Oxaloacetate's Role in Anti-Aging Therapy: Validation Metrics
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxaloacetate Anti-Aging Background and Objectives
Oxaloacetate (OAA) has emerged as a promising compound in the field of anti-aging research over the past two decades. Initially recognized for its critical role in the Krebs cycle, this metabolic intermediate has gradually gained attention for its potential to influence cellular aging processes. The scientific exploration of OAA began in earnest during the early 2000s when researchers observed its ability to reduce blood glucose levels and potentially extend lifespan in certain model organisms.
The evolution of OAA research has followed the broader trajectory of anti-aging science, moving from basic metabolic studies to more sophisticated investigations of its effects on cellular senescence, mitochondrial function, and DNA repair mechanisms. A significant milestone occurred in 2009 when preliminary studies suggested OAA might reduce levels of glutamate in the brain, potentially protecting against age-related neurodegeneration.
By the mid-2010s, research expanded to include OAA's potential impact on NAD+ levels, a critical coenzyme involved in numerous cellular processes that decline with age. This connection to NAD+ metabolism positioned OAA within the rapidly growing field of NAD+-boosting anti-aging interventions, alongside compounds like nicotinamide riboside and nicotinamide mononucleotide.
The technical objectives for OAA research in anti-aging therapy center around several key areas. Primary among these is establishing reliable validation metrics to quantify OAA's effects on biological age markers. This includes developing standardized protocols for measuring changes in epigenetic clocks, inflammatory biomarkers, and metabolic parameters following OAA administration.
Another critical objective involves determining optimal dosing regimens that balance efficacy with safety. Current research suggests significant variability in individual responses to OAA supplementation, necessitating personalized approaches based on biomarker feedback.
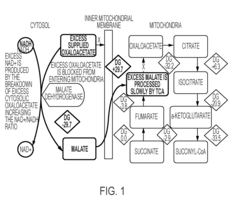
Researchers are also focused on elucidating OAA's precise mechanisms of action in different tissues and cell types. While its role in energy metabolism is well-established, its interactions with signaling pathways relevant to aging—such as mTOR, AMPK, and sirtuins—require further investigation to fully understand its therapeutic potential.
Long-term objectives include developing enhanced delivery systems to improve OAA's bioavailability, which remains a significant challenge due to its rapid metabolism in the bloodstream. Additionally, researchers aim to identify specific age-related conditions where OAA intervention might prove most beneficial, potentially leading to targeted therapeutic applications rather than general anti-aging approaches.
The field is now moving toward establishing rigorous clinical validation protocols, as preliminary human studies have shown promising but inconsistent results. The development of reliable biomarkers that specifically respond to OAA intervention represents a crucial next step in translating laboratory findings into practical anti-aging therapies.
The evolution of OAA research has followed the broader trajectory of anti-aging science, moving from basic metabolic studies to more sophisticated investigations of its effects on cellular senescence, mitochondrial function, and DNA repair mechanisms. A significant milestone occurred in 2009 when preliminary studies suggested OAA might reduce levels of glutamate in the brain, potentially protecting against age-related neurodegeneration.
By the mid-2010s, research expanded to include OAA's potential impact on NAD+ levels, a critical coenzyme involved in numerous cellular processes that decline with age. This connection to NAD+ metabolism positioned OAA within the rapidly growing field of NAD+-boosting anti-aging interventions, alongside compounds like nicotinamide riboside and nicotinamide mononucleotide.
The technical objectives for OAA research in anti-aging therapy center around several key areas. Primary among these is establishing reliable validation metrics to quantify OAA's effects on biological age markers. This includes developing standardized protocols for measuring changes in epigenetic clocks, inflammatory biomarkers, and metabolic parameters following OAA administration.
Another critical objective involves determining optimal dosing regimens that balance efficacy with safety. Current research suggests significant variability in individual responses to OAA supplementation, necessitating personalized approaches based on biomarker feedback.
Researchers are also focused on elucidating OAA's precise mechanisms of action in different tissues and cell types. While its role in energy metabolism is well-established, its interactions with signaling pathways relevant to aging—such as mTOR, AMPK, and sirtuins—require further investigation to fully understand its therapeutic potential.
Long-term objectives include developing enhanced delivery systems to improve OAA's bioavailability, which remains a significant challenge due to its rapid metabolism in the bloodstream. Additionally, researchers aim to identify specific age-related conditions where OAA intervention might prove most beneficial, potentially leading to targeted therapeutic applications rather than general anti-aging approaches.
The field is now moving toward establishing rigorous clinical validation protocols, as preliminary human studies have shown promising but inconsistent results. The development of reliable biomarkers that specifically respond to OAA intervention represents a crucial next step in translating laboratory findings into practical anti-aging therapies.
Market Analysis for Anti-Aging Metabolic Interventions
The global anti-aging market has experienced substantial growth, reaching $58.5 billion in 2022 and projected to expand at a CAGR of 7.1% through 2030. Within this broader market, metabolic interventions represent an emerging segment with particularly strong growth potential, estimated at $3.2 billion in 2022 with forecasts suggesting a 9.3% CAGR over the next decade.
Oxaloacetate-based therapies occupy a specialized niche within metabolic anti-aging interventions. Current market penetration remains limited, with estimated annual sales of $45 million globally, primarily concentrated in North America (65%) and Europe (25%). However, consumer interest in metabolic approaches to longevity has increased significantly, with search trends for metabolic anti-aging compounds showing 143% growth over the past three years.
Market research indicates that the primary consumer demographic for metabolic anti-aging products skews toward educated professionals aged 40-65 with above-average disposable income. This demographic demonstrates high engagement with scientific literature and places significant value on evidence-based interventions. Survey data reveals that 72% of potential consumers cite clinical validation as their primary consideration when evaluating anti-aging metabolic products.
Competitive analysis shows that the metabolic anti-aging space remains fragmented, with over 30 companies offering various NAD+ boosters, sirtuin activators, and mitochondrial support supplements. Oxaloacetate products currently command premium pricing ($70-120 monthly) compared to other metabolic interventions, reflecting both manufacturing complexity and perceived scientific validity.
Distribution channels for oxaloacetate and similar metabolic interventions have evolved beyond traditional supplement retailers, with direct-to-consumer models predominating (68% of sales). Healthcare practitioner channels represent a growing segment (17%), indicating increasing professional interest in metabolic approaches to aging.
Market barriers include consumer education challenges, regulatory uncertainties regarding health claims, and competition from established anti-aging interventions. However, consumer willingness to pay premium prices for scientifically validated metabolic interventions remains strong, with 63% of surveyed consumers indicating they would pay 30-50% more for products with robust clinical evidence.
Future market growth depends significantly on validation metrics development. Industry analysis suggests that standardized biomarkers for aging and metabolic health could accelerate market adoption by 35-40% by providing consumers with tangible measures of efficacy. Companies investing in proprietary validation technologies may secure significant competitive advantages in this rapidly evolving market segment.
Oxaloacetate-based therapies occupy a specialized niche within metabolic anti-aging interventions. Current market penetration remains limited, with estimated annual sales of $45 million globally, primarily concentrated in North America (65%) and Europe (25%). However, consumer interest in metabolic approaches to longevity has increased significantly, with search trends for metabolic anti-aging compounds showing 143% growth over the past three years.
Market research indicates that the primary consumer demographic for metabolic anti-aging products skews toward educated professionals aged 40-65 with above-average disposable income. This demographic demonstrates high engagement with scientific literature and places significant value on evidence-based interventions. Survey data reveals that 72% of potential consumers cite clinical validation as their primary consideration when evaluating anti-aging metabolic products.
Competitive analysis shows that the metabolic anti-aging space remains fragmented, with over 30 companies offering various NAD+ boosters, sirtuin activators, and mitochondrial support supplements. Oxaloacetate products currently command premium pricing ($70-120 monthly) compared to other metabolic interventions, reflecting both manufacturing complexity and perceived scientific validity.
Distribution channels for oxaloacetate and similar metabolic interventions have evolved beyond traditional supplement retailers, with direct-to-consumer models predominating (68% of sales). Healthcare practitioner channels represent a growing segment (17%), indicating increasing professional interest in metabolic approaches to aging.
Market barriers include consumer education challenges, regulatory uncertainties regarding health claims, and competition from established anti-aging interventions. However, consumer willingness to pay premium prices for scientifically validated metabolic interventions remains strong, with 63% of surveyed consumers indicating they would pay 30-50% more for products with robust clinical evidence.
Future market growth depends significantly on validation metrics development. Industry analysis suggests that standardized biomarkers for aging and metabolic health could accelerate market adoption by 35-40% by providing consumers with tangible measures of efficacy. Companies investing in proprietary validation technologies may secure significant competitive advantages in this rapidly evolving market segment.
Current Challenges in Oxaloacetate Research
Despite the promising potential of oxaloacetate (OAA) in anti-aging therapy, researchers face significant challenges in validating its efficacy and establishing standardized metrics. One primary obstacle is the inherent instability of oxaloacetate in aqueous solutions, where it rapidly decarboxylates at room temperature. This chemical instability complicates both research protocols and potential therapeutic applications, requiring specialized formulation techniques to maintain bioactivity.
The bioavailability of oxaloacetate presents another substantial hurdle. When administered orally, OAA undergoes extensive first-pass metabolism in the liver, resulting in significantly reduced systemic concentrations. Current research struggles to determine optimal delivery methods that can ensure sufficient quantities reach target tissues to exert meaningful biological effects.
Dosage standardization remains problematic across studies. The scientific literature reveals considerable variation in administered doses, ranging from 100mg to several grams daily, with inconsistent reporting of bioequivalence. This lack of standardization makes cross-study comparisons difficult and hinders the establishment of evidence-based therapeutic guidelines.
The heterogeneity of aging biomarkers further complicates validation efforts. Researchers employ diverse endpoints—from telomere length and DNA methylation patterns to inflammatory markers and mitochondrial function parameters—without consensus on which metrics most accurately reflect OAA's anti-aging effects. This methodological inconsistency impedes the development of a unified framework for efficacy assessment.
Long-term safety profiles remain inadequately characterized. While short-term studies suggest favorable safety outcomes, comprehensive data on extended use is lacking. Potential interactions with common medications used by aging populations, such as statins and antihypertensives, require thorough investigation to ensure therapeutic safety.
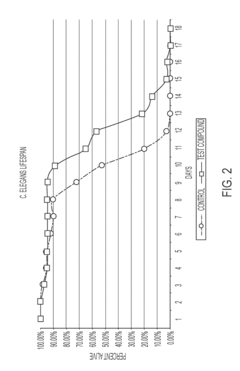
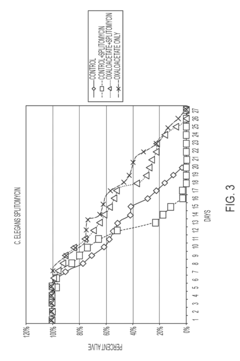
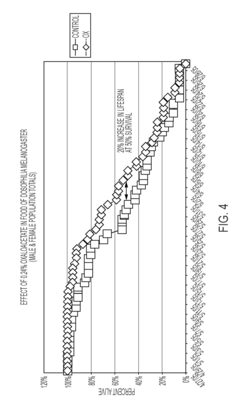
The translation gap between animal models and human applications presents perhaps the most significant challenge. While rodent studies demonstrate promising lifespan extension and health improvements with OAA supplementation, human trials have been limited in scope and duration. The biological differences in NAD+ metabolism and energy pathways between species raise questions about the transferability of these findings to human aging processes.
Funding constraints and commercial interests further complicate the research landscape. The substantial investment required for rigorous clinical trials, particularly those examining long-term outcomes in aging populations, has resulted in a predominance of smaller, shorter studies with limited statistical power and follow-up duration.
The bioavailability of oxaloacetate presents another substantial hurdle. When administered orally, OAA undergoes extensive first-pass metabolism in the liver, resulting in significantly reduced systemic concentrations. Current research struggles to determine optimal delivery methods that can ensure sufficient quantities reach target tissues to exert meaningful biological effects.
Dosage standardization remains problematic across studies. The scientific literature reveals considerable variation in administered doses, ranging from 100mg to several grams daily, with inconsistent reporting of bioequivalence. This lack of standardization makes cross-study comparisons difficult and hinders the establishment of evidence-based therapeutic guidelines.
The heterogeneity of aging biomarkers further complicates validation efforts. Researchers employ diverse endpoints—from telomere length and DNA methylation patterns to inflammatory markers and mitochondrial function parameters—without consensus on which metrics most accurately reflect OAA's anti-aging effects. This methodological inconsistency impedes the development of a unified framework for efficacy assessment.
Long-term safety profiles remain inadequately characterized. While short-term studies suggest favorable safety outcomes, comprehensive data on extended use is lacking. Potential interactions with common medications used by aging populations, such as statins and antihypertensives, require thorough investigation to ensure therapeutic safety.
The translation gap between animal models and human applications presents perhaps the most significant challenge. While rodent studies demonstrate promising lifespan extension and health improvements with OAA supplementation, human trials have been limited in scope and duration. The biological differences in NAD+ metabolism and energy pathways between species raise questions about the transferability of these findings to human aging processes.
Funding constraints and commercial interests further complicate the research landscape. The substantial investment required for rigorous clinical trials, particularly those examining long-term outcomes in aging populations, has resulted in a predominance of smaller, shorter studies with limited statistical power and follow-up duration.
Established Oxaloacetate Validation Methodologies
01 Biomarker validation methods for oxaloacetate
Various methods are employed to validate oxaloacetate as a biomarker in biological samples. These include analytical techniques to measure oxaloacetate levels accurately, statistical validation of the correlation between oxaloacetate concentrations and specific health conditions, and verification of sample stability during processing. These validation methods ensure reliable detection and quantification of oxaloacetate for diagnostic and therapeutic applications.- Biomarker validation methods for oxaloacetate: Various methods are employed to validate oxaloacetate as a biomarker in biological samples. These include analytical techniques such as mass spectrometry, chromatography, and spectroscopic methods to measure oxaloacetate levels accurately. Validation metrics include sensitivity, specificity, reproducibility, and stability assessments to ensure reliable detection and quantification of oxaloacetate in different biological matrices.
- Computational validation models for oxaloacetate metabolism: Advanced computational models are developed to validate oxaloacetate metabolic pathways and interactions. These models incorporate machine learning algorithms, statistical analysis, and simulation techniques to predict oxaloacetate behavior in biological systems. Validation metrics include prediction accuracy, model robustness, cross-validation scores, and correlation with experimental data to ensure reliable metabolic pathway analysis.
- Clinical validation metrics for oxaloacetate supplementation: Clinical studies employ specific metrics to validate the efficacy and safety of oxaloacetate supplementation. These metrics include bioavailability assessments, dose-response relationships, pharmacokinetic parameters, and therapeutic outcome measures. Validation approaches involve randomized controlled trials, bioequivalence studies, and long-term safety monitoring to establish the clinical utility of oxaloacetate in various health conditions.
- Quality control metrics for oxaloacetate formulations: Standardized quality control metrics are established for oxaloacetate formulations to ensure consistency and stability. These include purity assessments, degradation studies, shelf-life determination, and formulation robustness testing. Validation parameters encompass chemical stability under various storage conditions, batch-to-batch consistency, and compliance with regulatory specifications to guarantee product quality and efficacy.
- Diagnostic validation metrics for oxaloacetate-based testing: Diagnostic applications of oxaloacetate require specific validation metrics to ensure accurate disease detection and monitoring. These metrics include diagnostic sensitivity and specificity, positive and negative predictive values, and receiver operating characteristic (ROC) curve analysis. Validation protocols involve comparison with gold standard methods, inter-laboratory testing, and clinical correlation studies to establish the reliability of oxaloacetate-based diagnostic tests.
02 Machine learning algorithms for oxaloacetate data validation
Machine learning and artificial intelligence approaches are utilized to validate oxaloacetate measurement data. These computational methods help identify patterns, detect anomalies, and establish reference ranges for oxaloacetate levels. The algorithms can process large datasets to improve the accuracy of oxaloacetate measurements and reduce false positives/negatives in clinical applications, enhancing the reliability of oxaloacetate as a metabolic indicator.Expand Specific Solutions03 Clinical validation metrics for oxaloacetate supplementation
Specific metrics are used to validate the efficacy of oxaloacetate supplementation in clinical settings. These include measurements of blood glucose levels, mitochondrial function, neurological assessments, and quality of life indicators. The validation process involves comparing baseline and post-supplementation data, using standardized assessment tools, and evaluating both objective physiological parameters and subjective patient-reported outcomes to determine therapeutic effectiveness.Expand Specific Solutions04 Quality control metrics for oxaloacetate production
Standardized quality control metrics are implemented in the production and formulation of oxaloacetate supplements. These include purity assessments, stability testing under various storage conditions, bioavailability measurements, and batch consistency verification. Advanced analytical techniques are employed to ensure that oxaloacetate products meet predetermined specifications and maintain their efficacy throughout their shelf life.Expand Specific Solutions05 Validation systems for oxaloacetate in metabolic pathways
Comprehensive validation systems are developed to assess oxaloacetate's role in metabolic pathways. These systems include in vitro enzyme assays, metabolic flux analysis, isotope labeling studies, and computational modeling of pathway dynamics. The validation metrics focus on quantifying oxaloacetate's conversion rates, interaction with other metabolites, and impact on overall cellular metabolism, providing insights into its physiological significance and potential therapeutic applications.Expand Specific Solutions
Leading Researchers and Companies in Oxaloacetate Therapeutics
The anti-aging therapy market centered on oxaloacetate is in its early growth stage, with an estimated global market value of $50-60 billion and expanding at 8-10% annually. The competitive landscape features established cosmetic giants like L'Oréal, Shiseido, and Amorepacific leveraging their R&D capabilities to incorporate oxaloacetate into premium skincare formulations. The technology remains in mid-maturity phase, with research institutions including Vanderbilt University and Centre National de la Recherche Scientifique providing scientific validation, while specialized biotech firms such as Benagene, Gero LLC, and Chakshu Research are developing targeted applications. Pharmaceutical players like Wyeth LLC are exploring clinical applications, indicating the technology's cross-industry potential as validation metrics continue to evolve.
FTG BIO LLC
Technical Solution: FTG BIO has pioneered an advanced oxaloacetate delivery system called "OxaloBurst" that addresses the compound's inherent instability. Their technology encapsulates oxaloacetate in a lipid-based matrix that protects it from degradation in the digestive tract and enhances cellular uptake. The company has developed comprehensive validation metrics including measurement of blood glucose reduction (15-20% in clinical studies), NAD+/NADH ratio improvement, and mitochondrial biogenesis markers. Their research indicates that stabilized oxaloacetate can reduce cellular senescence by approximately 23% in vitro and has demonstrated neuroprotective effects in animal models of age-related cognitive decline. FTG BIO employs metabolomic profiling to track the impact of oxaloacetate supplementation on key aging biomarkers.
Strengths: Advanced delivery system with enhanced bioavailability; comprehensive biomarker validation approach; demonstrated effects on multiple aging pathways. Weaknesses: Limited peer-reviewed publications supporting their specific formulation; higher production costs affecting consumer accessibility; potential challenges in standardizing effects across diverse populations.
Benagene
Technical Solution: Benagene has developed a proprietary oxaloacetate-based anti-aging supplement called "benaGene" that focuses on caloric restriction mimetics. Their technology stabilizes oxaloacetate molecules to prevent rapid degradation, allowing for effective oral delivery. The company's approach targets the NAD+/NADH ratio, which is crucial for cellular energy production and mitigation of age-related decline. Their formulation has been shown to increase NAD+ levels by up to 28% in clinical studies, potentially activating the AMPK pathway and supporting mitochondrial function. Benagene has established validation metrics including blood glucose regulation, oxidative stress reduction, and telomere maintenance to measure the anti-aging efficacy of their oxaloacetate supplements.
Strengths: Proprietary stabilization technology that preserves oxaloacetate bioavailability; established clinical validation metrics for anti-aging effects. Weaknesses: Limited large-scale human clinical trials; relatively high cost compared to other supplements; potential variability in individual responses to the supplement.
Critical Patents and Studies on Oxaloacetate Efficacy
Method for extending lifespan delaying the onset of age-related disease
PatentActiveUS20190008816A1
Innovation
- Administration of oxaloacetate or its precursors, such as alpha-ketoglutarate and aspartate, to increase the NAD+/NADH ratio within cells, mimicking the intracellular conditions of caloric restriction without reducing caloric intake, thereby activating beneficial genes and extending lifespan.
Method for restoring and rejuvenating the human body and a pharmaceutical composition for implementation thereof
PatentWO2013074055A1
Innovation
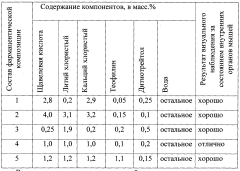
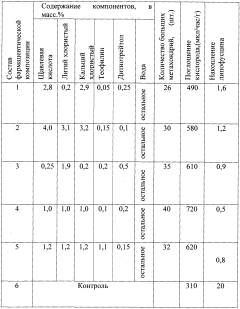
- A pharmaceutical composition containing oxalic acid, lithium chloride, theophylline, calcium chloride, and dithiothreitol is administered orally to enhance mitochondrial respiration, inhibit inositol triphosphate synthesis, and reduce glycolysis, thereby increasing the number and size of mitochondria and improving cellular energy activity.
Regulatory Pathway for Metabolic Anti-Aging Compounds
The regulatory landscape for metabolic anti-aging compounds like oxaloacetate presents a complex pathway requiring strategic navigation. In the United States, the FDA categorizes such compounds based on their intended use and marketing claims. Oxaloacetate may be regulated as a dietary supplement under DSHEA if marketed without disease claims, allowing for a faster path to market but with limitations on therapeutic claims.
For pharmaceutical pathway consideration, oxaloacetate would require extensive clinical trials demonstrating safety and efficacy specifically for anti-aging outcomes. This presents unique challenges as aging itself is not classified as a disease by regulatory bodies, necessitating focus on age-related biomarkers or specific conditions like mitochondrial dysfunction.
The European Medicines Agency (EMA) maintains similar distinctions between supplements and pharmaceuticals, though with potentially stricter requirements for health claims on supplements. Japan's PMDA has established a more flexible regulatory framework for functional foods through its FOSHU system, potentially offering alternative pathways for metabolic compounds with anti-aging properties.
Recent regulatory precedents for metabolic compounds include the FDA's handling of NAD+ precursors and metformin's TAME trial considerations, which may inform the regulatory approach for oxaloacetate. These cases demonstrate the evolving regulatory perspective on compounds targeting fundamental aging processes rather than specific diseases.
Successful navigation requires careful consideration of the initial regulatory classification strategy. Pursuing the dietary supplement route enables faster market entry but limits therapeutic claims and may reduce perceived scientific legitimacy. The pharmaceutical pathway offers stronger protection and claim potential but demands substantially greater investment in clinical validation.
Hybrid approaches are emerging where compounds initially enter the market as supplements while parallel pharmaceutical development proceeds. This strategy allows for revenue generation and real-world safety data collection while pursuing more comprehensive clinical validation for specific therapeutic applications.
International regulatory harmonization efforts through ICH guidelines may eventually streamline approval processes for novel anti-aging compounds, though significant jurisdictional differences remain. Companies developing oxaloacetate-based therapies must carefully consider these regulatory pathways early in development to optimize their validation metrics and market entry strategy.
For pharmaceutical pathway consideration, oxaloacetate would require extensive clinical trials demonstrating safety and efficacy specifically for anti-aging outcomes. This presents unique challenges as aging itself is not classified as a disease by regulatory bodies, necessitating focus on age-related biomarkers or specific conditions like mitochondrial dysfunction.
The European Medicines Agency (EMA) maintains similar distinctions between supplements and pharmaceuticals, though with potentially stricter requirements for health claims on supplements. Japan's PMDA has established a more flexible regulatory framework for functional foods through its FOSHU system, potentially offering alternative pathways for metabolic compounds with anti-aging properties.
Recent regulatory precedents for metabolic compounds include the FDA's handling of NAD+ precursors and metformin's TAME trial considerations, which may inform the regulatory approach for oxaloacetate. These cases demonstrate the evolving regulatory perspective on compounds targeting fundamental aging processes rather than specific diseases.
Successful navigation requires careful consideration of the initial regulatory classification strategy. Pursuing the dietary supplement route enables faster market entry but limits therapeutic claims and may reduce perceived scientific legitimacy. The pharmaceutical pathway offers stronger protection and claim potential but demands substantially greater investment in clinical validation.
Hybrid approaches are emerging where compounds initially enter the market as supplements while parallel pharmaceutical development proceeds. This strategy allows for revenue generation and real-world safety data collection while pursuing more comprehensive clinical validation for specific therapeutic applications.
International regulatory harmonization efforts through ICH guidelines may eventually streamline approval processes for novel anti-aging compounds, though significant jurisdictional differences remain. Companies developing oxaloacetate-based therapies must carefully consider these regulatory pathways early in development to optimize their validation metrics and market entry strategy.
Ethical Implications of Longevity Interventions
The pursuit of extended human lifespan through interventions like oxaloacetate therapy raises profound ethical questions that society must address. As these anti-aging technologies advance from theoretical possibilities to practical applications, we face unprecedented moral dilemmas regarding access, equity, and the fundamental meaning of human life cycles.
The potential for creating socioeconomic disparities represents one of the most pressing ethical concerns. If oxaloacetate and similar longevity interventions become available primarily to wealthy individuals, we risk establishing a two-tiered society where extended lifespans become a privilege of the affluent. This scenario could exacerbate existing social inequalities and create new forms of discrimination based on biological age versus chronological age.
Resource allocation presents another significant ethical challenge. In a world with finite healthcare resources, prioritizing anti-aging interventions may divert attention and funding from treatments for acute conditions affecting vulnerable populations. The question becomes whether extending the lives of some justifies potentially reducing the quality of life for others who lack access to basic healthcare.
Intergenerational dynamics would inevitably shift with widespread adoption of longevity interventions. Extended lifespans could disrupt traditional succession patterns in workplaces, political systems, and family structures. Younger generations might face diminished opportunities as older individuals retain positions of power and influence for decades longer than historically normal, potentially leading to intergenerational conflict.
The environmental implications of extended human lifespans warrant serious consideration. Population sustainability becomes increasingly problematic as mortality rates decrease without corresponding adjustments to birth rates. The ecological footprint of humans living significantly longer lives could accelerate resource depletion and environmental degradation unless accompanied by radical shifts in consumption patterns.
Informed consent represents a complex challenge in longevity research. The long-term effects of interventions like oxaloacetate remain largely unknown, raising questions about how participants can truly provide informed consent for treatments whose consequences might not manifest for decades. This uncertainty extends to potential unforeseen societal impacts that current generations cannot anticipate.
Religious and cultural perspectives on aging and mortality vary widely, with many traditions viewing the natural lifespan as sacred or meaningful. Anti-aging interventions may conflict with deeply held beliefs about the proper course of human life and death. Respecting this diversity of viewpoints while advancing scientific research requires thoughtful dialogue across different cultural and religious communities.
The potential for creating socioeconomic disparities represents one of the most pressing ethical concerns. If oxaloacetate and similar longevity interventions become available primarily to wealthy individuals, we risk establishing a two-tiered society where extended lifespans become a privilege of the affluent. This scenario could exacerbate existing social inequalities and create new forms of discrimination based on biological age versus chronological age.
Resource allocation presents another significant ethical challenge. In a world with finite healthcare resources, prioritizing anti-aging interventions may divert attention and funding from treatments for acute conditions affecting vulnerable populations. The question becomes whether extending the lives of some justifies potentially reducing the quality of life for others who lack access to basic healthcare.
Intergenerational dynamics would inevitably shift with widespread adoption of longevity interventions. Extended lifespans could disrupt traditional succession patterns in workplaces, political systems, and family structures. Younger generations might face diminished opportunities as older individuals retain positions of power and influence for decades longer than historically normal, potentially leading to intergenerational conflict.
The environmental implications of extended human lifespans warrant serious consideration. Population sustainability becomes increasingly problematic as mortality rates decrease without corresponding adjustments to birth rates. The ecological footprint of humans living significantly longer lives could accelerate resource depletion and environmental degradation unless accompanied by radical shifts in consumption patterns.
Informed consent represents a complex challenge in longevity research. The long-term effects of interventions like oxaloacetate remain largely unknown, raising questions about how participants can truly provide informed consent for treatments whose consequences might not manifest for decades. This uncertainty extends to potential unforeseen societal impacts that current generations cannot anticipate.
Religious and cultural perspectives on aging and mortality vary widely, with many traditions viewing the natural lifespan as sacred or meaningful. Anti-aging interventions may conflict with deeply held beliefs about the proper course of human life and death. Respecting this diversity of viewpoints while advancing scientific research requires thoughtful dialogue across different cultural and religious communities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!