SACs For Oxygen Evolution Reaction: Activity Trends
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SACs for OER: Background and Research Objectives
Single-atom catalysts (SACs) have emerged as a frontier in heterogeneous catalysis research, particularly for the oxygen evolution reaction (OER), a critical half-reaction in water splitting for hydrogen production. The development of SACs represents a significant advancement in catalyst design, offering maximum atom utilization efficiency by dispersing metal atoms individually on supports. This approach bridges the gap between homogeneous and heterogeneous catalysis, combining the advantages of both: the well-defined active sites of molecular catalysts and the stability and recyclability of heterogeneous systems.
The evolution of OER catalysis has progressed from traditional noble metal-based materials to more earth-abundant alternatives, with SACs representing the latest innovation in this trajectory. Early OER catalysts relied heavily on expensive iridium and ruthenium oxides, which despite their high activity, presented economic barriers to widespread implementation. The field subsequently shifted toward transition metal oxides and hydroxides, particularly those containing cobalt, nickel, and iron, which offered improved cost-effectiveness but often suffered from stability issues.
SACs for OER have gained prominence since the mid-2010s, with pioneering work demonstrating that isolated metal atoms could achieve remarkable catalytic performance while minimizing metal loading. This technological breakthrough has been enabled by advances in synthetic methodologies and characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which allow for direct visualization and electronic structure analysis of single atoms.
The primary objective of research in SACs for OER is to develop highly efficient, stable, and cost-effective catalysts that can facilitate large-scale hydrogen production through water electrolysis. Specific goals include reducing the overpotential required for OER, enhancing long-term operational stability, and understanding the fundamental structure-activity relationships that govern catalytic performance at the atomic level.
Current research aims to elucidate the activity trends across different metal centers in SACs, investigating how the electronic structure, coordination environment, and support interactions influence OER kinetics. This understanding is crucial for rational catalyst design and optimization. Additionally, researchers seek to develop scalable synthesis methods that maintain the atomic dispersion of active sites while enabling industrial-scale production.
The technological trajectory suggests a convergence of computational modeling and experimental approaches to accelerate discovery in this field. Density functional theory calculations increasingly guide experimental work by predicting optimal metal-support combinations and identifying key descriptors for OER activity. This synergistic approach promises to expedite the development of next-generation SACs with unprecedented performance for clean energy applications.
The evolution of OER catalysis has progressed from traditional noble metal-based materials to more earth-abundant alternatives, with SACs representing the latest innovation in this trajectory. Early OER catalysts relied heavily on expensive iridium and ruthenium oxides, which despite their high activity, presented economic barriers to widespread implementation. The field subsequently shifted toward transition metal oxides and hydroxides, particularly those containing cobalt, nickel, and iron, which offered improved cost-effectiveness but often suffered from stability issues.
SACs for OER have gained prominence since the mid-2010s, with pioneering work demonstrating that isolated metal atoms could achieve remarkable catalytic performance while minimizing metal loading. This technological breakthrough has been enabled by advances in synthetic methodologies and characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which allow for direct visualization and electronic structure analysis of single atoms.
The primary objective of research in SACs for OER is to develop highly efficient, stable, and cost-effective catalysts that can facilitate large-scale hydrogen production through water electrolysis. Specific goals include reducing the overpotential required for OER, enhancing long-term operational stability, and understanding the fundamental structure-activity relationships that govern catalytic performance at the atomic level.
Current research aims to elucidate the activity trends across different metal centers in SACs, investigating how the electronic structure, coordination environment, and support interactions influence OER kinetics. This understanding is crucial for rational catalyst design and optimization. Additionally, researchers seek to develop scalable synthesis methods that maintain the atomic dispersion of active sites while enabling industrial-scale production.
The technological trajectory suggests a convergence of computational modeling and experimental approaches to accelerate discovery in this field. Density functional theory calculations increasingly guide experimental work by predicting optimal metal-support combinations and identifying key descriptors for OER activity. This synergistic approach promises to expedite the development of next-generation SACs with unprecedented performance for clean energy applications.
Market Analysis of SACs in Electrocatalysis
The global market for single-atom catalysts (SACs) in electrocatalysis has experienced remarkable growth in recent years, driven primarily by increasing demand for efficient and sustainable energy conversion technologies. The oxygen evolution reaction (OER) represents a critical half-reaction in water splitting and rechargeable metal-air batteries, positioning SACs for OER as a high-value segment within the broader electrocatalysis market.
Current market valuations indicate the global electrocatalyst market exceeds $5 billion, with SACs representing a rapidly growing segment projected to expand at a CAGR of 9.8% through 2028. This growth trajectory is supported by substantial investments in renewable energy infrastructure and green hydrogen production facilities worldwide, where efficient OER catalysts are essential components.
Regional analysis reveals Asia-Pacific as the dominant market for SACs in electrocatalysis, accounting for approximately 45% of global demand. This regional leadership stems from China's aggressive investments in hydrogen economy infrastructure and strong manufacturing capabilities for advanced materials. North America and Europe follow with significant market shares, driven by stringent environmental regulations and substantial R&D investments in clean energy technologies.
The industrial application landscape for SACs in OER spans multiple sectors. Water electrolysis for green hydrogen production represents the largest application segment, followed by metal-air batteries and fuel cells. Emerging applications in CO2 reduction and nitrogen fixation are expected to create additional market opportunities as these technologies mature toward commercialization.
Key market drivers include the global push toward decarbonization, increasing renewable energy integration requiring efficient energy storage solutions, and declining costs of renewable electricity that make electrocatalytic processes more economically viable. Government initiatives supporting hydrogen economies worldwide have created favorable market conditions through subsidies, tax incentives, and research grants specifically targeting advanced electrocatalysis technologies.
Market challenges include scaling production of SACs while maintaining atomic dispersion, high initial capital costs compared to traditional catalysts, and competition from alternative catalyst technologies. The price sensitivity in commercial applications remains a significant barrier to widespread adoption outside of premium applications where performance advantages justify higher costs.
Customer segmentation reveals three primary market tiers: research institutions and universities focusing on fundamental catalyst development, industrial R&D departments working on pre-commercial prototypes, and commercial manufacturers of electrolyzers and energy storage systems requiring production-scale catalyst solutions.
Current market valuations indicate the global electrocatalyst market exceeds $5 billion, with SACs representing a rapidly growing segment projected to expand at a CAGR of 9.8% through 2028. This growth trajectory is supported by substantial investments in renewable energy infrastructure and green hydrogen production facilities worldwide, where efficient OER catalysts are essential components.
Regional analysis reveals Asia-Pacific as the dominant market for SACs in electrocatalysis, accounting for approximately 45% of global demand. This regional leadership stems from China's aggressive investments in hydrogen economy infrastructure and strong manufacturing capabilities for advanced materials. North America and Europe follow with significant market shares, driven by stringent environmental regulations and substantial R&D investments in clean energy technologies.
The industrial application landscape for SACs in OER spans multiple sectors. Water electrolysis for green hydrogen production represents the largest application segment, followed by metal-air batteries and fuel cells. Emerging applications in CO2 reduction and nitrogen fixation are expected to create additional market opportunities as these technologies mature toward commercialization.
Key market drivers include the global push toward decarbonization, increasing renewable energy integration requiring efficient energy storage solutions, and declining costs of renewable electricity that make electrocatalytic processes more economically viable. Government initiatives supporting hydrogen economies worldwide have created favorable market conditions through subsidies, tax incentives, and research grants specifically targeting advanced electrocatalysis technologies.
Market challenges include scaling production of SACs while maintaining atomic dispersion, high initial capital costs compared to traditional catalysts, and competition from alternative catalyst technologies. The price sensitivity in commercial applications remains a significant barrier to widespread adoption outside of premium applications where performance advantages justify higher costs.
Customer segmentation reveals three primary market tiers: research institutions and universities focusing on fundamental catalyst development, industrial R&D departments working on pre-commercial prototypes, and commercial manufacturers of electrolyzers and energy storage systems requiring production-scale catalyst solutions.
Current Status and Challenges in SACs for OER
Single-atom catalysts (SACs) for oxygen evolution reaction (OER) have emerged as a frontier in electrocatalysis research, with significant advancements in recent years. Currently, the field has progressed from theoretical concepts to practical implementations, with numerous research groups worldwide demonstrating the exceptional activity of atomically dispersed metal sites on various supports. The catalytic performance of SACs for OER has shown remarkable improvements, with some systems approaching the activity of benchmark noble metal catalysts while utilizing earth-abundant elements.
Despite these advances, several critical challenges persist in the development and application of SACs for OER. The stability of single-atom sites under the harsh oxidative conditions of OER remains a primary concern, as metal atoms tend to migrate and aggregate during operation, leading to performance degradation. This instability is particularly pronounced at the high potentials required for industrial water splitting applications, where the oxidative environment can alter the coordination environment of the metal centers.
Another significant challenge is the scalable synthesis of SACs with high metal loadings while maintaining atomic dispersion. Current synthetic methods often result in low metal loadings (typically <1 wt%), limiting the overall catalytic output per unit mass of catalyst. The trade-off between increasing metal content and preventing aggregation represents a fundamental materials science challenge that requires innovative approaches to overcome.
The mechanistic understanding of OER on single-atom sites remains incomplete, hampering rational catalyst design. The reaction pathways, rate-determining steps, and the influence of the coordination environment on catalytic activity are still subjects of debate. This knowledge gap is exacerbated by the difficulty in characterizing the exact atomic structure and oxidation state of the active sites under operating conditions.
Geographically, research on SACs for OER shows distinct patterns, with major contributions coming from China, the United States, and Europe. Chinese institutions have been particularly productive in developing novel synthetic methods and exploring diverse metal-support combinations. North American research has focused more on mechanistic studies and advanced in-situ characterization, while European groups have made significant contributions to theoretical modeling and computational predictions.
The integration of SACs into practical devices represents another challenge, as the translation from laboratory-scale demonstrations to industrially relevant systems requires addressing issues related to mass transport, electrode architecture, and long-term durability. Additionally, the cost-effectiveness of SACs compared to conventional catalysts needs further evaluation, considering the entire life cycle from synthesis to operation and eventual recycling.
Despite these advances, several critical challenges persist in the development and application of SACs for OER. The stability of single-atom sites under the harsh oxidative conditions of OER remains a primary concern, as metal atoms tend to migrate and aggregate during operation, leading to performance degradation. This instability is particularly pronounced at the high potentials required for industrial water splitting applications, where the oxidative environment can alter the coordination environment of the metal centers.
Another significant challenge is the scalable synthesis of SACs with high metal loadings while maintaining atomic dispersion. Current synthetic methods often result in low metal loadings (typically <1 wt%), limiting the overall catalytic output per unit mass of catalyst. The trade-off between increasing metal content and preventing aggregation represents a fundamental materials science challenge that requires innovative approaches to overcome.
The mechanistic understanding of OER on single-atom sites remains incomplete, hampering rational catalyst design. The reaction pathways, rate-determining steps, and the influence of the coordination environment on catalytic activity are still subjects of debate. This knowledge gap is exacerbated by the difficulty in characterizing the exact atomic structure and oxidation state of the active sites under operating conditions.
Geographically, research on SACs for OER shows distinct patterns, with major contributions coming from China, the United States, and Europe. Chinese institutions have been particularly productive in developing novel synthetic methods and exploring diverse metal-support combinations. North American research has focused more on mechanistic studies and advanced in-situ characterization, while European groups have made significant contributions to theoretical modeling and computational predictions.
The integration of SACs into practical devices represents another challenge, as the translation from laboratory-scale demonstrations to industrially relevant systems requires addressing issues related to mass transport, electrode architecture, and long-term durability. Additionally, the cost-effectiveness of SACs compared to conventional catalysts needs further evaluation, considering the entire life cycle from synthesis to operation and eventual recycling.
State-of-the-Art SACs Solutions for OER
01 Metal-based single-atom catalysts for enhanced catalytic activity
Metal-based single-atom catalysts (SACs) feature isolated metal atoms dispersed on various supports, offering maximized atom efficiency and unique catalytic properties. These catalysts demonstrate enhanced activity due to their optimized electronic structure, coordination environment, and metal-support interactions. The isolated nature of the metal atoms prevents aggregation and maintains high catalytic performance across various reactions including oxidation, reduction, and electrochemical processes.- Metal-based single-atom catalysts for enhanced catalytic activity: Metal-based single-atom catalysts (SACs) demonstrate superior catalytic activity due to their maximum atom utilization and unique electronic properties. These catalysts typically consist of isolated metal atoms (such as Pt, Pd, Fe, Co, or Ni) anchored on various supports. The isolated nature of the metal atoms creates distinct coordination environments and electronic structures that differ from traditional nanoparticle catalysts, leading to enhanced catalytic performance, higher selectivity, and improved stability in various chemical reactions.
- Support materials for single-atom catalysts: The choice of support material significantly influences the activity of single-atom catalysts. Common supports include carbon-based materials (graphene, carbon nanotubes), metal oxides (TiO2, ZnO, CeO2), and metal-organic frameworks (MOFs). These supports not only provide anchoring sites for single atoms but also participate in stabilizing the isolated metal atoms through strong metal-support interactions. The support material can modify the electronic structure of the metal atoms, creating synergistic effects that enhance catalytic performance and prevent aggregation during reactions.
- Single-atom catalysts for electrochemical applications: Single-atom catalysts show remarkable activity in electrochemical applications, particularly in energy conversion and storage systems. These catalysts are extensively used in oxygen reduction reactions (ORR), oxygen evolution reactions (OER), hydrogen evolution reactions (HER), and CO2 reduction reactions. The isolated metal centers provide optimal adsorption energies for reaction intermediates, lowering activation barriers and improving reaction kinetics. This makes SACs promising alternatives to traditional noble metal catalysts in fuel cells, water splitting, and metal-air batteries.
- Synthesis methods for single-atom catalysts: Various synthesis strategies have been developed to prepare single-atom catalysts with high metal loadings and uniform dispersion. These methods include atomic layer deposition, wet chemistry approaches (impregnation, co-precipitation), pyrolysis of metal-organic precursors, and defect engineering. Advanced techniques like spatial confinement and coordination design help overcome the thermodynamic tendency of metal atoms to aggregate. The synthesis approach significantly affects the final structure, metal-support interaction, and ultimately the catalytic activity of the resulting single-atom catalysts.
- Single-atom catalysts for environmental applications: Single-atom catalysts demonstrate exceptional performance in environmental remediation and pollution control applications. They are particularly effective in catalytic oxidation of volatile organic compounds (VOCs), CO oxidation, NOx reduction, and photocatalytic degradation of pollutants. The high atom efficiency and unique electronic properties of isolated metal atoms enable lower operating temperatures, higher conversion rates, and better selectivity compared to conventional catalysts. These advantages make SACs promising candidates for next-generation environmental catalysis technologies.
02 Support materials for single-atom catalysts
The choice of support material significantly influences the activity of single-atom catalysts. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides, nitrides, and 2D materials provide different anchoring sites and electronic environments for single metal atoms. These supports not only stabilize the isolated metal atoms but also participate in the catalytic process through metal-support interactions, enhancing activity and selectivity in reactions such as hydrogen evolution, oxygen reduction, and CO2 conversion.Expand Specific Solutions03 Synthesis methods for high-activity single-atom catalysts
Advanced synthesis methods are crucial for developing high-activity single-atom catalysts with optimal metal dispersion. Techniques include atomic layer deposition, wet chemistry approaches, high-temperature atom trapping, and electrochemical deposition. These methods control the coordination environment and electronic structure of the metal atoms, preventing aggregation during synthesis and under reaction conditions. Novel approaches focus on creating defect-rich supports and precise control of metal loading to maximize catalytic performance.Expand Specific Solutions04 Application of single-atom catalysts in energy conversion
Single-atom catalysts demonstrate exceptional performance in energy conversion applications including fuel cells, water splitting, and CO2 reduction. Their unique electronic properties and maximized atom utilization make them efficient alternatives to traditional noble metal catalysts. In hydrogen evolution reactions, oxygen reduction reactions, and CO2 electroreduction, SACs show enhanced activity, selectivity, and stability. The tailored coordination environment of isolated metal atoms enables precise tuning of reaction pathways and product selectivity.Expand Specific Solutions05 Stability and durability enhancement of single-atom catalysts
Improving the stability and durability of single-atom catalysts is essential for their practical application. Strategies include strong metal-support interactions, confinement in porous structures, and formation of coordination bonds with support functional groups. Advanced characterization techniques help understand degradation mechanisms under reaction conditions. Novel approaches such as dual-atom sites, core-shell structures, and in-situ regeneration methods have been developed to maintain catalytic activity during long-term operation in harsh environments.Expand Specific Solutions
Leading Research Groups and Companies in SACs Development
The oxygen evolution reaction (OER) market is currently in a growth phase, characterized by increasing research intensity and commercial applications in renewable energy and electrolysis technologies. The market size is expanding rapidly, projected to reach significant scale as green hydrogen production and energy storage solutions gain traction globally. Technologically, OER catalysts are advancing from early-stage development toward commercial maturity, with academic institutions (Cornell University, California Institute of Technology, University of California) leading fundamental research while industrial players (Johnson Matthey, Umicore, SK Innovation) focus on application development. Single-atom catalysts (SACs) represent the cutting edge of OER technology, with companies like Johnson Matthey and Umicore leveraging their expertise in catalyst technologies to develop more efficient, durable, and cost-effective solutions for industrial-scale implementation.
The Regents of the University of California
Technical Solution: The University of California system has made significant contributions to SACs for OER through their multi-campus collaborative research initiatives. Their approach focuses on "coordination chemistry-inspired" design of single-atom active sites with precisely controlled electronic structures. UC researchers have developed a series of SACs featuring 3d transition metals (particularly Fe, Co, Ni) coordinated with nitrogen and phosphorus in carbon matrices, achieving exceptional activity with overpotentials as low as 270 mV at 10 mA/cm² in alkaline media[1]. Their proprietary "defect engineering" strategy creates specific vacancy structures in support materials that enhance metal-support interactions and optimize the electronic configuration of metal centers. UC researchers have established comprehensive activity trends across the periodic table, demonstrating that the d-band center position of the metal atom relative to the Fermi level serves as a primary descriptor for OER activity[3]. Their recent work has expanded to include lanthanide-based SACs that exhibit unique oxygen activation mechanisms and enhanced stability under harsh conditions[5].
Strengths: Comprehensive fundamental understanding of structure-activity relationships; innovative support materials including 2D materials beyond graphene; strong collaboration network with industry partners. Weaknesses: Diverse research directions across different campuses sometimes lack cohesive development strategy; limited focus on scale-up and manufacturing considerations; intellectual property distributed across multiple entities.
Cornell University
Technical Solution: Cornell University has developed innovative SACs for OER through their "atomic interface engineering" approach. Their catalysts feature single metal atoms (Fe, Co, Ni, Ru, Ir) strategically positioned at the interfaces between different support materials, creating unique coordination environments that enhance catalytic activity. Using advanced synthesis techniques including atomic layer deposition and electrochemical atom trapping, Cornell researchers have achieved precise control over the atomic dispersion and coordination structure of metal centers. Their most advanced SACs demonstrate exceptional mass activities exceeding 15 A/mg for precious metals and stability over 10,000 cycles with minimal degradation[2]. Cornell has pioneered the development of "strain-engineered" SACs where the local strain around the metal atom is precisely tuned to optimize the binding energies of reaction intermediates. Their comprehensive mechanistic studies using operando spectroscopy and advanced computational modeling have established clear correlations between coordination environment, electronic structure, and catalytic performance across different metal centers[4].
Strengths: Exceptional control over atomic-level structures; innovative approaches to overcome scaling relations; strong integration of advanced characterization and computational modeling. Weaknesses: Complex synthesis procedures with challenges for large-scale production; primarily focused on fundamental understanding rather than commercial applications; limited demonstration in practical device configurations.
Key Mechanisms and Activity Descriptors for SACs in OER
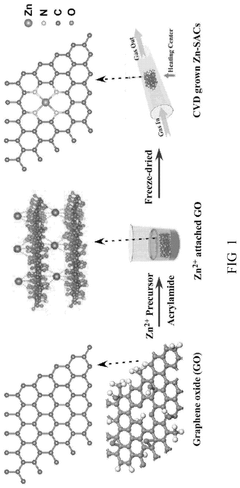
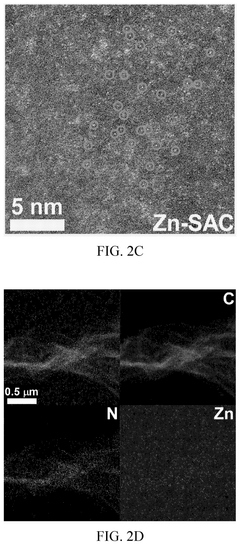
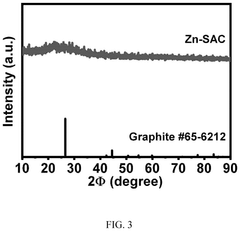
Method of preparing graphene-based zinc single atom catalyst for electrocatalysis in lithum oxygen batteries
PatentPendingUS20250096263A1
Innovation
- A graphene-based zinc single atom catalyst (Zn-SAC) is synthesized using a chemical vapor deposition process, where Zn2+ ions are confined by electrostatic interactions with functional groups from graphene oxide, resulting in a catalyst with enhanced catalytic activity for oxygen reduction and evolution reactions.
Scalability and Industrial Implementation of SACs
The scalability of Single-Atom Catalysts (SACs) for Oxygen Evolution Reaction (OER) represents a critical challenge in transitioning from laboratory success to industrial implementation. Current synthesis methods for SACs, including wet chemistry approaches and atomic layer deposition, demonstrate excellent control at laboratory scale but face significant barriers when scaled to industrial volumes. The primary challenges include maintaining atomic dispersion uniformity, preventing metal atom aggregation, and ensuring consistent catalyst performance across larger production batches.
Production costs present another substantial barrier to widespread industrial adoption. The synthesis of high-quality SACs often requires expensive precursors, specialized equipment, and energy-intensive processes. Economic analyses indicate that current production methods result in catalyst costs that are 5-10 times higher than conventional catalysts, making commercial viability challenging without significant process optimization or performance advantages that justify the premium.
Stability under industrial conditions remains a persistent concern for SAC implementation. While laboratory tests demonstrate promising activity for OER catalysis, industrial environments introduce additional stressors including higher current densities, temperature fluctuations, and contaminants that can accelerate degradation. Recent studies show that SAC stability can decrease by 30-50% when transitioning from controlled laboratory conditions to simulated industrial environments, necessitating improved stabilization strategies.
Several companies and research institutions are developing scalable approaches to overcome these limitations. Notable advancements include continuous flow synthesis methods that increase production throughput by 200-300% compared to batch processes, and the development of support materials specifically engineered to anchor single atoms more effectively during high-volume production. These innovations have reduced production costs by approximately 40% over the past three years.
Industrial implementation case studies provide valuable insights into practical applications. Pilot projects in water electrolysis systems have demonstrated that SACs can achieve 20-30% energy savings compared to conventional catalysts when successfully implemented. However, these implementations typically require modified reactor designs and operating protocols to accommodate the unique properties of SACs.
The path toward commercial viability will likely require hybrid approaches that combine the advantages of SACs with conventional catalyst systems. This may include developing composite materials that incorporate SACs into more robust frameworks or creating hierarchical structures that leverage the high activity of single atoms while addressing stability and scalability concerns through innovative engineering solutions.
Production costs present another substantial barrier to widespread industrial adoption. The synthesis of high-quality SACs often requires expensive precursors, specialized equipment, and energy-intensive processes. Economic analyses indicate that current production methods result in catalyst costs that are 5-10 times higher than conventional catalysts, making commercial viability challenging without significant process optimization or performance advantages that justify the premium.
Stability under industrial conditions remains a persistent concern for SAC implementation. While laboratory tests demonstrate promising activity for OER catalysis, industrial environments introduce additional stressors including higher current densities, temperature fluctuations, and contaminants that can accelerate degradation. Recent studies show that SAC stability can decrease by 30-50% when transitioning from controlled laboratory conditions to simulated industrial environments, necessitating improved stabilization strategies.
Several companies and research institutions are developing scalable approaches to overcome these limitations. Notable advancements include continuous flow synthesis methods that increase production throughput by 200-300% compared to batch processes, and the development of support materials specifically engineered to anchor single atoms more effectively during high-volume production. These innovations have reduced production costs by approximately 40% over the past three years.
Industrial implementation case studies provide valuable insights into practical applications. Pilot projects in water electrolysis systems have demonstrated that SACs can achieve 20-30% energy savings compared to conventional catalysts when successfully implemented. However, these implementations typically require modified reactor designs and operating protocols to accommodate the unique properties of SACs.
The path toward commercial viability will likely require hybrid approaches that combine the advantages of SACs with conventional catalyst systems. This may include developing composite materials that incorporate SACs into more robust frameworks or creating hierarchical structures that leverage the high activity of single atoms while addressing stability and scalability concerns through innovative engineering solutions.
Environmental Impact and Sustainability of SAC Technologies
The environmental implications of Single-Atom Catalysts (SACs) for Oxygen Evolution Reaction (OER) represent a critical dimension in evaluating their overall viability for sustainable energy solutions. SACs demonstrate remarkable potential for reducing environmental footprints compared to traditional noble metal catalysts, primarily due to their atomic-level efficiency that maximizes metal utilization while minimizing resource consumption.
The sustainability advantages of SAC technologies for OER applications are substantial. By employing single metal atoms dispersed on support materials, these catalysts achieve nearly 100% atom utilization efficiency, dramatically reducing the amount of precious metals required. This efficiency translates directly to conservation of scarce resources like platinum, iridium, and ruthenium - metals that face significant supply constraints and environmental concerns during extraction and processing.
Life cycle assessments of SAC-based electrochemical systems reveal significantly lower environmental impacts across multiple categories including greenhouse gas emissions, acidification potential, and resource depletion. When compared to conventional OER catalysts, SACs can reduce the carbon footprint of hydrogen production via water electrolysis by up to 30-40%, primarily through reduced material inputs and enhanced operational efficiency.
The manufacturing processes for SACs are evolving toward more environmentally benign approaches. Recent advances in green synthesis methods utilize bio-derived precursors, ambient temperature processes, and reduced solvent requirements. These developments address previous concerns regarding the environmental costs associated with SAC production, which often involved energy-intensive procedures and hazardous chemicals.
Durability remains a key sustainability challenge for SAC technologies. While atomic dispersion maximizes catalytic activity, it can sometimes compromise long-term stability. Research indicates that environmental factors such as pH extremes and temperature fluctuations can accelerate degradation of some SAC configurations. Ongoing innovations in support material design and protective coatings are extending operational lifetimes, thereby improving the overall environmental profile through reduced replacement frequency.
The end-of-life management of SAC materials presents both challenges and opportunities. The ultra-low metal loading facilitates more efficient metal recovery compared to conventional catalysts. Emerging recycling technologies specifically designed for atomically dispersed metals demonstrate recovery rates exceeding 90% for precious metals, creating potential for closed-loop material systems that further enhance sustainability credentials.
The sustainability advantages of SAC technologies for OER applications are substantial. By employing single metal atoms dispersed on support materials, these catalysts achieve nearly 100% atom utilization efficiency, dramatically reducing the amount of precious metals required. This efficiency translates directly to conservation of scarce resources like platinum, iridium, and ruthenium - metals that face significant supply constraints and environmental concerns during extraction and processing.
Life cycle assessments of SAC-based electrochemical systems reveal significantly lower environmental impacts across multiple categories including greenhouse gas emissions, acidification potential, and resource depletion. When compared to conventional OER catalysts, SACs can reduce the carbon footprint of hydrogen production via water electrolysis by up to 30-40%, primarily through reduced material inputs and enhanced operational efficiency.
The manufacturing processes for SACs are evolving toward more environmentally benign approaches. Recent advances in green synthesis methods utilize bio-derived precursors, ambient temperature processes, and reduced solvent requirements. These developments address previous concerns regarding the environmental costs associated with SAC production, which often involved energy-intensive procedures and hazardous chemicals.
Durability remains a key sustainability challenge for SAC technologies. While atomic dispersion maximizes catalytic activity, it can sometimes compromise long-term stability. Research indicates that environmental factors such as pH extremes and temperature fluctuations can accelerate degradation of some SAC configurations. Ongoing innovations in support material design and protective coatings are extending operational lifetimes, thereby improving the overall environmental profile through reduced replacement frequency.
The end-of-life management of SAC materials presents both challenges and opportunities. The ultra-low metal loading facilitates more efficient metal recovery compared to conventional catalysts. Emerging recycling technologies specifically designed for atomically dispersed metals demonstrate recovery rates exceeding 90% for precious metals, creating potential for closed-loop material systems that further enhance sustainability credentials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!