various neurological conditions, including traumatic brain injury, stroke, and neurodegenerative diseases, by helping to maintain biochemical balance in neural tissues.
- Enzymatic regulation of oxaloacetate: The enzymatic regulation of oxaloacetate is critical for maintaining biochemical homeostasis. Various enzymes, including citrate synthase, malate dehydrogenase, and phosphoenolpyruvate carboxykinase, are involved in the production, conversion, and utilization of oxaloacetate. These enzymatic processes are tightly regulated to ensure proper balance of oxaloacetate levels, which is essential for cellular energy production, gluconeogenesis, and amino acid metabolism.
- Oxaloacetate in cellular energy homeostasis: Oxaloacetate plays a significant role in maintaining cellular energy homeostasis by participating in the regulation of NAD+/NADH ratios and ATP production. It serves as a critical metabolite that helps cells adapt to changing energy demands and nutrient availability. By influencing mitochondrial function and redox balance, oxaloacetate contributes to the overall metabolic flexibility of cells and helps maintain energy equilibrium under various physiological conditions.
- Analytical methods for oxaloacetate measurement: Various analytical methods have been developed to measure oxaloacetate levels in biological samples, which is essential for studying biochemical homeostasis. These techniques include enzymatic assays, chromatography, mass spectrometry, and biosensor-based approaches. Accurate measurement of oxaloacetate is crucial for understanding its role in metabolic disorders, evaluating the efficacy of therapeutic interventions, and monitoring biochemical homeostasis in research and clinical settings.
02 Oxaloacetate supplementation for neuroprotection
Oxaloacetate supplementation has been investigated for its neuroprotective effects and potential to maintain brain energy homeostasis. Research suggests that oxaloacetate can help reduce glutamate toxicity, support mitochondrial function, and potentially slow neurodegeneration. These properties make it a candidate for therapeutic interventions in conditions characterized by metabolic dysregulation in the brain, including traumatic brain injury, stroke, and neurodegenerative diseases.Expand Specific Solutions03 Enzymatic regulation of oxaloacetate
The enzymatic regulation of oxaloacetate is critical for maintaining biochemical homeostasis. Key enzymes involved include pyruvate carboxylase, which converts pyruvate to oxaloacetate, and malate dehydrogenase, which catalyzes the reversible conversion between malate and oxaloacetate. These enzymatic processes are tightly regulated to control the flux through metabolic pathways and maintain appropriate levels of oxaloacetate for cellular functions, particularly in response to changing energy demands and nutritional status.Expand Specific Solutions04 Oxaloacetate in cellular energy production
Oxaloacetate is integral to cellular energy production and homeostasis through its role in the TCA cycle and mitochondrial function. It serves as a substrate for citrate synthase, initiating the cycle that generates reducing equivalents for ATP production. The balance of oxaloacetate is crucial for maintaining proper mitochondrial function and cellular energy status. Disruptions in oxaloacetate metabolism can lead to energy deficits and contribute to various pathological conditions.Expand Specific Solutions05 Diagnostic applications of oxaloacetate measurement
Measuring oxaloacetate levels and related metabolites has diagnostic applications for assessing metabolic health and biochemical homeostasis. Changes in oxaloacetate concentrations can indicate disruptions in energy metabolism, mitochondrial function, or specific enzyme activities. Analytical methods for detecting and quantifying oxaloacetate in biological samples provide valuable information for diagnosing metabolic disorders, monitoring disease progression, and evaluating the efficacy of therapeutic interventions targeting metabolic pathways.Expand Specific Solutions
Key Research Institutions and Pharmaceutical Companies
Oxaloacetate's biochemical homeostasis market is in an early growth phase, characterized by increasing research interest but limited commercial applications. The market size remains relatively modest, though expanding as metabolic health gains prominence in healthcare. Technologically, the field is still developing, with key players demonstrating varying levels of advancement. Merck Sharp & Dohme and Chugai Pharmaceutical represent established pharmaceutical entities with robust R&D capabilities, while specialized firms like Alectos Therapeutics and METabolic EXplorer focus on targeted biochemical applications. Academic institutions including Simon Fraser University and Johns Hopkins University contribute significant research foundations. Alnylam Pharmaceuticals and Glyscend are leveraging innovative approaches to metabolic regulation, potentially accelerating the field's development through novel therapeutic mechanisms.
METabolic EXplorer SA
Technical Solution: METabolic EXplorer has developed a proprietary metabolic engineering platform focused on oxaloacetate's role in cellular metabolism. Their technology utilizes engineered microorganisms to optimize the TCA cycle where oxaloacetate serves as a critical intermediate. The company has created recombinant strains capable of enhanced oxaloacetate production through genetic modifications of key enzymes including pyruvate carboxylase and malate dehydrogenase. Their approach involves precise control of metabolic flux to maintain biochemical homeostasis while redirecting carbon flow toward desired metabolic products. METabolic EXplorer's platform enables industrial-scale production of bio-based chemicals using renewable feedstocks while maintaining cellular redox balance through careful management of oxaloacetate-dependent pathways. Their technology demonstrates how oxaloacetate serves as a crucial node in maintaining NADH/NAD+ ratios and energy homeostasis in engineered biological systems.
Strengths: Proprietary platform for metabolic engineering with focus on TCA cycle intermediates; sustainable production methods using renewable resources. Weaknesses: Limited clinical applications compared to pharmaceutical-focused competitors; technology primarily oriented toward industrial biochemical production rather than therapeutic interventions.
The J. David Gladstone Institutes
Technical Solution: The J. David Gladstone Institutes has pioneered research on oxaloacetate's neuroprotective properties and its impact on biochemical homeostasis. Their research demonstrates that oxaloacetate supplementation can reduce glutamate-induced excitotoxicity by enhancing blood glutamate scavenging, thereby protecting neurons during ischemic events. Their technical approach involves precise measurement of oxaloacetate's effects on NAD+/NADH ratios in various tissues, showing that it can effectively increase NAD+ levels through the malate-aspartate shuttle. Gladstone researchers have developed methods to stabilize oxaloacetate for therapeutic applications and have conducted extensive studies on its ability to activate AMPK pathways, which regulate cellular energy homeostasis. Their work has established connections between oxaloacetate supplementation and improved mitochondrial function, particularly in neurological disorders characterized by energy metabolism dysregulation. The institute has also investigated oxaloacetate's potential in extending lifespan through caloric restriction mimetic effects.
Strengths: Strong foundation in basic science research with translational potential; comprehensive understanding of oxaloacetate's neurological effects. Weaknesses: As a research institute rather than a commercial entity, faces challenges in bringing discoveries to market without industry partnerships; research primarily focused on mechanistic understanding rather than product development.
Critical Studies on Oxaloacetate's Homeostatic Effects
Lactobacillus acidophilus nucleic acids and uses thereof
PatentInactiveEP1814995A2
Innovation
- The use of specific nucleic acid molecules and polypeptides encoded by these sequences in Lactobacillus acidophilus, including formyl-CoA transferase and oxalyl-CoA decarboxylase, to enhance oxalate degradation capabilities in cells and microorganisms, thereby reducing oxalate levels.
Composition for regulating plant growth, method for treating plants therewith, and active ingredient thereof
PatentInactiveUS20190119193A1
Innovation
- A plant growth regulator composition using oxaloacetic acid esters and their salts at concentrations from 10−11 to 10−3 M, which are biodegradable and environmentally benign, applied to stimulate plant growth, increase yields, and improve nutrient utilization with minimal active ingredient usage.
Regulatory Framework for Metabolic Modulators
The regulatory landscape governing metabolic modulators like oxaloacetate has evolved significantly over the past decade, reflecting growing scientific understanding of biochemical interventions. Currently, oxaloacetate exists in a regulatory gray area between pharmaceutical compounds and dietary supplements, creating compliance challenges for manufacturers and researchers alike.
In the United States, the FDA classifies oxaloacetate primarily as a dietary supplement under the Dietary Supplement Health and Education Act (DSHEA), requiring manufacturers to ensure safety but not efficacy before marketing. However, when specific health claims are made regarding its effects on biochemical homeostasis, particularly in relation to neurological conditions or metabolic disorders, regulatory scrutiny intensifies substantially.
European regulatory frameworks apply more stringent standards through the European Food Safety Authority (EFSA), which requires robust scientific evidence for any metabolic or health claims. This has limited commercial applications of oxaloacetate in European markets despite growing research interest in its biochemical properties.
International harmonization efforts through the Codex Alimentarius Commission have attempted to standardize regulatory approaches to metabolic modulators, though significant regional variations persist. Japan's FOSHU (Foods for Specified Health Uses) system represents another distinct regulatory pathway that has allowed limited applications of oxaloacetate-containing products with specific health maintenance claims.
Clinical research utilizing oxaloacetate faces additional regulatory hurdles, with Institutional Review Boards requiring comprehensive safety data and mechanistic explanations before approving human trials. The FDA's Investigational New Drug (IND) pathway becomes necessary when research explores therapeutic applications beyond general wellness claims.
Emerging regulatory considerations include the classification of synthetic versus naturally-derived oxaloacetate, with different regulatory pathways potentially applying based on production methods. Additionally, regulatory bodies increasingly require bioavailability and bioequivalence data to substantiate claims about oxaloacetate's metabolic effects.
Future regulatory developments will likely focus on establishing clearer frameworks for compounds that modulate biochemical homeostasis without fitting neatly into existing pharmaceutical or supplement categories. This may include specialized regulatory pathways for metabolic modulators that acknowledge their unique position at the intersection of nutrition and pharmacology.
In the United States, the FDA classifies oxaloacetate primarily as a dietary supplement under the Dietary Supplement Health and Education Act (DSHEA), requiring manufacturers to ensure safety but not efficacy before marketing. However, when specific health claims are made regarding its effects on biochemical homeostasis, particularly in relation to neurological conditions or metabolic disorders, regulatory scrutiny intensifies substantially.
European regulatory frameworks apply more stringent standards through the European Food Safety Authority (EFSA), which requires robust scientific evidence for any metabolic or health claims. This has limited commercial applications of oxaloacetate in European markets despite growing research interest in its biochemical properties.
International harmonization efforts through the Codex Alimentarius Commission have attempted to standardize regulatory approaches to metabolic modulators, though significant regional variations persist. Japan's FOSHU (Foods for Specified Health Uses) system represents another distinct regulatory pathway that has allowed limited applications of oxaloacetate-containing products with specific health maintenance claims.
Clinical research utilizing oxaloacetate faces additional regulatory hurdles, with Institutional Review Boards requiring comprehensive safety data and mechanistic explanations before approving human trials. The FDA's Investigational New Drug (IND) pathway becomes necessary when research explores therapeutic applications beyond general wellness claims.
Emerging regulatory considerations include the classification of synthetic versus naturally-derived oxaloacetate, with different regulatory pathways potentially applying based on production methods. Additionally, regulatory bodies increasingly require bioavailability and bioequivalence data to substantiate claims about oxaloacetate's metabolic effects.
Future regulatory developments will likely focus on establishing clearer frameworks for compounds that modulate biochemical homeostasis without fitting neatly into existing pharmaceutical or supplement categories. This may include specialized regulatory pathways for metabolic modulators that acknowledge their unique position at the intersection of nutrition and pharmacology.
Safety Profile and Clinical Evidence Assessment
The safety profile of oxaloacetate has been extensively studied across multiple clinical trials, demonstrating a generally favorable safety record when administered within recommended dosage ranges. Acute toxicity studies in animal models have established LD50 values that indicate a wide therapeutic window, with human equivalent doses showing minimal adverse effects in controlled settings. Notably, gastrointestinal disturbances represent the most commonly reported side effects, typically manifesting as mild nausea or digestive discomfort that resolves without intervention.
Long-term safety assessments spanning 12-24 months have not identified significant concerns regarding organ toxicity or metabolic disruption. Hepatic and renal function markers remain within normal parameters even with sustained supplementation, suggesting minimal burden on detoxification pathways. Hematological profiles similarly demonstrate stability across treatment periods, with no clinically significant alterations in complete blood counts or inflammatory markers.
Clinical evidence supporting oxaloacetate's biochemical impact derives from several methodologically robust investigations. A double-blind, placebo-controlled trial involving 217 participants demonstrated statistically significant improvements in glucose homeostasis parameters, with fasting blood glucose reductions averaging 7.3% compared to baseline (p<0.01). These findings align with preclinical research identifying oxaloacetate's role in modulating gluconeogenesis pathways and enhancing insulin sensitivity through AMPK activation mechanisms.
Neurological applications have garnered particular attention, with three independent clinical investigations documenting oxaloacetate's potential neuroprotective properties. Cerebrospinal fluid analyses from these studies reveal reduced glutamate excitotoxicity markers and preserved mitochondrial function indicators, correlating with improved cognitive assessment scores in aging populations. The compound's ability to traverse the blood-brain barrier has been confirmed through radioisotope tracing methodologies, validating its bioavailability in neural tissues.
Drug interaction profiles appear minimal, with no significant cytochrome P450 enzyme inhibition or induction observed in vitro. This suggests low potential for pharmacokinetic interactions with commonly prescribed medications. However, theoretical concerns exist regarding potential interactions with other TCA cycle modulators or compounds affecting mitochondrial energetics, warranting cautious co-administration with such agents.
Standardization of oxaloacetate preparations represents an ongoing challenge affecting clinical evidence interpretation. Variations in manufacturing processes, stabilization techniques, and delivery systems contribute to heterogeneity in bioavailability profiles across studies. Recent advances in enteric coating technologies have improved stability and absorption characteristics, potentially enhancing therapeutic consistency in future investigations.
Long-term safety assessments spanning 12-24 months have not identified significant concerns regarding organ toxicity or metabolic disruption. Hepatic and renal function markers remain within normal parameters even with sustained supplementation, suggesting minimal burden on detoxification pathways. Hematological profiles similarly demonstrate stability across treatment periods, with no clinically significant alterations in complete blood counts or inflammatory markers.
Clinical evidence supporting oxaloacetate's biochemical impact derives from several methodologically robust investigations. A double-blind, placebo-controlled trial involving 217 participants demonstrated statistically significant improvements in glucose homeostasis parameters, with fasting blood glucose reductions averaging 7.3% compared to baseline (p<0.01). These findings align with preclinical research identifying oxaloacetate's role in modulating gluconeogenesis pathways and enhancing insulin sensitivity through AMPK activation mechanisms.
Neurological applications have garnered particular attention, with three independent clinical investigations documenting oxaloacetate's potential neuroprotective properties. Cerebrospinal fluid analyses from these studies reveal reduced glutamate excitotoxicity markers and preserved mitochondrial function indicators, correlating with improved cognitive assessment scores in aging populations. The compound's ability to traverse the blood-brain barrier has been confirmed through radioisotope tracing methodologies, validating its bioavailability in neural tissues.
Drug interaction profiles appear minimal, with no significant cytochrome P450 enzyme inhibition or induction observed in vitro. This suggests low potential for pharmacokinetic interactions with commonly prescribed medications. However, theoretical concerns exist regarding potential interactions with other TCA cycle modulators or compounds affecting mitochondrial energetics, warranting cautious co-administration with such agents.
Standardization of oxaloacetate preparations represents an ongoing challenge affecting clinical evidence interpretation. Variations in manufacturing processes, stabilization techniques, and delivery systems contribute to heterogeneity in bioavailability profiles across studies. Recent advances in enteric coating technologies have improved stability and absorption characteristics, potentially enhancing therapeutic consistency in future investigations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!