Poisoning Mechanisms And Regeneration Strategies
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalyst Poisoning Background and Objectives
Catalyst poisoning represents a significant challenge in industrial catalytic processes, affecting efficiency, selectivity, and catalyst lifespan. The phenomenon has been observed since the early applications of catalysts in the petroleum and chemical industries during the early 20th century. As industrial processes evolved, the understanding of poisoning mechanisms has grown from empirical observations to sophisticated molecular-level analyses.
The evolution of catalyst technology has been marked by continuous efforts to mitigate poisoning effects. Initially, catalyst poisoning was primarily addressed through frequent replacement of deactivated catalysts, resulting in significant operational costs. By the mid-20th century, researchers began developing more poison-resistant catalyst formulations, incorporating structural modifications and protective components to shield active sites.
Recent decades have witnessed remarkable advancements in analytical techniques, enabling researchers to characterize poisoning mechanisms at atomic and molecular levels. Techniques such as X-ray photoelectron spectroscopy (XPS), temperature-programmed desorption (TPD), and advanced microscopy methods have revolutionized our understanding of how poisons interact with catalytic surfaces.
The primary objective of studying poisoning mechanisms is to develop more resilient catalytic systems that maintain performance under challenging industrial conditions. This includes understanding the chemical and physical interactions between poison molecules and catalyst active sites, identifying the kinetics of poisoning processes, and developing predictive models for catalyst deactivation.
Regeneration strategies have evolved from simple thermal treatments to sophisticated multi-step processes involving controlled oxidation, reduction, and chemical treatments. The goal is to restore catalytic activity while minimizing structural damage to the catalyst. Modern regeneration approaches aim to be more environmentally sustainable and energy-efficient compared to traditional methods.
Current research trends focus on developing "smart" catalysts with self-regenerating capabilities, poison-trapping components, and enhanced resistance to multiple types of poisons simultaneously. There is also growing interest in in-situ regeneration technologies that minimize process downtime and extend catalyst lifetime.
The economic implications of catalyst poisoning are substantial, with global industries spending billions annually on catalyst replacement and regeneration. Improved understanding of poisoning mechanisms and more effective regeneration strategies could significantly reduce these costs while enhancing process sustainability and reducing environmental impact.
This technical research aims to comprehensively review current knowledge of poisoning mechanisms across various catalytic systems, evaluate the effectiveness of existing regeneration strategies, and identify promising directions for future innovations in poison-resistant catalyst design and regeneration technologies.
The evolution of catalyst technology has been marked by continuous efforts to mitigate poisoning effects. Initially, catalyst poisoning was primarily addressed through frequent replacement of deactivated catalysts, resulting in significant operational costs. By the mid-20th century, researchers began developing more poison-resistant catalyst formulations, incorporating structural modifications and protective components to shield active sites.
Recent decades have witnessed remarkable advancements in analytical techniques, enabling researchers to characterize poisoning mechanisms at atomic and molecular levels. Techniques such as X-ray photoelectron spectroscopy (XPS), temperature-programmed desorption (TPD), and advanced microscopy methods have revolutionized our understanding of how poisons interact with catalytic surfaces.
The primary objective of studying poisoning mechanisms is to develop more resilient catalytic systems that maintain performance under challenging industrial conditions. This includes understanding the chemical and physical interactions between poison molecules and catalyst active sites, identifying the kinetics of poisoning processes, and developing predictive models for catalyst deactivation.
Regeneration strategies have evolved from simple thermal treatments to sophisticated multi-step processes involving controlled oxidation, reduction, and chemical treatments. The goal is to restore catalytic activity while minimizing structural damage to the catalyst. Modern regeneration approaches aim to be more environmentally sustainable and energy-efficient compared to traditional methods.
Current research trends focus on developing "smart" catalysts with self-regenerating capabilities, poison-trapping components, and enhanced resistance to multiple types of poisons simultaneously. There is also growing interest in in-situ regeneration technologies that minimize process downtime and extend catalyst lifetime.
The economic implications of catalyst poisoning are substantial, with global industries spending billions annually on catalyst replacement and regeneration. Improved understanding of poisoning mechanisms and more effective regeneration strategies could significantly reduce these costs while enhancing process sustainability and reducing environmental impact.
This technical research aims to comprehensively review current knowledge of poisoning mechanisms across various catalytic systems, evaluate the effectiveness of existing regeneration strategies, and identify promising directions for future innovations in poison-resistant catalyst design and regeneration technologies.
Market Analysis of Catalyst Regeneration Technologies
The global catalyst regeneration market is experiencing robust growth, valued at approximately 4.5 billion USD in 2022 and projected to reach 6.8 billion USD by 2028, representing a compound annual growth rate of 7.2%. This growth is primarily driven by increasing environmental regulations, rising demand for petroleum products, and the expanding chemical manufacturing sector worldwide.
Petroleum refining remains the dominant application segment, accounting for nearly 45% of the total market share. This dominance stems from the critical role catalysts play in various refining processes, including fluid catalytic cracking (FCC), hydroprocessing, and reforming. The chemical synthesis sector follows closely, representing approximately 30% of the market, with applications in polymer production, fine chemicals, and pharmaceutical manufacturing.
Geographically, North America and Europe currently lead the catalyst regeneration market, collectively holding about 55% of the global share. This leadership position is attributed to their established refining infrastructure and stringent environmental regulations. However, the Asia-Pacific region is witnessing the fastest growth rate at approximately 9% annually, driven by rapid industrialization in China and India, alongside expanding refining capacities.
The market structure features a mix of integrated oil companies with in-house regeneration capabilities and specialized third-party service providers. Major players include Porocel Corporation, Catalyst Services LLC, Eurecat S.A., and Nippon Ketjen Co., Ltd., who collectively control approximately 40% of the global market. Recent years have seen increasing consolidation through mergers and acquisitions as companies seek to expand their technological capabilities and geographical presence.
Technological segmentation reveals that thermal regeneration methods dominate with approximately 65% market share, followed by chemical (20%) and biological methods (15%). However, emerging technologies focusing on energy-efficient processes and reduced environmental impact are gaining traction, particularly those addressing complex poisoning mechanisms such as sulfur, nitrogen, and heavy metal contamination.
Customer demand is increasingly shifting toward regeneration services that can handle multiple poison types simultaneously while maintaining catalyst performance close to fresh catalyst levels. This trend is particularly evident in industries where catalyst costs represent a significant operational expense, such as petroleum refining and petrochemical production, where regeneration can offer cost savings of 40-60% compared to fresh catalyst replacement.
Petroleum refining remains the dominant application segment, accounting for nearly 45% of the total market share. This dominance stems from the critical role catalysts play in various refining processes, including fluid catalytic cracking (FCC), hydroprocessing, and reforming. The chemical synthesis sector follows closely, representing approximately 30% of the market, with applications in polymer production, fine chemicals, and pharmaceutical manufacturing.
Geographically, North America and Europe currently lead the catalyst regeneration market, collectively holding about 55% of the global share. This leadership position is attributed to their established refining infrastructure and stringent environmental regulations. However, the Asia-Pacific region is witnessing the fastest growth rate at approximately 9% annually, driven by rapid industrialization in China and India, alongside expanding refining capacities.
The market structure features a mix of integrated oil companies with in-house regeneration capabilities and specialized third-party service providers. Major players include Porocel Corporation, Catalyst Services LLC, Eurecat S.A., and Nippon Ketjen Co., Ltd., who collectively control approximately 40% of the global market. Recent years have seen increasing consolidation through mergers and acquisitions as companies seek to expand their technological capabilities and geographical presence.
Technological segmentation reveals that thermal regeneration methods dominate with approximately 65% market share, followed by chemical (20%) and biological methods (15%). However, emerging technologies focusing on energy-efficient processes and reduced environmental impact are gaining traction, particularly those addressing complex poisoning mechanisms such as sulfur, nitrogen, and heavy metal contamination.
Customer demand is increasingly shifting toward regeneration services that can handle multiple poison types simultaneously while maintaining catalyst performance close to fresh catalyst levels. This trend is particularly evident in industries where catalyst costs represent a significant operational expense, such as petroleum refining and petrochemical production, where regeneration can offer cost savings of 40-60% compared to fresh catalyst replacement.
Current Poisoning Challenges and Technical Limitations
Catalyst poisoning represents one of the most significant challenges in industrial catalytic processes, causing substantial economic losses and operational inefficiencies. Current poisoning mechanisms primarily involve chemical, physical, and thermal deactivation pathways. Chemical poisoning occurs when contaminants form strong chemical bonds with active catalyst sites, effectively blocking access to reactants. Common poisons include sulfur compounds, heavy metals, and nitrogen-containing molecules that demonstrate high affinity for catalyst surfaces.
Physical poisoning mechanisms involve the deposition of carbonaceous materials (coking) or inorganic substances that physically cover active sites without necessarily forming chemical bonds. This phenomenon is particularly problematic in petroleum refining and petrochemical processes where carbon-rich feedstocks are processed at elevated temperatures, leading to progressive catalyst deactivation over time.
Thermal deactivation presents another significant limitation, occurring when catalysts are exposed to temperatures exceeding their thermal stability thresholds. This results in sintering, phase transformations, and structural collapse that permanently damages catalyst architecture and reduces active surface area. The challenge is particularly acute in high-temperature applications like automotive catalytic converters and industrial reforming processes.
A major technical limitation in addressing catalyst poisoning is the difficulty in developing universal regeneration strategies. Current regeneration approaches are highly catalyst-specific and process-dependent, requiring customized protocols that may not be easily transferable across different industrial applications. This specificity increases operational complexity and maintenance costs.
Real-time monitoring of catalyst poisoning represents another significant technical gap. Existing analytical techniques often provide delayed feedback, making it difficult to implement preventive measures before significant catalyst damage occurs. The lack of in-situ monitoring capabilities forces many operations to rely on scheduled maintenance rather than condition-based interventions.
Regeneration processes themselves present technical challenges, as they often involve harsh conditions that can cause secondary damage to catalyst structures. Oxidative treatments to remove carbonaceous deposits, for instance, may simultaneously accelerate sintering or promote unwanted phase transformations in the catalyst material.
The economic feasibility of regeneration versus replacement remains a complex decision point for many industrial operations. Current regeneration technologies frequently fail to restore catalyst performance to original levels, particularly after multiple poisoning-regeneration cycles, leading to diminishing returns on regeneration investments and eventual mandatory replacement.
Physical poisoning mechanisms involve the deposition of carbonaceous materials (coking) or inorganic substances that physically cover active sites without necessarily forming chemical bonds. This phenomenon is particularly problematic in petroleum refining and petrochemical processes where carbon-rich feedstocks are processed at elevated temperatures, leading to progressive catalyst deactivation over time.
Thermal deactivation presents another significant limitation, occurring when catalysts are exposed to temperatures exceeding their thermal stability thresholds. This results in sintering, phase transformations, and structural collapse that permanently damages catalyst architecture and reduces active surface area. The challenge is particularly acute in high-temperature applications like automotive catalytic converters and industrial reforming processes.
A major technical limitation in addressing catalyst poisoning is the difficulty in developing universal regeneration strategies. Current regeneration approaches are highly catalyst-specific and process-dependent, requiring customized protocols that may not be easily transferable across different industrial applications. This specificity increases operational complexity and maintenance costs.
Real-time monitoring of catalyst poisoning represents another significant technical gap. Existing analytical techniques often provide delayed feedback, making it difficult to implement preventive measures before significant catalyst damage occurs. The lack of in-situ monitoring capabilities forces many operations to rely on scheduled maintenance rather than condition-based interventions.
Regeneration processes themselves present technical challenges, as they often involve harsh conditions that can cause secondary damage to catalyst structures. Oxidative treatments to remove carbonaceous deposits, for instance, may simultaneously accelerate sintering or promote unwanted phase transformations in the catalyst material.
The economic feasibility of regeneration versus replacement remains a complex decision point for many industrial operations. Current regeneration technologies frequently fail to restore catalyst performance to original levels, particularly after multiple poisoning-regeneration cycles, leading to diminishing returns on regeneration investments and eventual mandatory replacement.
Established Catalyst Regeneration Methodologies
01 Mechanisms of catalyst poisoning
Catalyst poisoning occurs when substances bind to active sites, reducing catalytic activity. Common poisons include sulfur compounds, heavy metals, and carbon deposits. Poisoning can be reversible or irreversible depending on the strength of binding between the poison and catalyst surface. Understanding these mechanisms is crucial for developing effective regeneration strategies and designing poison-resistant catalysts.- Catalyst poisoning mechanisms and prevention: Catalyst poisoning occurs when certain substances bind to active sites, reducing catalytic activity. Common poisons include sulfur compounds, heavy metals, and carbon deposits. Prevention strategies include feedstock purification, protective barriers, and selective poison traps that capture contaminants before they reach the catalyst surface. Understanding the specific poisoning mechanisms allows for the development of more poison-resistant catalyst formulations and operating conditions that minimize deactivation.
- Thermal regeneration techniques: Thermal regeneration involves controlled heating of deactivated catalysts to remove deposited contaminants. This process typically uses high-temperature oxidation to burn off carbon deposits (coke) and other organic poisons. The temperature, atmosphere composition, and heating rate must be carefully controlled to prevent catalyst sintering or structural damage. Thermal regeneration can be performed in-situ within the reactor or ex-situ in dedicated regeneration units, depending on the catalyst system and process requirements.
- Chemical regeneration methods: Chemical regeneration employs specific reagents to dissolve or transform catalyst poisons into removable forms. This approach includes acid washing to remove metal deposits, solvent extraction for organic contaminants, and chelating agents for heavy metal removal. Chemical treatments can be more selective than thermal methods, preserving catalyst structure while targeting specific poisons. Sequential treatments with different chemicals may be necessary for catalysts affected by multiple poison types, with careful control of pH, concentration, and contact time to maximize effectiveness.
- Continuous regeneration systems: Continuous regeneration systems allow for catalyst renewal without process interruption, maintaining consistent performance in industrial operations. These systems typically involve moving bed reactors where a portion of the catalyst is continuously withdrawn, regenerated, and returned to the process. Advanced designs incorporate multiple regeneration zones addressing different deactivation mechanisms sequentially. Continuous regeneration is particularly valuable in processes where catalyst deactivation is rapid or where production interruptions for batch regeneration would be economically prohibitive.
- Novel regeneration technologies and monitoring: Emerging regeneration technologies include plasma-assisted regeneration, supercritical fluid extraction, and microwave heating that offer improved efficiency and reduced environmental impact. These approaches can achieve more complete poison removal with lower energy consumption than conventional methods. Advanced monitoring techniques such as real-time spectroscopy and predictive modeling help optimize regeneration timing and conditions. Monitoring catalyst activity and poison accumulation allows for data-driven decisions about when and how to regenerate, maximizing catalyst lifetime and process economics.
02 Thermal regeneration techniques
Thermal regeneration involves heating poisoned catalysts to high temperatures to remove contaminants. This process can include oxidation of carbon deposits, desorption of adsorbed poisons, or decomposition of deactivating compounds. Temperature control is critical to prevent sintering or structural damage to the catalyst. Thermal regeneration can be performed in-situ or ex-situ depending on the catalyst system and process requirements.Expand Specific Solutions03 Chemical regeneration methods
Chemical regeneration uses specific reagents to remove or transform catalyst poisons. This may involve acid/base treatments, chelating agents for metal poison removal, or selective solvents to dissolve contaminants. Chemical washing can restore catalyst activity without the high energy requirements of thermal methods. The selection of regeneration chemicals depends on the nature of the poison and the catalyst composition to avoid damaging the catalyst structure.Expand Specific Solutions04 Innovative regeneration equipment and systems
Specialized equipment has been developed for efficient catalyst regeneration, including fluidized bed regenerators, continuous regeneration units, and pulse regeneration systems. These technologies optimize the regeneration process by ensuring uniform treatment, minimizing catalyst damage, and reducing downtime. Advanced monitoring systems can detect early signs of poisoning and automatically initiate regeneration procedures when necessary.Expand Specific Solutions05 Poison-resistant catalyst design
Developing catalysts with inherent resistance to poisoning is a preventive approach to the poisoning problem. Strategies include creating physical barriers to protect active sites, incorporating sacrificial components that preferentially bind poisons, and designing multi-functional catalysts that maintain activity even when partially poisoned. Novel materials and structures such as core-shell catalysts and alloys with specific electronic properties can significantly improve poison resistance and extend catalyst lifetime.Expand Specific Solutions
Leading Companies and Research Institutions in Catalysis
The poisoning mechanisms and regeneration strategies market is currently in a growth phase, with increasing research and development activities across academic institutions and pharmaceutical companies. The global market for catalyst regeneration and poisoning prevention technologies is expanding, driven by environmental regulations and efficiency demands in industrial processes. Leading academic institutions like Yale University, Harvard College, and Institut Curie are advancing fundamental research, while pharmaceutical companies including Allergan, Boehringer Ingelheim, and AgeX Therapeutics are developing commercial applications. Technology maturity varies significantly across different sectors, with established players like GLOBALFOUNDRIES implementing mature regeneration processes in semiconductor manufacturing, while biotechnology firms such as Synlogic and Vedanta Biosciences are pioneering novel biological regeneration approaches. Environmental applications are being advanced by companies like Viva Blu and Shanghai Taihe Water Technology.
President & Fellows of Harvard College
Technical Solution: Harvard College has developed sophisticated poisoning mechanisms and regeneration strategies centered on catalyst preservation and environmental remediation. Their approach incorporates molecular-level understanding of catalyst poisoning, particularly in heterogeneous catalysis where they've identified specific binding mechanisms of sulfur, nitrogen, and carbon-based poisons. Their regeneration platform utilizes controlled oxidation environments with precisely calibrated oxygen partial pressures to selectively remove carbon deposits while preserving catalyst structure. Harvard researchers have pioneered electrochemical regeneration techniques that apply controlled potential to catalyst surfaces, effectively oxidizing and removing poisoning species without thermal damage. Additionally, they've developed novel solvent extraction methods using tailored ionic liquids that can selectively dissolve and remove specific poisoning compounds while leaving the catalyst structure intact, achieving regeneration efficiencies exceeding 90% in some applications.
Strengths: Exceptional fundamental research capabilities; sophisticated analytical techniques for poison identification; innovative non-thermal regeneration approaches. Weaknesses: Some techniques remain laboratory-scale and face challenges in industrial implementation; certain regeneration methods require specialized equipment and expertise; higher costs associated with advanced regeneration technologies compared to conventional replacement strategies.
Sanford Burnham Prebys Medical Discovery Institute
Technical Solution: Sanford Burnham Prebys Medical Discovery Institute has developed innovative approaches to poisoning mechanisms and regeneration strategies focused on cellular and tissue regeneration after toxic exposure. Their platform centers on understanding molecular mechanisms of cellular poisoning, particularly how environmental toxins, pharmaceutical compounds, and metabolic byproducts can disrupt cellular function. Their regeneration strategies employ small molecule activators of endogenous repair pathways, including compounds that can stimulate mitochondrial biogenesis after mitochondrial poisoning. They've pioneered techniques to enhance cellular detoxification mechanisms through targeted activation of Nrf2 and related transcription factors that upregulate antioxidant response elements. Their research extends to tissue-specific regeneration after toxic injury, with particular focus on liver and kidney regeneration following exposure to nephrotoxic and hepatotoxic compounds. Additionally, they've developed screening platforms to identify compounds that can selectively reverse specific poisoning mechanisms, such as heavy metal chelators optimized for cellular penetration and minimal side effects.
Strengths: Strong focus on translational applications; comprehensive understanding of cellular detoxification pathways; innovative drug discovery platform for regenerative compounds; expertise in tissue-specific regeneration. Weaknesses: Many approaches remain in preclinical development stages; biological complexity creates challenges for targeted interventions; regeneration strategies may have variable efficacy depending on extent of toxic damage.
Critical Poisoning Mechanism Research Breakthroughs
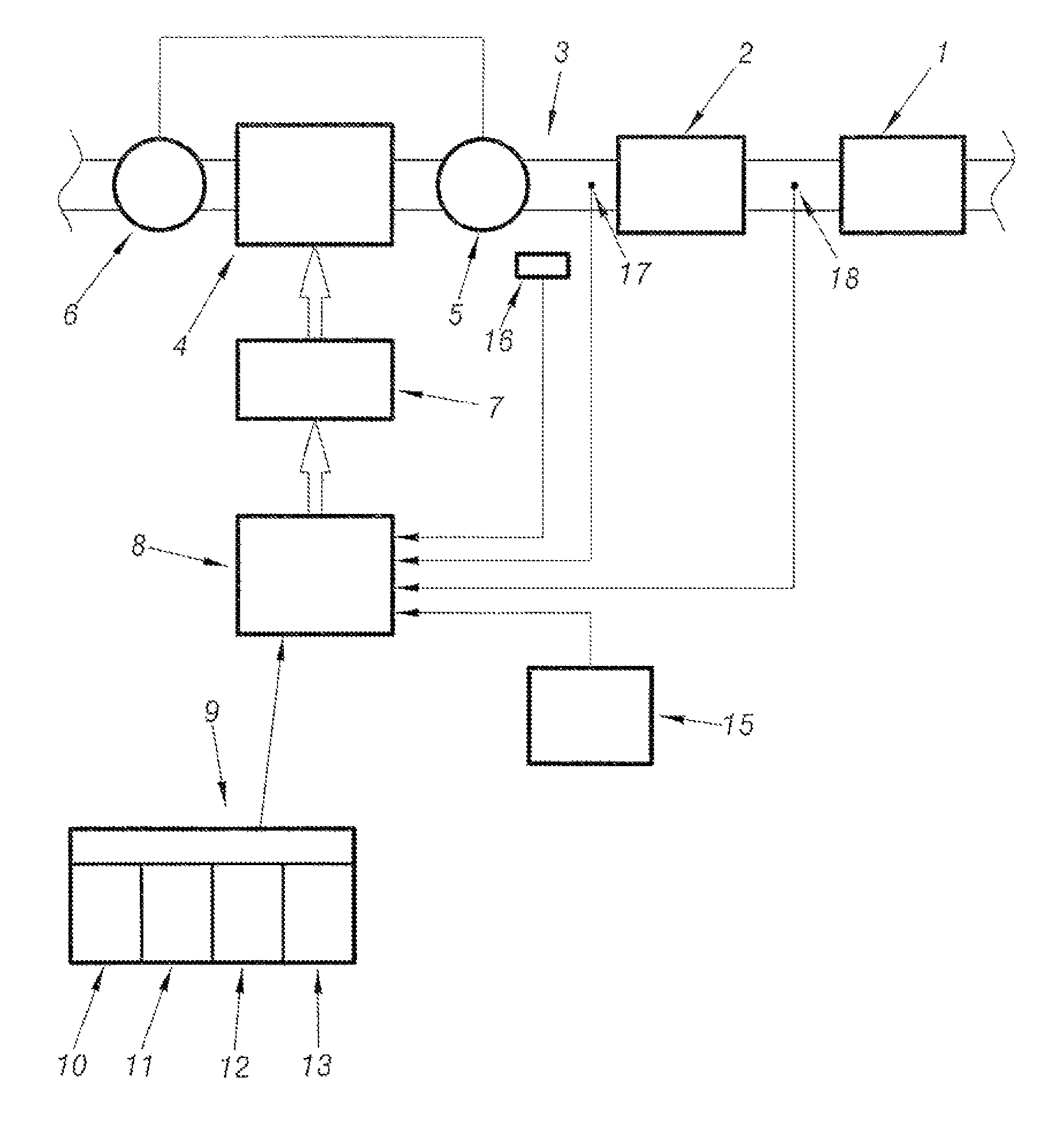
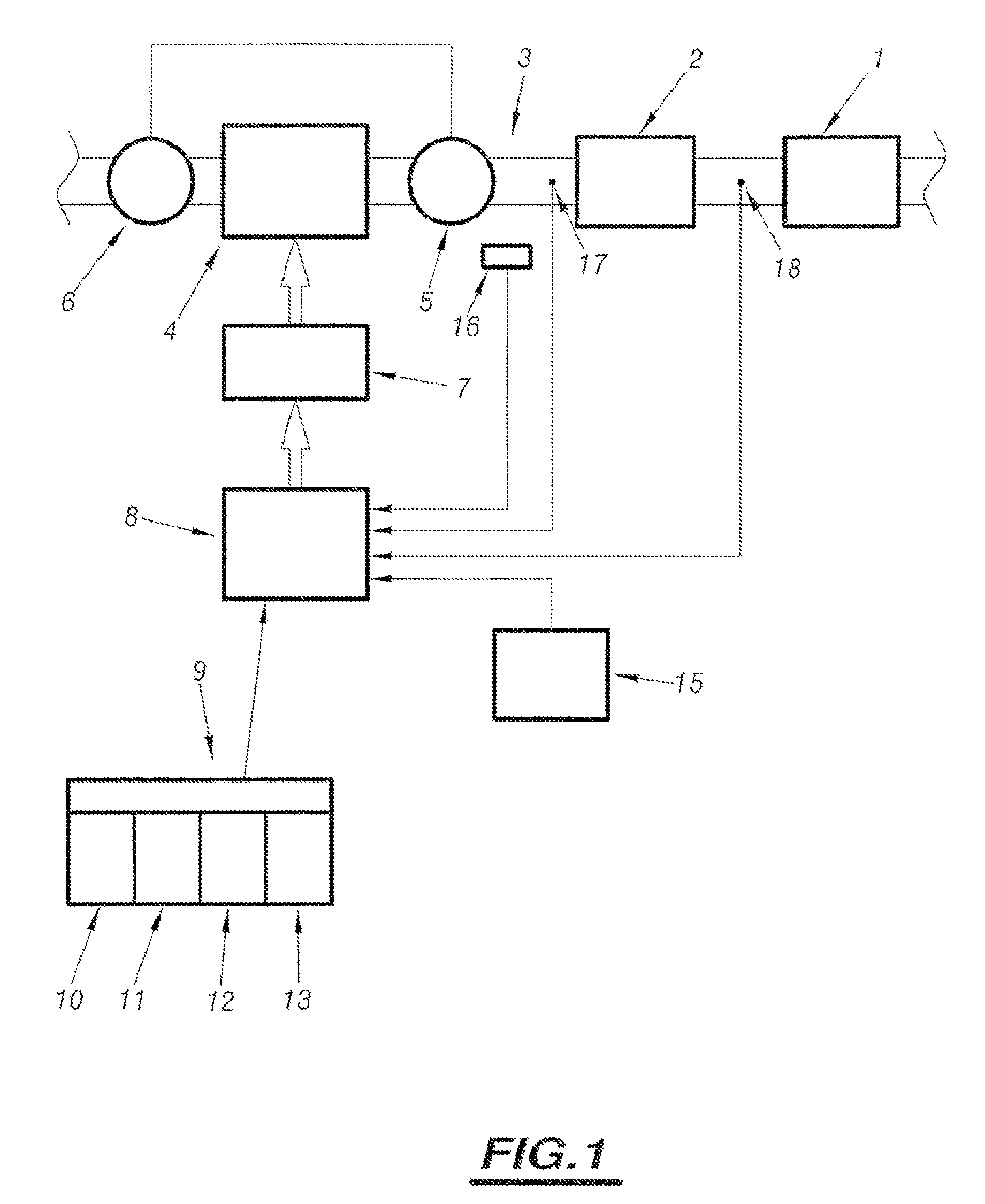
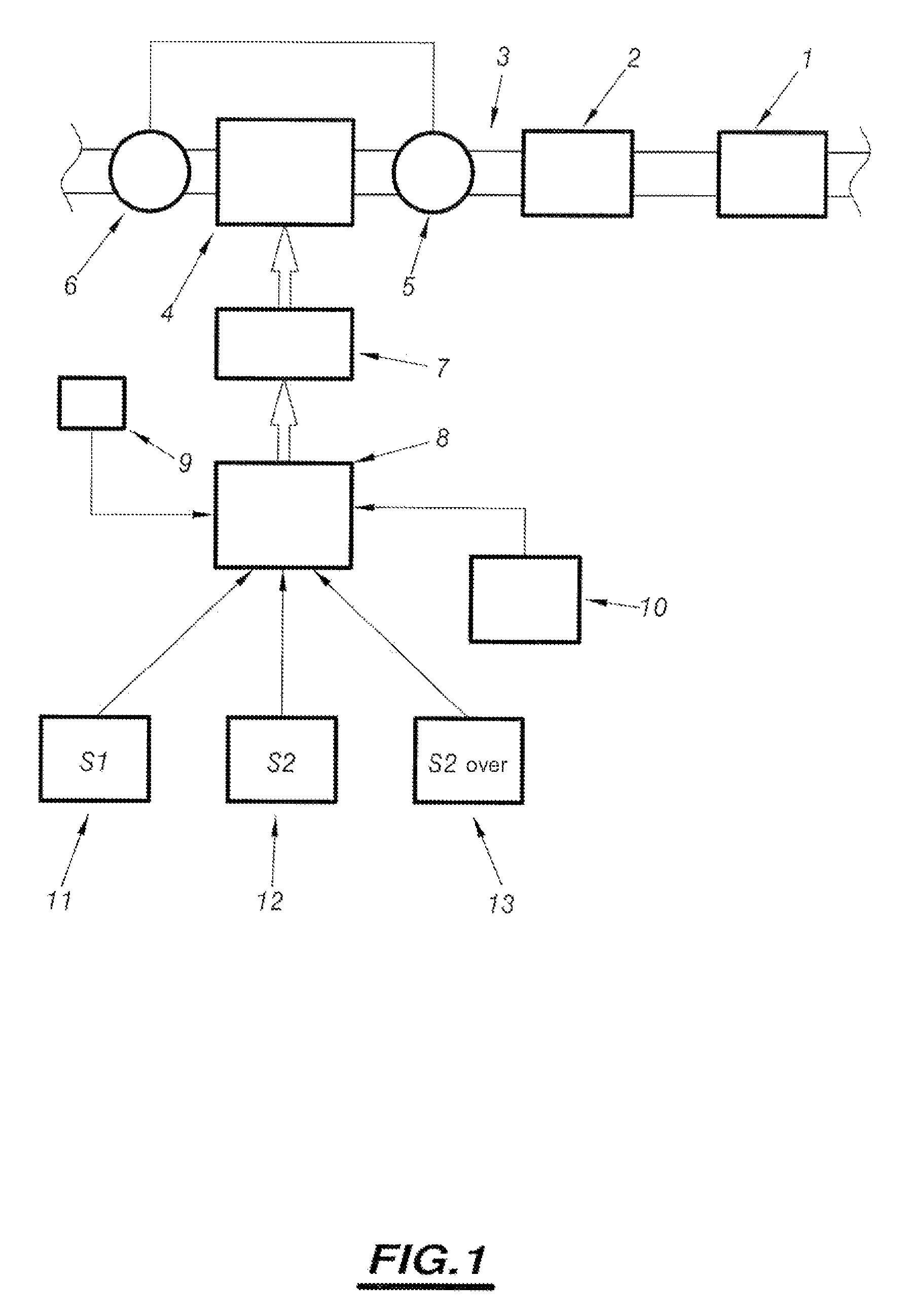
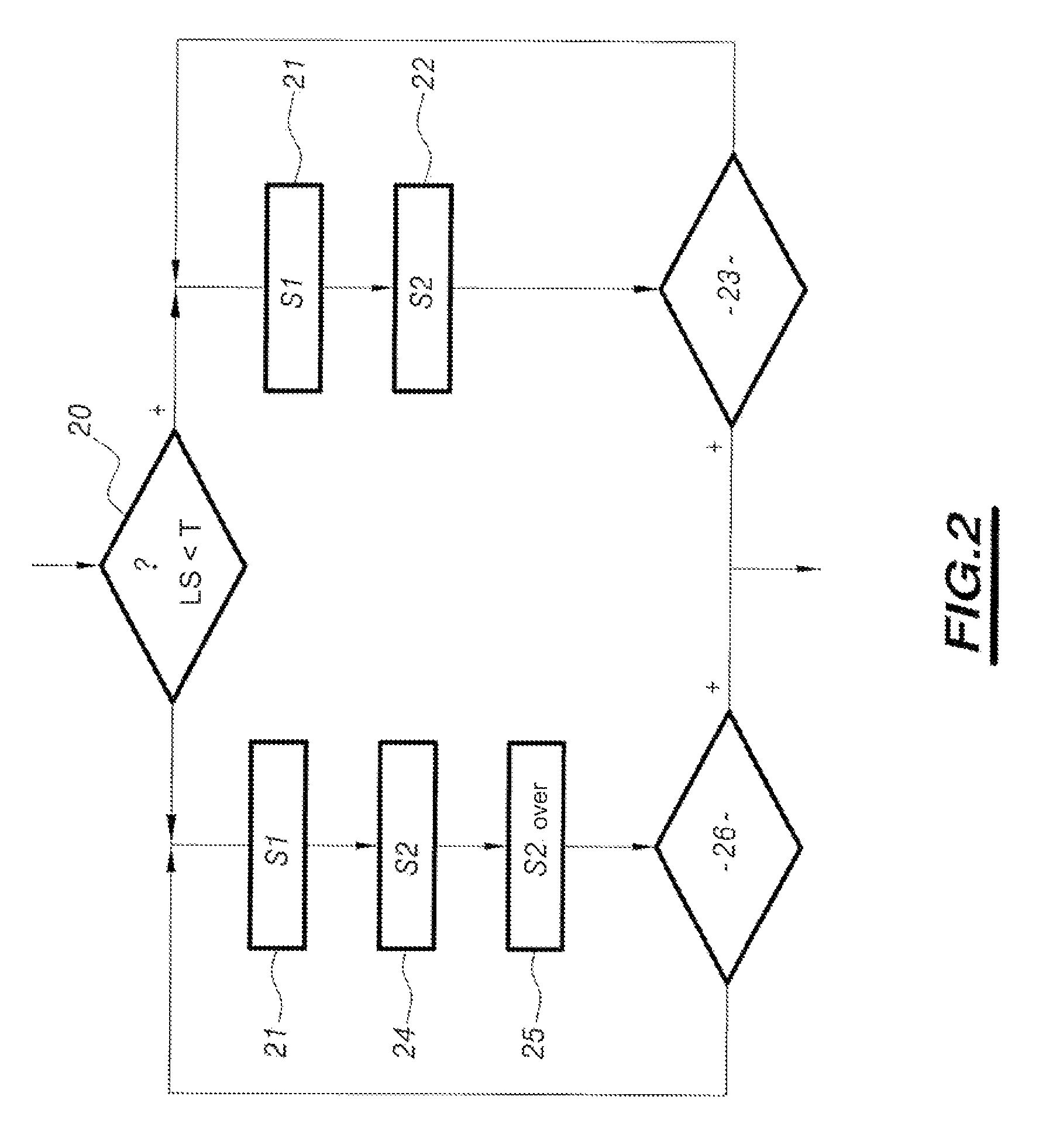
Method of assisting regeneration of pollution management means associated with catalyst forming means
PatentInactiveUS7802422B2
Innovation
- A system that adjusts engine operation through common rail fuel supply to shift between four regeneration strategies, monitoring thermal levels and catalyst priming to optimize exhaust line temperatures, ensuring efficient and complete regeneration by dynamically switching between normal, level 1, level 2, and over-calibrated level 2 strategies.
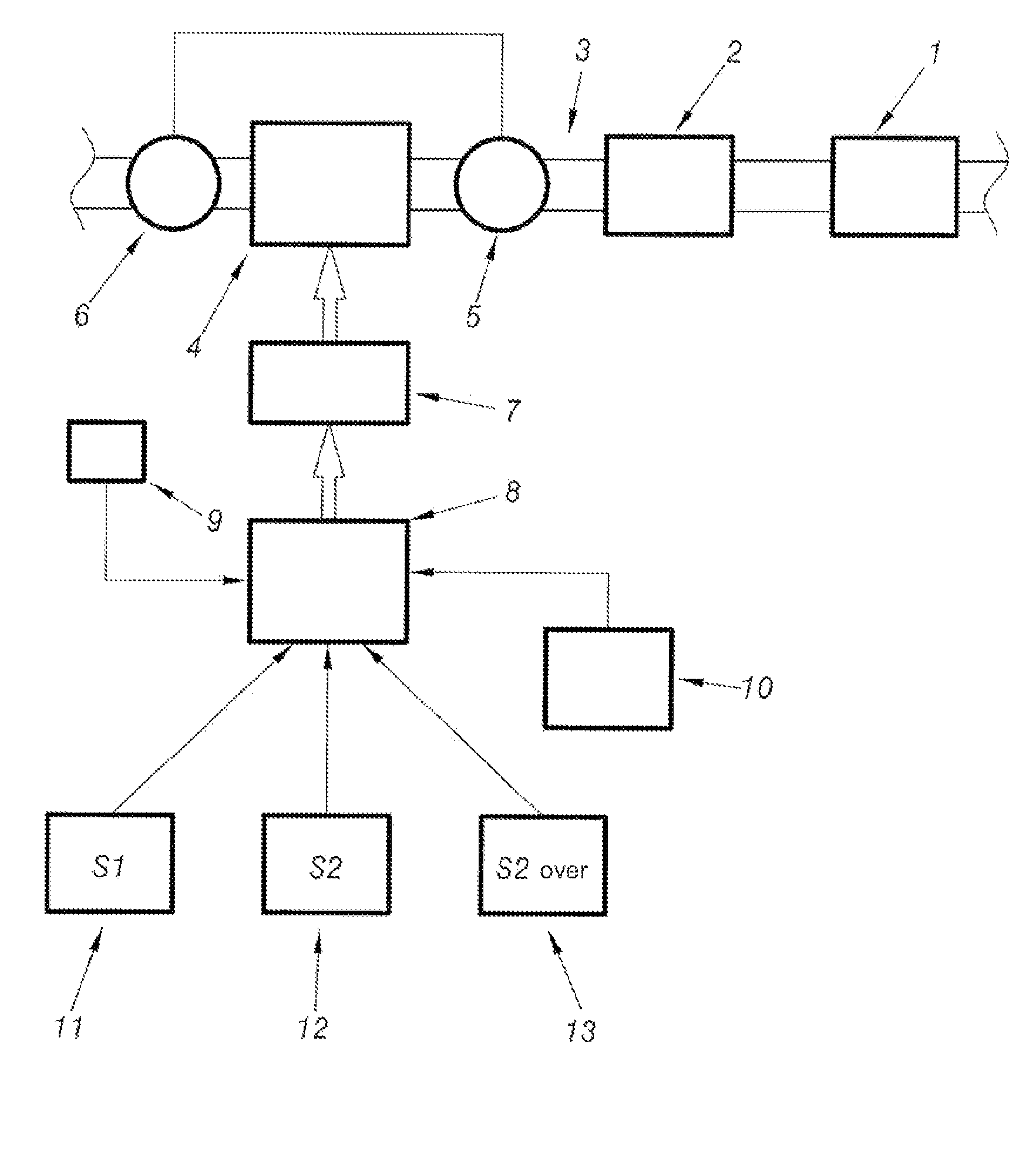
System for assisting regeneration of pollution management means for motor vehicle engine
PatentInactiveUS7596942B2
Innovation
- A system that analyzes the loading state of depollution means and compares it to predetermined threshold values to adapt engine operation parameters, implementing different regeneration strategies to achieve optimal thermal levels in the exhaust line, including level 1 and over-calibrated level 2 strategies, ensuring complete and efficient regeneration.
Environmental Impact of Catalyst Poisoning and Regeneration
Catalyst poisoning processes have significant environmental implications that extend beyond mere technical performance issues. The release of deactivated catalysts into the environment can lead to soil and water contamination with heavy metals and toxic compounds. For instance, automotive catalytic converters contain platinum group metals that, if improperly disposed of, can leach into groundwater systems. Similarly, industrial catalysts often contain elements like nickel, cobalt, and molybdenum that pose environmental risks when released without proper treatment.
Regeneration processes themselves create substantial environmental footprints. Thermal regeneration methods, commonly employed for catalyst revival, consume significant energy and produce greenhouse gas emissions. Studies indicate that the regeneration of fluid catalytic cracking (FCC) catalysts can account for up to 30% of a refinery's carbon dioxide emissions. Chemical regeneration approaches often utilize hazardous solvents and acids, creating waste streams that require specialized treatment and disposal protocols.
Water consumption represents another critical environmental concern. Wet regeneration techniques can consume between 3-10 gallons of water per pound of catalyst processed, placing pressure on water resources in water-stressed regions. The wastewater generated typically contains dissolved metals, organic contaminants, and treatment chemicals that require extensive purification before discharge.
Recent regulatory frameworks have begun addressing these environmental challenges. The European Union's REACH regulations and the United States EPA's RCRA guidelines now classify spent catalysts as hazardous waste requiring specialized handling. This regulatory landscape has driven innovation toward more environmentally sustainable approaches to catalyst management.
Emerging green regeneration technologies show promising environmental benefits. Supercritical fluid regeneration using CO2 reduces solvent waste by 85% compared to conventional methods. Bioleaching techniques employ microorganisms to recover precious metals from spent catalysts, reducing chemical usage and energy requirements by approximately 40%. Additionally, closed-loop regeneration systems that capture and reuse process chemicals have demonstrated 70% reductions in waste generation.
Life cycle assessment studies reveal that despite the environmental impacts of regeneration, the practice typically produces 60-80% lower environmental footprints compared to manufacturing new catalysts. This favorable comparison stems primarily from avoided mining activities and reduced energy-intensive refining processes required for virgin catalyst production.
Regeneration processes themselves create substantial environmental footprints. Thermal regeneration methods, commonly employed for catalyst revival, consume significant energy and produce greenhouse gas emissions. Studies indicate that the regeneration of fluid catalytic cracking (FCC) catalysts can account for up to 30% of a refinery's carbon dioxide emissions. Chemical regeneration approaches often utilize hazardous solvents and acids, creating waste streams that require specialized treatment and disposal protocols.
Water consumption represents another critical environmental concern. Wet regeneration techniques can consume between 3-10 gallons of water per pound of catalyst processed, placing pressure on water resources in water-stressed regions. The wastewater generated typically contains dissolved metals, organic contaminants, and treatment chemicals that require extensive purification before discharge.
Recent regulatory frameworks have begun addressing these environmental challenges. The European Union's REACH regulations and the United States EPA's RCRA guidelines now classify spent catalysts as hazardous waste requiring specialized handling. This regulatory landscape has driven innovation toward more environmentally sustainable approaches to catalyst management.
Emerging green regeneration technologies show promising environmental benefits. Supercritical fluid regeneration using CO2 reduces solvent waste by 85% compared to conventional methods. Bioleaching techniques employ microorganisms to recover precious metals from spent catalysts, reducing chemical usage and energy requirements by approximately 40%. Additionally, closed-loop regeneration systems that capture and reuse process chemicals have demonstrated 70% reductions in waste generation.
Life cycle assessment studies reveal that despite the environmental impacts of regeneration, the practice typically produces 60-80% lower environmental footprints compared to manufacturing new catalysts. This favorable comparison stems primarily from avoided mining activities and reduced energy-intensive refining processes required for virgin catalyst production.
Economic Feasibility of Advanced Regeneration Strategies
The economic viability of advanced catalyst regeneration strategies represents a critical factor in industrial decision-making processes. When evaluating the financial feasibility of implementing sophisticated regeneration techniques for poisoned catalysts, organizations must consider both direct costs and long-term economic benefits. Initial capital expenditures for advanced regeneration equipment typically range from $500,000 to several million dollars, depending on the scale of operations and technological complexity.
Operational expenses present another significant economic consideration, with labor costs, energy consumption, and specialized chemical reagents contributing to ongoing financial commitments. However, these costs must be weighed against the substantial economic benefits of extending catalyst lifespans. Industry data suggests that effective regeneration can extend catalyst life by 30-70%, translating to significant savings in replacement costs, which can exceed $10,000 per kilogram for precious metal catalysts.
Return-on-investment (ROI) analyses indicate that advanced regeneration technologies typically achieve payback periods of 12-36 months in large-scale industrial applications. This favorable economic profile is further enhanced when considering production continuity benefits, as regeneration can often be performed without complete system shutdowns, minimizing costly production interruptions.
Environmental compliance costs also factor into economic feasibility calculations. Traditional disposal methods for spent catalysts face increasingly stringent regulations, with associated penalties and disposal fees. Advanced regeneration strategies that minimize waste generation can significantly reduce these compliance costs while simultaneously enhancing corporate sustainability profiles.
Market analysis reveals growing economic incentives for regeneration technologies, with the global catalyst regeneration market projected to reach $5.8 billion by 2027, growing at a CAGR of 5.7%. This growth is driven by rising catalyst prices, particularly for precious metal-based formulations, and increasing environmental regulations worldwide.
Scale considerations demonstrate that economic feasibility improves significantly with operational scale. While smaller operations may struggle to justify dedicated regeneration facilities, collaborative models including third-party regeneration services have emerged, offering economically viable solutions across various operational scales with service fees typically representing 15-30% of new catalyst costs.
Operational expenses present another significant economic consideration, with labor costs, energy consumption, and specialized chemical reagents contributing to ongoing financial commitments. However, these costs must be weighed against the substantial economic benefits of extending catalyst lifespans. Industry data suggests that effective regeneration can extend catalyst life by 30-70%, translating to significant savings in replacement costs, which can exceed $10,000 per kilogram for precious metal catalysts.
Return-on-investment (ROI) analyses indicate that advanced regeneration technologies typically achieve payback periods of 12-36 months in large-scale industrial applications. This favorable economic profile is further enhanced when considering production continuity benefits, as regeneration can often be performed without complete system shutdowns, minimizing costly production interruptions.
Environmental compliance costs also factor into economic feasibility calculations. Traditional disposal methods for spent catalysts face increasingly stringent regulations, with associated penalties and disposal fees. Advanced regeneration strategies that minimize waste generation can significantly reduce these compliance costs while simultaneously enhancing corporate sustainability profiles.
Market analysis reveals growing economic incentives for regeneration technologies, with the global catalyst regeneration market projected to reach $5.8 billion by 2027, growing at a CAGR of 5.7%. This growth is driven by rising catalyst prices, particularly for precious metal-based formulations, and increasing environmental regulations worldwide.
Scale considerations demonstrate that economic feasibility improves significantly with operational scale. While smaller operations may struggle to justify dedicated regeneration facilities, collaborative models including third-party regeneration services have emerged, offering economically viable solutions across various operational scales with service fees typically representing 15-30% of new catalyst costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!