Bimetallic Single-Atom Sites And Synergistic Effects
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bimetallic SAC Development History and Objectives
Single-atom catalysts (SACs) emerged in the early 2000s as a revolutionary concept in heterogeneous catalysis, offering maximum atom efficiency by dispersing metal atoms individually on supports. The evolution of bimetallic single-atom catalysts (SACs) represents a significant advancement in this field, combining the advantages of single-atom dispersion with synergistic effects between different metal species.
The development of bimetallic SACs can be traced through several key phases. Initially, researchers focused on monometallic SACs, with pioneering work by Zhang and colleagues in 2011 demonstrating the feasibility of anchoring isolated platinum atoms on iron oxide. By 2015, the field expanded to explore dual-metal systems, with early attempts primarily focusing on random distributions of two different metal atoms on supports without controlled proximity or interaction.
A breakthrough occurred around 2017-2018 when researchers began successfully synthesizing true bimetallic single-atom sites, where two different metal atoms were positioned in close proximity to enable direct interaction and electronic coupling. This marked the transition from simply co-dispersed single atoms to genuinely synergistic bimetallic sites.
The technological progression has been driven by advances in synthetic methodologies, including atomic layer deposition, galvanic replacement, and coordination chemistry approaches. Simultaneously, characterization techniques have evolved dramatically, with aberration-corrected electron microscopy, X-ray absorption spectroscopy, and advanced computational modeling enabling precise identification of bimetallic configurations at the atomic scale.
Current research objectives in this field focus on several key areas. First, developing precise synthetic control over the spatial arrangement of heterometallic atoms to optimize their synergistic effects. Second, understanding the fundamental mechanisms of electronic interaction between different metal centers and how these influence catalytic performance. Third, expanding the range of metal combinations to discover novel synergistic pairs with enhanced properties.
The long-term technological goals include designing bimetallic SACs with tailored electronic structures for specific reactions, particularly those relevant to renewable energy applications such as water splitting, CO2 reduction, and nitrogen fixation. Additionally, researchers aim to develop scalable synthesis methods that maintain atomic precision while enabling industrial-scale production, addressing the gap between laboratory demonstrations and practical applications.
The evolution of this field reflects broader trends in nanocatalysis toward atomic-level precision and rational design principles, moving beyond empirical approaches to theory-guided catalyst development with predictable performance characteristics.
The development of bimetallic SACs can be traced through several key phases. Initially, researchers focused on monometallic SACs, with pioneering work by Zhang and colleagues in 2011 demonstrating the feasibility of anchoring isolated platinum atoms on iron oxide. By 2015, the field expanded to explore dual-metal systems, with early attempts primarily focusing on random distributions of two different metal atoms on supports without controlled proximity or interaction.
A breakthrough occurred around 2017-2018 when researchers began successfully synthesizing true bimetallic single-atom sites, where two different metal atoms were positioned in close proximity to enable direct interaction and electronic coupling. This marked the transition from simply co-dispersed single atoms to genuinely synergistic bimetallic sites.
The technological progression has been driven by advances in synthetic methodologies, including atomic layer deposition, galvanic replacement, and coordination chemistry approaches. Simultaneously, characterization techniques have evolved dramatically, with aberration-corrected electron microscopy, X-ray absorption spectroscopy, and advanced computational modeling enabling precise identification of bimetallic configurations at the atomic scale.
Current research objectives in this field focus on several key areas. First, developing precise synthetic control over the spatial arrangement of heterometallic atoms to optimize their synergistic effects. Second, understanding the fundamental mechanisms of electronic interaction between different metal centers and how these influence catalytic performance. Third, expanding the range of metal combinations to discover novel synergistic pairs with enhanced properties.
The long-term technological goals include designing bimetallic SACs with tailored electronic structures for specific reactions, particularly those relevant to renewable energy applications such as water splitting, CO2 reduction, and nitrogen fixation. Additionally, researchers aim to develop scalable synthesis methods that maintain atomic precision while enabling industrial-scale production, addressing the gap between laboratory demonstrations and practical applications.
The evolution of this field reflects broader trends in nanocatalysis toward atomic-level precision and rational design principles, moving beyond empirical approaches to theory-guided catalyst development with predictable performance characteristics.
Market Applications and Demand Analysis for Bimetallic SACs
The market for bimetallic single-atom catalysts (SACs) is experiencing rapid growth driven by increasing demands for more efficient, sustainable, and cost-effective catalytic solutions across multiple industries. Current market analysis indicates that the global catalyst market exceeds $30 billion annually, with precious metal catalysts representing a significant portion of this value. Bimetallic SACs offer substantial economic advantages by maximizing atom efficiency and reducing precious metal usage by up to 95% compared to traditional catalysts.
The automotive sector represents one of the largest application areas, with stringent emission regulations worldwide driving demand for more efficient catalytic converters. Bimetallic SACs demonstrate superior performance in reducing NOx, CO, and hydrocarbon emissions while using significantly less platinum group metals. This application alone could represent a market opportunity of several billion dollars annually as manufacturers seek to meet Euro 7, China 6, and equivalent standards.
The chemical manufacturing industry shows growing interest in bimetallic SACs for selective hydrogenation, oxidation, and coupling reactions. These catalysts enable more selective transformations, reduced energy requirements, and fewer byproducts. Market research indicates that specialty chemical manufacturers are willing to pay premium prices for catalysts that can improve yield by even 2-3% or reduce energy consumption by 5-10%.
Renewable energy applications represent the fastest-growing segment for bimetallic SACs. In hydrogen production via water splitting, bimetallic single-atom catalysts have demonstrated activity approaching platinum while using earth-abundant metals. The hydrogen economy is projected to reach $500 billion by 2030, with electrolyzers and fuel cells requiring advanced catalysts as critical components.
Environmental remediation presents another substantial market, with bimetallic SACs showing promise for water purification, CO2 reduction, and pollutant degradation. Municipal water treatment facilities and industrial wastewater management systems represent a combined market exceeding $200 billion globally, with growing regulatory pressure to adopt more effective treatment technologies.
Pharmaceutical manufacturing is increasingly adopting continuous flow processes where bimetallic SACs excel due to their stability and selectivity. The ability to perform challenging transformations under milder conditions translates to significant cost savings and improved product quality, addressing the pharmaceutical industry's $1.3 trillion global market.
Market adoption faces challenges including scaling production methods, ensuring long-term stability under industrial conditions, and developing standardized characterization protocols. However, the demonstrated performance advantages and potential for significant cost reductions across multiple high-value applications indicate strong market growth potential for bimetallic SACs over the next decade.
The automotive sector represents one of the largest application areas, with stringent emission regulations worldwide driving demand for more efficient catalytic converters. Bimetallic SACs demonstrate superior performance in reducing NOx, CO, and hydrocarbon emissions while using significantly less platinum group metals. This application alone could represent a market opportunity of several billion dollars annually as manufacturers seek to meet Euro 7, China 6, and equivalent standards.
The chemical manufacturing industry shows growing interest in bimetallic SACs for selective hydrogenation, oxidation, and coupling reactions. These catalysts enable more selective transformations, reduced energy requirements, and fewer byproducts. Market research indicates that specialty chemical manufacturers are willing to pay premium prices for catalysts that can improve yield by even 2-3% or reduce energy consumption by 5-10%.
Renewable energy applications represent the fastest-growing segment for bimetallic SACs. In hydrogen production via water splitting, bimetallic single-atom catalysts have demonstrated activity approaching platinum while using earth-abundant metals. The hydrogen economy is projected to reach $500 billion by 2030, with electrolyzers and fuel cells requiring advanced catalysts as critical components.
Environmental remediation presents another substantial market, with bimetallic SACs showing promise for water purification, CO2 reduction, and pollutant degradation. Municipal water treatment facilities and industrial wastewater management systems represent a combined market exceeding $200 billion globally, with growing regulatory pressure to adopt more effective treatment technologies.
Pharmaceutical manufacturing is increasingly adopting continuous flow processes where bimetallic SACs excel due to their stability and selectivity. The ability to perform challenging transformations under milder conditions translates to significant cost savings and improved product quality, addressing the pharmaceutical industry's $1.3 trillion global market.
Market adoption faces challenges including scaling production methods, ensuring long-term stability under industrial conditions, and developing standardized characterization protocols. However, the demonstrated performance advantages and potential for significant cost reductions across multiple high-value applications indicate strong market growth potential for bimetallic SACs over the next decade.
Current Status and Technical Challenges in Bimetallic SACs
Bimetallic single-atom catalysts (SACs) represent a frontier in heterogeneous catalysis research, combining two distinct metal elements at the atomic level to create unique active sites with enhanced catalytic properties. Currently, significant progress has been made in synthesizing and characterizing these advanced materials, with several methodologies emerging as predominant approaches, including wet chemistry methods, atomic layer deposition, and high-temperature atom trapping.
The global research landscape shows concentrated efforts in China, the United States, and Europe, with Chinese institutions leading in publication volume while Western institutions often pioneering fundamental breakthroughs. Recent advancements have demonstrated remarkable activity improvements in reactions including oxygen reduction, CO2 reduction, and nitrogen fixation, where bimetallic synergies often outperform their monometallic counterparts by orders of magnitude.
Despite these achievements, the field faces substantial technical challenges. Precise structural control remains elusive, as achieving consistent atomic dispersion of two different metals with predetermined spatial relationships presents significant synthetic difficulties. The tendency of metal atoms to aggregate during synthesis or catalytic operation undermines stability, particularly under harsh reaction conditions such as high temperatures or corrosive environments.
Characterization limitations constitute another major obstacle. Even advanced techniques like aberration-corrected transmission electron microscopy (AC-TEM) and X-ray absorption spectroscopy (XAS) struggle to definitively identify the coordination environment and oxidation states of neighboring metal atoms. This creates uncertainty in structure-property relationships that is difficult to resolve with current analytical capabilities.
The mechanistic understanding of synergistic effects between different metal atoms remains incomplete. While enhanced performance is frequently observed, the electronic and geometric factors driving these improvements are often speculative rather than conclusively established. Computational models frequently diverge from experimental results due to the complexity of accurately representing these atomic interactions.
Scalability presents perhaps the most significant barrier to industrial implementation. Current synthetic approaches typically yield milligram quantities under laboratory conditions, whereas commercial applications would require kilogram-scale production with consistent quality. The specialized equipment and precise control needed for synthesis further complicate economic viability.
Environmental considerations also pose challenges, as some synthesis methods rely on toxic precursors or generate hazardous waste. Additionally, the long-term environmental impact of these novel nanomaterials remains inadequately studied, raising potential regulatory concerns for future applications.
The global research landscape shows concentrated efforts in China, the United States, and Europe, with Chinese institutions leading in publication volume while Western institutions often pioneering fundamental breakthroughs. Recent advancements have demonstrated remarkable activity improvements in reactions including oxygen reduction, CO2 reduction, and nitrogen fixation, where bimetallic synergies often outperform their monometallic counterparts by orders of magnitude.
Despite these achievements, the field faces substantial technical challenges. Precise structural control remains elusive, as achieving consistent atomic dispersion of two different metals with predetermined spatial relationships presents significant synthetic difficulties. The tendency of metal atoms to aggregate during synthesis or catalytic operation undermines stability, particularly under harsh reaction conditions such as high temperatures or corrosive environments.
Characterization limitations constitute another major obstacle. Even advanced techniques like aberration-corrected transmission electron microscopy (AC-TEM) and X-ray absorption spectroscopy (XAS) struggle to definitively identify the coordination environment and oxidation states of neighboring metal atoms. This creates uncertainty in structure-property relationships that is difficult to resolve with current analytical capabilities.
The mechanistic understanding of synergistic effects between different metal atoms remains incomplete. While enhanced performance is frequently observed, the electronic and geometric factors driving these improvements are often speculative rather than conclusively established. Computational models frequently diverge from experimental results due to the complexity of accurately representing these atomic interactions.
Scalability presents perhaps the most significant barrier to industrial implementation. Current synthetic approaches typically yield milligram quantities under laboratory conditions, whereas commercial applications would require kilogram-scale production with consistent quality. The specialized equipment and precise control needed for synthesis further complicate economic viability.
Environmental considerations also pose challenges, as some synthesis methods rely on toxic precursors or generate hazardous waste. Additionally, the long-term environmental impact of these novel nanomaterials remains inadequately studied, raising potential regulatory concerns for future applications.
Current Synthetic Strategies for Bimetallic Single-Atom Sites
01 Bimetallic single-atom catalysts for electrochemical applications
Bimetallic single-atom catalysts demonstrate enhanced electrochemical performance through synergistic effects between different metal atoms. These catalysts exhibit improved activity, selectivity, and stability for various electrochemical reactions including oxygen reduction, hydrogen evolution, and CO2 reduction. The synergistic interaction between the two metals creates unique electronic structures and active sites that are not achievable with monometallic catalysts, leading to optimized adsorption energies of reaction intermediates and accelerated reaction kinetics.- Bimetallic single-atom catalysts for electrochemical applications: Bimetallic single-atom catalysts demonstrate enhanced electrochemical performance through synergistic effects between different metal atoms. These catalysts show improved activity, selectivity, and stability in various electrochemical reactions such as oxygen reduction, hydrogen evolution, and CO2 reduction. The synergistic interaction between the two metals creates unique electronic structures and active sites that are not achievable with monometallic catalysts, leading to optimized adsorption energies of reaction intermediates and accelerated reaction kinetics.
- Synthesis methods for bimetallic single-atom catalysts: Various synthesis strategies have been developed to prepare bimetallic single-atom catalysts with precise control over the atomic distribution and coordination environment. These methods include atomic layer deposition, wet chemical approaches, pyrolysis of metal-organic frameworks, and electrochemical deposition. The synthesis techniques focus on achieving uniform dispersion of both metal atoms, preventing aggregation, and creating specific metal-metal or metal-support interactions that maximize synergistic effects for catalytic applications.
- Synergistic effects in bimetallic single-atom catalysts for energy conversion: The synergistic effects in bimetallic single-atom catalysts significantly enhance energy conversion processes. These effects arise from charge transfer between different metal atoms, altered electronic structures, and optimized binding energies with reactants. In applications such as fuel cells, water splitting, and metal-air batteries, the bimetallic systems demonstrate superior performance compared to their monometallic counterparts due to complementary functions of the different metal atoms working in concert to facilitate multi-step reaction pathways.
- Support materials and their influence on bimetallic single-atom catalyst performance: The choice of support material plays a crucial role in determining the performance of bimetallic single-atom catalysts. Supports such as carbon-based materials, metal oxides, nitrides, and 2D materials can significantly influence the electronic properties, stability, and synergistic effects between metal atoms. The support-metal interaction affects the charge distribution, coordination environment, and dispersion of metal atoms, thereby tuning the catalytic activity and selectivity. Engineered supports with specific defects or functional groups can further enhance the synergistic effects between bimetallic single atoms.
- Characterization and theoretical understanding of synergistic effects: Advanced characterization techniques and theoretical calculations are essential for understanding the synergistic effects in bimetallic single-atom catalysts. Methods such as X-ray absorption spectroscopy, scanning transmission electron microscopy, and X-ray photoelectron spectroscopy provide insights into the local atomic structure and electronic properties. Density functional theory calculations help elucidate the nature of metal-metal interactions, reaction mechanisms, and the origin of enhanced catalytic performance. These combined experimental and theoretical approaches guide the rational design of more efficient bimetallic single-atom catalysts by revealing structure-property relationships.
02 Synthesis methods for bimetallic single-atom catalysts
Various synthesis strategies have been developed to prepare bimetallic single-atom catalysts with controlled distribution and coordination environments. These methods include atomic layer deposition, wet chemical approaches, pyrolysis of metal-organic frameworks, and electrochemical deposition. The synthesis techniques focus on achieving precise control over the atomic dispersion of both metals, their proximity, and coordination environment to maximize synergistic effects. These methods enable the creation of well-defined bimetallic single-atom sites with tunable electronic properties and catalytic performance.Expand Specific Solutions03 Bimetallic single-atom catalysts for environmental applications
Bimetallic single-atom catalysts show exceptional performance in environmental remediation and pollution control applications. The synergistic effects between different metal atoms enhance catalytic activity for pollutant degradation, CO oxidation, and NOx reduction. These catalysts demonstrate improved low-temperature activity, resistance to poisoning, and long-term stability compared to conventional catalysts. The unique electronic properties created by the interaction between two different metal atoms at the atomic level enable more efficient activation of reactant molecules and facilitate reaction pathways with lower energy barriers.Expand Specific Solutions04 Theoretical understanding of synergistic effects in bimetallic single-atom catalysts
Computational studies and theoretical models have been developed to understand the fundamental mechanisms of synergistic effects in bimetallic single-atom catalysts. Density functional theory calculations reveal how the electronic interaction between two different metal atoms modifies the d-band center, charge distribution, and binding energies of reaction intermediates. These theoretical insights guide the rational design of bimetallic single-atom catalysts by predicting optimal metal combinations, coordination environments, and support materials to maximize synergistic effects for specific reactions.Expand Specific Solutions05 Support effects on bimetallic single-atom catalysts
The choice of support material significantly influences the synergistic effects in bimetallic single-atom catalysts. Various supports including carbon-based materials, metal oxides, nitrides, and 2D materials have been investigated to optimize the performance of bimetallic single-atom catalysts. The support not only stabilizes the single-atom sites but also participates in the synergistic interaction by modulating the electronic properties of the metal atoms. Strong metal-support interactions can enhance the stability of single-atom sites and further tune their catalytic properties, leading to improved activity and selectivity for target reactions.Expand Specific Solutions
Leading Research Groups and Industrial Players in SAC Field
The research on bimetallic single-atom sites and synergistic effects is currently in an emerging growth phase, characterized by increasing academic interest and industrial applications. The market for this technology is expanding rapidly, with applications in catalysis, energy conversion, and chemical manufacturing, though precise market size remains difficult to quantify. From a technical maturity perspective, the field shows varying degrees of development across institutions. Academic leaders include Dalian Institute of Chemical Physics, Tsinghua University, and King Abdullah University of Science & Technology, while specialized companies like Beijing Single Atom Site Catalysis Technology Co. and IFP Energies Nouvelles are advancing commercial applications. Major chemical corporations such as Sanofi, Mitsui Chemicals, and SCG Chemicals are also investing in this technology, indicating its growing industrial relevance and potential for transformative impact.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered innovative approaches in bimetallic single-atom catalysts (SACs) research, focusing on precise synthesis methods and atomic-level characterization. Their technical solution involves developing atomically dispersed bimetallic sites on various supports like metal oxides and carbon materials using controlled co-precipitation and atomic layer deposition techniques. DICP has successfully demonstrated synergistic effects between different metal atoms (particularly Pt-Fe, Au-Co, and Pd-Cu combinations) that significantly enhance catalytic performance in reactions such as CO oxidation, water-gas shift, and hydrogenation processes. Their advanced in-situ characterization techniques, including aberration-corrected STEM and XAFS, allow for real-time observation of catalytic mechanisms at the atomic level[1][3]. Recent breakthroughs include achieving over 95% selectivity in acetylene hydrogenation using Pd-Cu single-atom alloys and developing stable Pt-Fe catalysts that maintain activity for over 100 hours in harsh reaction conditions.
Strengths: Exceptional expertise in atomic-level characterization techniques and advanced synthesis methods for precise control of bimetallic structures. Their catalysts demonstrate superior stability and selectivity compared to conventional catalysts. Weaknesses: Scale-up challenges persist for industrial applications, and some of their most effective catalysts rely on precious metals, raising cost concerns for commercial implementation.
King Abdullah University of Science & Technology
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed an integrated platform for bimetallic single-atom catalyst design focusing on energy conversion applications. Their technical approach combines advanced computational screening with precise synthetic methodologies to create atomically dispersed bimetallic sites with optimized geometric and electronic structures. KAUST researchers have pioneered the use of metal-organic frameworks and covalent organic frameworks as templates for creating isolated bimetallic sites with controlled metal-metal interactions. Their most significant innovation involves developing "tandem" bimetallic sites where two different metals work cooperatively to facilitate multi-step reaction pathways, particularly in CO2 reduction and oxygen evolution reactions[5]. Using operando X-ray absorption spectroscopy and environmental TEM, they've elucidated the dynamic structural changes during catalysis that contribute to synergistic effects. Recent breakthroughs include Ru-Co bimetallic single-atom catalysts achieving turnover frequencies exceeding 7,800 h−1 for CO2 hydrogenation and Fe-Ni sites demonstrating exceptional stability for over 200 hours in alkaline oxygen evolution reactions with overpotentials below 250 mV at 10 mA/cm².
Strengths: Exceptional integration of computational and experimental approaches enabling rational catalyst design with predictable performance metrics. Their catalysts demonstrate remarkable stability under harsh reaction conditions. Weaknesses: Some of their most effective systems rely on rare or precious metals, creating potential cost barriers for widespread adoption, and certain synthetic approaches require specialized equipment limiting broader implementation.
Key Mechanisms of Metal-Metal Synergistic Effects
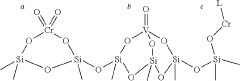
Supported three-center catalyst and preparation method and application
PatentActiveUS20220056165A1
Innovation
- A supported three-center catalyst comprising a porous inorganic carrier and three catalyst active centers: organic chromium, inorganic chromium, and inorganic vanadium active components, along with a catalyst modification component, which enables homopolymerization and copolymerization of ethylene and α-olefins, resulting in polymers with trimodal molecular weight distribution.
Characterization Techniques for Bimetallic Single-Atom Sites
The characterization of bimetallic single-atom sites represents one of the most challenging aspects in the field of heterogeneous catalysis. Advanced microscopy techniques, particularly aberration-corrected scanning transmission electron microscopy (AC-STEM), have emerged as essential tools for direct visualization of these atomic structures. AC-STEM with high-angle annular dark-field (HAADF) imaging provides atomic-resolution Z-contrast images, enabling researchers to distinguish between different metal atoms based on their atomic numbers.
X-ray absorption spectroscopy (XAS) techniques, including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offer complementary information about the local electronic structure and coordination environment of metal atoms. These techniques are particularly valuable for bimetallic systems as they can probe element-specific properties and reveal the nature of metal-metal interactions at the atomic level.
Synchrotron-based techniques have revolutionized the field by providing unprecedented spatial and temporal resolution. X-ray photoelectron spectroscopy (XPS) has become instrumental in determining the oxidation states of metal atoms and analyzing surface compositions, which is crucial for understanding catalytic mechanisms involving bimetallic single-atom sites.
Advanced computational methods have been integrated with experimental characterization to enhance data interpretation. Density functional theory (DFT) calculations, combined with experimental spectroscopic data, enable researchers to construct accurate atomic models of bimetallic single-atom catalysts and predict their electronic properties and catalytic behaviors.
In-situ and operando characterization techniques represent the frontier of this field, allowing researchers to observe dynamic changes in bimetallic structures under reaction conditions. Environmental transmission electron microscopy (E-TEM) and in-situ XAS provide real-time information about structural transformations and electronic state changes during catalytic processes, offering insights into the synergistic effects between different metal atoms.
Recent developments in atom probe tomography (APT) and low-temperature scanning tunneling microscopy (LT-STM) have further expanded the toolbox for characterizing bimetallic single-atom sites. These techniques offer three-dimensional atomic-scale mapping and precise electronic structure analysis, respectively, providing complementary information to traditional spectroscopic and microscopic methods.
The combination of multiple characterization techniques has become standard practice in the field, as no single method can provide complete information about these complex systems. This multi-technique approach, often referred to as correlative microscopy, has proven essential for establishing structure-property relationships in bimetallic single-atom catalysts and understanding the fundamental mechanisms behind their synergistic effects.
X-ray absorption spectroscopy (XAS) techniques, including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offer complementary information about the local electronic structure and coordination environment of metal atoms. These techniques are particularly valuable for bimetallic systems as they can probe element-specific properties and reveal the nature of metal-metal interactions at the atomic level.
Synchrotron-based techniques have revolutionized the field by providing unprecedented spatial and temporal resolution. X-ray photoelectron spectroscopy (XPS) has become instrumental in determining the oxidation states of metal atoms and analyzing surface compositions, which is crucial for understanding catalytic mechanisms involving bimetallic single-atom sites.
Advanced computational methods have been integrated with experimental characterization to enhance data interpretation. Density functional theory (DFT) calculations, combined with experimental spectroscopic data, enable researchers to construct accurate atomic models of bimetallic single-atom catalysts and predict their electronic properties and catalytic behaviors.
In-situ and operando characterization techniques represent the frontier of this field, allowing researchers to observe dynamic changes in bimetallic structures under reaction conditions. Environmental transmission electron microscopy (E-TEM) and in-situ XAS provide real-time information about structural transformations and electronic state changes during catalytic processes, offering insights into the synergistic effects between different metal atoms.
Recent developments in atom probe tomography (APT) and low-temperature scanning tunneling microscopy (LT-STM) have further expanded the toolbox for characterizing bimetallic single-atom sites. These techniques offer three-dimensional atomic-scale mapping and precise electronic structure analysis, respectively, providing complementary information to traditional spectroscopic and microscopic methods.
The combination of multiple characterization techniques has become standard practice in the field, as no single method can provide complete information about these complex systems. This multi-technique approach, often referred to as correlative microscopy, has proven essential for establishing structure-property relationships in bimetallic single-atom catalysts and understanding the fundamental mechanisms behind their synergistic effects.
Sustainability and Green Chemistry Implications of SACs
Single-atom catalysts (SACs) represent a significant advancement in sustainable chemistry, offering unprecedented atom efficiency by utilizing nearly every metal atom as an active catalytic site. This efficiency directly addresses one of the core principles of green chemistry: maximizing atom economy. Bimetallic single-atom sites further enhance this advantage by creating synergistic effects that often reduce the required catalyst loading while maintaining or improving catalytic performance.
The environmental implications of bimetallic SACs are substantial. These catalysts frequently demonstrate superior activity at lower temperatures compared to conventional catalysts, thereby reducing energy requirements for chemical processes. This translates to lower carbon footprints across various industrial applications, from hydrogen production to carbon dioxide conversion, supporting global decarbonization efforts.
Waste reduction constitutes another critical sustainability benefit. The precise atomic architecture of bimetallic SACs enables highly selective transformations, minimizing unwanted by-products and reducing separation costs. This selectivity is particularly valuable in pharmaceutical manufacturing, where it can dramatically decrease the environmental impact of complex synthesis routes.
Resource conservation represents a compelling advantage of bimetallic SACs. Many conventional catalysts rely heavily on precious metals like platinum and palladium, which face supply constraints and environmental concerns related to mining. Bimetallic SACs often incorporate earth-abundant metals as one component, reducing dependence on scarce resources while maintaining catalytic efficiency through synergistic interactions between the metal centers.
The recyclability of these catalysts further enhances their green chemistry profile. When properly designed, bimetallic SACs can maintain stability through multiple reaction cycles, extending their useful lifetime and reducing the frequency of catalyst replacement. This durability directly contributes to waste reduction and resource conservation in industrial settings.
From a life cycle assessment perspective, bimetallic SACs show promise in reducing the overall environmental footprint of chemical manufacturing. Their potential to operate under milder conditions, with higher selectivity and lower metal loading, addresses multiple environmental impact categories simultaneously, from energy use to waste generation.
The development of bimetallic SACs aligns with the principles of circular economy by enabling more efficient transformation of waste streams into valuable products. For instance, these catalysts show particular promise in converting biomass or plastic waste into chemical feedstocks, potentially closing material loops in industrial ecosystems.
The environmental implications of bimetallic SACs are substantial. These catalysts frequently demonstrate superior activity at lower temperatures compared to conventional catalysts, thereby reducing energy requirements for chemical processes. This translates to lower carbon footprints across various industrial applications, from hydrogen production to carbon dioxide conversion, supporting global decarbonization efforts.
Waste reduction constitutes another critical sustainability benefit. The precise atomic architecture of bimetallic SACs enables highly selective transformations, minimizing unwanted by-products and reducing separation costs. This selectivity is particularly valuable in pharmaceutical manufacturing, where it can dramatically decrease the environmental impact of complex synthesis routes.
Resource conservation represents a compelling advantage of bimetallic SACs. Many conventional catalysts rely heavily on precious metals like platinum and palladium, which face supply constraints and environmental concerns related to mining. Bimetallic SACs often incorporate earth-abundant metals as one component, reducing dependence on scarce resources while maintaining catalytic efficiency through synergistic interactions between the metal centers.
The recyclability of these catalysts further enhances their green chemistry profile. When properly designed, bimetallic SACs can maintain stability through multiple reaction cycles, extending their useful lifetime and reducing the frequency of catalyst replacement. This durability directly contributes to waste reduction and resource conservation in industrial settings.
From a life cycle assessment perspective, bimetallic SACs show promise in reducing the overall environmental footprint of chemical manufacturing. Their potential to operate under milder conditions, with higher selectivity and lower metal loading, addresses multiple environmental impact categories simultaneously, from energy use to waste generation.
The development of bimetallic SACs aligns with the principles of circular economy by enabling more efficient transformation of waste streams into valuable products. For instance, these catalysts show particular promise in converting biomass or plastic waste into chemical feedstocks, potentially closing material loops in industrial ecosystems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!