Support Materials For Stabilizing Single Metal Atoms
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single Atom Catalysis Background and Objectives
Single atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis, emerging as a bridge between homogeneous and heterogeneous catalytic systems. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining it. SAC involves individual metal atoms dispersed on support materials, maximizing atomic efficiency while exhibiting unique catalytic properties distinct from their nanoparticle counterparts.
The evolution of this field has been driven by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled direct visualization and electronic structure analysis of isolated metal atoms. This technological progress has transformed SAC from a theoretical concept to an experimentally verifiable and industrially relevant technology.
Current research trends focus on developing support materials that can effectively stabilize single metal atoms against aggregation under reaction conditions. Traditional supports like metal oxides (TiO2, CeO2), carbon-based materials, and zeolites have demonstrated varying degrees of success, but challenges remain in achieving long-term stability across diverse reaction environments.
The primary objective of research on support materials for stabilizing single metal atoms is to develop robust, scalable systems that maintain atomic dispersion under industrial conditions. This includes identifying optimal metal-support interactions that anchor single atoms while allowing them to maintain catalytic activity, understanding the fundamental mechanisms of stabilization, and developing synthetic methods that enable precise control over the atomic architecture.
Secondary objectives include enhancing the thermal and chemical stability of supported single atoms, expanding the range of metals that can be stabilized as isolated atoms, and developing multifunctional supports that can simultaneously stabilize different metal species for tandem catalytic processes.
The ultimate goal is to translate laboratory discoveries into practical applications by addressing scalability challenges and demonstrating performance advantages in industrially relevant reactions. Success in this field could revolutionize chemical manufacturing by dramatically reducing precious metal usage while potentially enabling new reaction pathways not accessible with conventional catalysts.
Research in this area aligns with global sustainability initiatives by promising more atom-efficient utilization of scarce metal resources and potentially enabling more energy-efficient chemical transformations through precisely tailored catalytic sites.
The evolution of this field has been driven by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled direct visualization and electronic structure analysis of isolated metal atoms. This technological progress has transformed SAC from a theoretical concept to an experimentally verifiable and industrially relevant technology.
Current research trends focus on developing support materials that can effectively stabilize single metal atoms against aggregation under reaction conditions. Traditional supports like metal oxides (TiO2, CeO2), carbon-based materials, and zeolites have demonstrated varying degrees of success, but challenges remain in achieving long-term stability across diverse reaction environments.
The primary objective of research on support materials for stabilizing single metal atoms is to develop robust, scalable systems that maintain atomic dispersion under industrial conditions. This includes identifying optimal metal-support interactions that anchor single atoms while allowing them to maintain catalytic activity, understanding the fundamental mechanisms of stabilization, and developing synthetic methods that enable precise control over the atomic architecture.
Secondary objectives include enhancing the thermal and chemical stability of supported single atoms, expanding the range of metals that can be stabilized as isolated atoms, and developing multifunctional supports that can simultaneously stabilize different metal species for tandem catalytic processes.
The ultimate goal is to translate laboratory discoveries into practical applications by addressing scalability challenges and demonstrating performance advantages in industrially relevant reactions. Success in this field could revolutionize chemical manufacturing by dramatically reducing precious metal usage while potentially enabling new reaction pathways not accessible with conventional catalysts.
Research in this area aligns with global sustainability initiatives by promising more atom-efficient utilization of scarce metal resources and potentially enabling more energy-efficient chemical transformations through precisely tailored catalytic sites.
Market Analysis for Single Atom Catalysts
The single atom catalyst (SAC) market is experiencing rapid growth, driven by increasing demand for sustainable and efficient catalytic solutions across various industries. The global market for advanced catalysts, including SACs, was valued at approximately 26.1 billion USD in 2022 and is projected to reach 38.6 billion USD by 2030, growing at a CAGR of around 5.0%. Single atom catalysts represent an emerging segment within this broader market, with particularly strong growth potential due to their superior performance characteristics.
The automotive sector constitutes a significant portion of the SAC market demand, primarily for emission control applications. With stringent environmental regulations being implemented globally, particularly in Europe and North America, the need for more efficient catalytic converters has intensified. SACs offer reduced precious metal usage while maintaining or improving catalytic performance, making them economically attractive alternatives to traditional catalysts.
Chemical manufacturing represents another substantial market segment, where SACs are increasingly utilized for fine chemical synthesis, hydrogenation reactions, and oxidation processes. The pharmaceutical industry is also showing growing interest in SACs for their ability to enable more selective and environmentally friendly synthesis routes for complex molecules.
Energy-related applications form a rapidly expanding market segment for SACs, particularly in fuel cells, hydrogen production, and CO2 conversion technologies. As global efforts to transition to renewable energy sources accelerate, the demand for efficient catalysts in these areas is expected to grow significantly over the next decade.
Regionally, Asia-Pacific dominates the SAC market, accounting for approximately 40% of global demand, with China leading in both research and commercial applications. North America and Europe follow closely, with substantial investments in research and development of novel SAC technologies for various applications.
Market barriers include high production costs, scalability challenges, and the need for specialized characterization techniques to verify single-atom dispersion. However, recent advancements in synthesis methods are gradually addressing these limitations, potentially accelerating market penetration.
The competitive landscape features both established catalyst manufacturers expanding into the SAC space and specialized startups focused exclusively on single-atom technology development. Strategic partnerships between academic institutions and industry players are becoming increasingly common, accelerating the commercialization of laboratory-scale discoveries.
The automotive sector constitutes a significant portion of the SAC market demand, primarily for emission control applications. With stringent environmental regulations being implemented globally, particularly in Europe and North America, the need for more efficient catalytic converters has intensified. SACs offer reduced precious metal usage while maintaining or improving catalytic performance, making them economically attractive alternatives to traditional catalysts.
Chemical manufacturing represents another substantial market segment, where SACs are increasingly utilized for fine chemical synthesis, hydrogenation reactions, and oxidation processes. The pharmaceutical industry is also showing growing interest in SACs for their ability to enable more selective and environmentally friendly synthesis routes for complex molecules.
Energy-related applications form a rapidly expanding market segment for SACs, particularly in fuel cells, hydrogen production, and CO2 conversion technologies. As global efforts to transition to renewable energy sources accelerate, the demand for efficient catalysts in these areas is expected to grow significantly over the next decade.
Regionally, Asia-Pacific dominates the SAC market, accounting for approximately 40% of global demand, with China leading in both research and commercial applications. North America and Europe follow closely, with substantial investments in research and development of novel SAC technologies for various applications.
Market barriers include high production costs, scalability challenges, and the need for specialized characterization techniques to verify single-atom dispersion. However, recent advancements in synthesis methods are gradually addressing these limitations, potentially accelerating market penetration.
The competitive landscape features both established catalyst manufacturers expanding into the SAC space and specialized startups focused exclusively on single-atom technology development. Strategic partnerships between academic institutions and industry players are becoming increasingly common, accelerating the commercialization of laboratory-scale discoveries.
Current Support Materials and Technical Challenges
The current landscape of support materials for single atom catalysts (SACs) is dominated by several key categories, each with distinct advantages and limitations. Metal oxides such as TiO2, CeO2, and Fe2O3 remain among the most widely utilized supports due to their excellent thermal stability and strong metal-support interactions. These materials provide abundant oxygen vacancies and defect sites that effectively anchor single metal atoms, preventing aggregation during catalytic reactions.
Carbon-based materials represent another significant category, including graphene, carbon nanotubes, and porous carbon structures. These supports offer high surface areas and excellent electrical conductivity, making them particularly valuable for electrocatalytic applications. The π-electron systems in carbon materials can form strong interactions with metal atoms, while their tunable pore structures facilitate mass transport during catalysis.
Metal-organic frameworks (MOFs) have emerged as promising supports due to their exceptional porosity and structural versatility. The well-defined coordination environments within MOFs enable precise control over the local environment of single metal atoms, though their relatively poor thermal and chemical stability remains problematic for high-temperature applications.
Despite these advances, significant technical challenges persist in the field. Stability under reaction conditions represents the foremost challenge, as single atoms possess high surface energy and tend to aggregate into nanoparticles, especially at elevated temperatures or in reducing environments. This aggregation dramatically reduces catalytic performance and selectivity.
The loading capacity of single atoms presents another critical limitation. Most current support materials can only accommodate low metal loadings (typically <2 wt%), severely restricting catalytic efficiency and commercial viability. Increasing metal loading without inducing aggregation remains a fundamental challenge.
Characterization difficulties further complicate research progress. The atomic dispersion of metal species makes them challenging to visualize and analyze, requiring advanced techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy, which are not universally accessible.
Scalable synthesis represents perhaps the most significant barrier to industrial implementation. Current preparation methods often involve complex procedures with poor reproducibility, making large-scale production economically unfeasible. Additionally, the rational design of support materials with specific binding sites for targeted catalytic reactions remains underdeveloped, limiting the ability to create application-specific catalysts with optimized performance.
Carbon-based materials represent another significant category, including graphene, carbon nanotubes, and porous carbon structures. These supports offer high surface areas and excellent electrical conductivity, making them particularly valuable for electrocatalytic applications. The π-electron systems in carbon materials can form strong interactions with metal atoms, while their tunable pore structures facilitate mass transport during catalysis.
Metal-organic frameworks (MOFs) have emerged as promising supports due to their exceptional porosity and structural versatility. The well-defined coordination environments within MOFs enable precise control over the local environment of single metal atoms, though their relatively poor thermal and chemical stability remains problematic for high-temperature applications.
Despite these advances, significant technical challenges persist in the field. Stability under reaction conditions represents the foremost challenge, as single atoms possess high surface energy and tend to aggregate into nanoparticles, especially at elevated temperatures or in reducing environments. This aggregation dramatically reduces catalytic performance and selectivity.
The loading capacity of single atoms presents another critical limitation. Most current support materials can only accommodate low metal loadings (typically <2 wt%), severely restricting catalytic efficiency and commercial viability. Increasing metal loading without inducing aggregation remains a fundamental challenge.
Characterization difficulties further complicate research progress. The atomic dispersion of metal species makes them challenging to visualize and analyze, requiring advanced techniques like aberration-corrected electron microscopy and X-ray absorption spectroscopy, which are not universally accessible.
Scalable synthesis represents perhaps the most significant barrier to industrial implementation. Current preparation methods often involve complex procedures with poor reproducibility, making large-scale production economically unfeasible. Additionally, the rational design of support materials with specific binding sites for targeted catalytic reactions remains underdeveloped, limiting the ability to create application-specific catalysts with optimized performance.
Current Support Material Solutions
01 Carbon-based support materials for single metal atoms
Carbon-based materials serve as excellent supports for single metal atoms due to their high surface area, conductivity, and chemical stability. These supports include graphene, carbon nanotubes, porous carbon, and carbon nitride structures. The carbon frameworks provide anchoring sites for metal atoms through defects, functional groups, or heteroatom doping, preventing aggregation and maintaining the atomic dispersion of metal catalysts under reaction conditions.- Carbon-based support materials for single metal atoms: Carbon-based materials serve as excellent supports for single metal atoms due to their high surface area, electrical conductivity, and chemical stability. These supports include graphene, carbon nanotubes, porous carbon, and carbon nitride structures. The carbon frameworks provide anchoring sites for metal atoms through defects, functional groups, or doping with heteroatoms like nitrogen, which can significantly enhance the stability of single metal atoms by preventing aggregation during catalytic reactions.
- Metal oxide supports for single atom catalysts: Metal oxides such as TiO2, CeO2, Al2O3, and ZnO provide strong metal-support interactions that stabilize single metal atoms. These materials offer oxygen vacancies and surface hydroxyl groups that serve as anchoring sites for metal atoms. The strong electronic interactions between the metal atoms and oxide supports prevent sintering and aggregation, even under harsh reaction conditions. Additionally, the redox properties of certain metal oxides can enhance the catalytic performance of the supported single atoms.
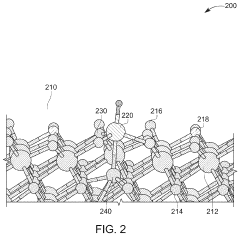
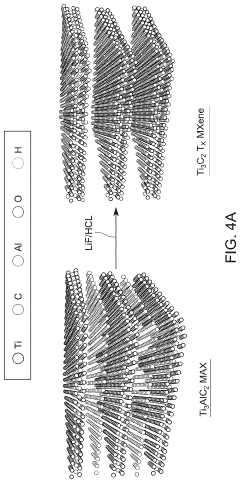
- Two-dimensional materials as supports for single metal atoms: Two-dimensional materials such as MXenes, transition metal dichalcogenides, and layered double hydroxides provide unique platforms for stabilizing single metal atoms. Their layered structures offer abundant surface sites for metal atom anchoring, while their tunable electronic properties allow for optimized metal-support interactions. The confined spaces between layers can effectively prevent metal atom migration and aggregation, leading to enhanced stability during catalytic processes.
- MOF-derived supports for single atom stabilization: Metal-organic frameworks (MOFs) and their derivatives serve as excellent precursors and templates for preparing stable single atom catalysts. The highly ordered structure of MOFs with well-defined metal nodes and organic linkers provides uniform coordination environments for anchoring single metal atoms. After controlled pyrolysis, MOF-derived materials retain high surface area and porosity while offering strong binding sites for metal atoms, resulting in enhanced thermal and chemical stability of the single atom catalysts.
- Hybrid and composite supports for enhanced single atom stability: Hybrid supports combining multiple materials such as metal oxide/carbon composites, metal sulfide/carbon hybrids, or oxide/nitride heterojunctions offer synergistic effects for stabilizing single metal atoms. These composite materials provide multiple anchoring sites with different binding energies, creating a more robust environment for single atoms. The interfaces between different components in these hybrid supports can trap metal atoms and prevent their migration, significantly improving their stability under reaction conditions while maintaining high catalytic activity.
02 Metal oxide supports for single atom catalysts
Metal oxides such as TiO2, CeO2, Al2O3, and ZnO provide strong metal-support interactions that enhance the stability of single metal atoms. These supports offer oxygen vacancies and surface hydroxyl groups that serve as anchoring sites for metal atoms. The strong electronic interactions between the metal atoms and oxide supports prevent sintering and maintain catalytic activity under harsh reaction conditions, making them suitable for high-temperature applications.Expand Specific Solutions03 Zeolite and MOF-based supports for single atom stabilization
Zeolites and Metal-Organic Frameworks (MOFs) provide well-defined porous structures that can confine and stabilize single metal atoms. Their uniform pore sizes and abundant coordination sites allow for precise control of metal atom location and coordination environment. The confined spaces within these materials prevent metal atom migration and aggregation, while the framework structure can be tailored to optimize the electronic properties of the supported metal atoms.Expand Specific Solutions04 Heteroatom-doped supports for enhanced metal atom anchoring
Introducing heteroatoms such as nitrogen, phosphorus, sulfur, or boron into support materials creates strong binding sites for single metal atoms. These dopants modify the electronic structure of the support, creating localized charge centers that form strong coordination bonds with metal atoms. The resulting enhanced metal-support interactions significantly improve the thermal and chemical stability of single atom catalysts, preventing aggregation during catalytic reactions.Expand Specific Solutions05 Composite and hybrid support materials for single atom stability
Hybrid supports combining multiple materials (such as metal oxide/carbon composites or mixed metal oxides) provide synergistic effects for stabilizing single metal atoms. These composite structures offer multiple anchoring mechanisms and can be designed to optimize both stability and catalytic performance. The interfaces between different components in these hybrid materials often create unique coordination environments that enhance metal atom binding while maintaining accessibility for catalytic reactions.Expand Specific Solutions
Leading Research Groups and Industrial Players
The single metal atom stabilization support materials field is currently in the growth phase, with an estimated market size of $500-700 million and expanding at 15-20% annually. The technology is approaching maturity with significant advancements from key players across academia and industry. Leading research institutions like Dalian Institute of Chemical Physics, Northwestern University, and KAUST are pioneering fundamental breakthroughs, while companies including 3M Innovative Properties, Phillips 66, and ConocoPhillips are developing commercial applications. The competitive landscape features strong collaboration between academic institutions (Beijing University of Chemical Technology, Nanjing University) and industrial partners, with increasing patent activity suggesting the technology is transitioning from laboratory research to commercial deployment in catalysis, energy storage, and environmental remediation applications.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative support materials for single-atom catalysts (SACs) focusing on metal-organic frameworks (MOFs) and nitrogen-doped carbon materials. Their approach involves precise control of coordination environments through atomic layer deposition techniques and defect engineering. DICP researchers have successfully stabilized various metal atoms (Pt, Pd, Au, Ru) on supports by creating tailored nitrogen-containing anchor sites that form strong metal-N bonds. Their recent breakthrough involves using hierarchical porous carbon materials with controlled micropore/mesopore ratios that prevent metal atom aggregation during high-temperature treatments. The institute has demonstrated remarkable stability of their SACs under harsh reaction conditions (800°C) while maintaining single-atom dispersion, addressing one of the field's most significant challenges.
Strengths: Superior thermal stability of supported single atoms through innovative nitrogen-doping strategies; excellent control over metal-support interactions; proven scalability of synthesis methods. Weaknesses: Some supports may have limited applicability in certain industrial environments; potential high production costs for specialized MOF-derived materials.
Northwestern University
Technical Solution: Northwestern University has pioneered the development of metal-organic frameworks (MOFs) as support materials for single-atom catalysts. Their research team has created a series of zirconium-based MOFs with engineered defect sites that serve as strong anchoring points for isolated metal atoms. The university's approach involves post-synthetic metalation of MOFs, where open metal sites or functional groups within the framework selectively bind single metal atoms through coordination chemistry. Their proprietary method includes controlled thermal treatment protocols that maintain the single-atom state while activating the catalyst. Northwestern researchers have demonstrated remarkable success in stabilizing platinum, palladium, and gold atoms with loadings up to 5 wt% without aggregation. Their MOF supports provide unique advantages through tunable pore environments that can be tailored to specific catalytic applications, creating a molecular-level controlled environment for single-atom catalysis.
Strengths: Exceptional control over the local coordination environment of metal atoms; high metal loading capacity without aggregation; tunable pore structure for application-specific optimization. Weaknesses: MOF stability under harsh reaction conditions remains challenging; scale-up of precisely engineered MOF supports can be complex and costly.
Key Innovations in Metal-Support Interactions
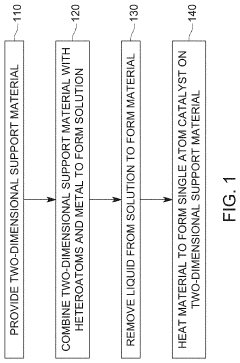
Method to produce high densities of isolated atoms on support substrates
PatentActiveUS12226754B2
Innovation
- A method involving the dissolution of a catalyst precursor in water, mixing with a support substrate, adding a metal precursor, using a non-water soluble chelating agent like 8-hydroxyquinoline to prevent agglomeration, and reducing the solution to anchor metal precursors to the support, resulting in high loadings of single atoms up to 1 atom per nm2.
Single atom catalyst having a two dimensional support material
PatentPendingUS20220111358A1
Innovation
- A single atom catalyst is formed by combining a two-dimensional support material with at least two heteroatoms and a metal, where the heteroatoms bind and stabilize the single atom metal on the support material, reducing the need for expensive metals and simplifying production.
Characterization Methods for Single Atom Catalysts
Characterization of single atom catalysts (SACs) requires sophisticated analytical techniques due to the atomic dispersion of metal atoms on support materials. Advanced microscopy techniques, particularly aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), have revolutionized SAC analysis by enabling direct visualization of individual metal atoms. This technique distinguishes metal atoms from support materials based on Z-contrast differences, providing crucial information about spatial distribution and coordination environments.
X-ray absorption spectroscopy (XAS), including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offers complementary information about the electronic structure and local coordination environment of single metal atoms. XANES reveals oxidation states and electronic configurations, while EXAFS provides detailed information about bond distances and coordination numbers, confirming the isolated nature of metal atoms.
Synchrotron radiation-based techniques have significantly enhanced characterization capabilities. X-ray photoelectron spectroscopy (XPS) determines the oxidation states and electronic interactions between metal atoms and supports, while temperature-programmed techniques (TPR/TPO/TPD) evaluate the redox properties and stability of single atoms under reaction conditions.
Advanced spectroscopic methods such as solid-state nuclear magnetic resonance (NMR) and Mössbauer spectroscopy provide element-specific information about the local environment of metal atoms. For certain elements, these techniques can reveal subtle changes in electronic structure and bonding that might be missed by other methods.
Computational approaches have become increasingly important in interpreting experimental data. Density functional theory (DFT) calculations help predict stable configurations of single atoms on various supports and simulate spectroscopic signatures, enabling more accurate interpretation of experimental results.
Multi-technique approaches combining microscopic and spectroscopic methods are now standard practice in SAC characterization. This integrated approach provides comprehensive understanding of structure-property relationships in single atom catalysts, correlating atomic dispersion with catalytic performance.
In-situ and operando characterization techniques represent the frontier of SAC analysis, allowing researchers to observe structural changes and dynamic behaviors of single atoms under realistic reaction conditions. These methods are crucial for understanding catalyst stability and deactivation mechanisms, particularly the tendency of single atoms to aggregate into nanoparticles during catalytic reactions.
X-ray absorption spectroscopy (XAS), including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offers complementary information about the electronic structure and local coordination environment of single metal atoms. XANES reveals oxidation states and electronic configurations, while EXAFS provides detailed information about bond distances and coordination numbers, confirming the isolated nature of metal atoms.
Synchrotron radiation-based techniques have significantly enhanced characterization capabilities. X-ray photoelectron spectroscopy (XPS) determines the oxidation states and electronic interactions between metal atoms and supports, while temperature-programmed techniques (TPR/TPO/TPD) evaluate the redox properties and stability of single atoms under reaction conditions.
Advanced spectroscopic methods such as solid-state nuclear magnetic resonance (NMR) and Mössbauer spectroscopy provide element-specific information about the local environment of metal atoms. For certain elements, these techniques can reveal subtle changes in electronic structure and bonding that might be missed by other methods.
Computational approaches have become increasingly important in interpreting experimental data. Density functional theory (DFT) calculations help predict stable configurations of single atoms on various supports and simulate spectroscopic signatures, enabling more accurate interpretation of experimental results.
Multi-technique approaches combining microscopic and spectroscopic methods are now standard practice in SAC characterization. This integrated approach provides comprehensive understanding of structure-property relationships in single atom catalysts, correlating atomic dispersion with catalytic performance.
In-situ and operando characterization techniques represent the frontier of SAC analysis, allowing researchers to observe structural changes and dynamic behaviors of single atoms under realistic reaction conditions. These methods are crucial for understanding catalyst stability and deactivation mechanisms, particularly the tendency of single atoms to aggregate into nanoparticles during catalytic reactions.
Sustainability Aspects of Support Materials
The sustainability of support materials for single atom catalysts (SACs) represents a critical dimension in their development trajectory. Environmental considerations have become increasingly prominent, with researchers focusing on reducing the ecological footprint of support material synthesis and application. Materials derived from renewable resources, such as biomass-based carbons and natural minerals, are gaining traction as sustainable alternatives to conventional synthetic supports.
Life cycle assessment (LCA) studies reveal that traditional support materials often involve energy-intensive production processes and hazardous chemicals. For instance, the synthesis of metal-organic frameworks (MOFs) typically requires organic solvents that pose environmental risks. Recent innovations have introduced greener synthesis routes, including solvent-free mechanochemical approaches and low-temperature aqueous methods, significantly reducing environmental impact.
Recyclability emerges as another crucial sustainability factor. Ideal support materials should maintain structural integrity through multiple catalytic cycles, allowing for metal atom recovery and redeposition. Advanced materials like graphene-based supports demonstrate exceptional recyclability, with some systems maintaining over 90% catalytic activity after five regeneration cycles.
The toxicity profiles of support materials also warrant careful consideration. Nanoscale materials may present unique environmental and health risks due to their high surface reactivity and potential bioaccumulation. Research indicates that carbon-based supports generally exhibit lower ecotoxicity compared to metal oxide counterparts, though comprehensive long-term studies remain limited.
Energy efficiency in catalyst operation represents another sustainability dimension. Support materials that enable catalytic reactions under milder conditions (lower temperatures and pressures) contribute significantly to reducing the overall energy demands of chemical processes. For example, nitrogen-doped carbon supports have demonstrated the ability to lower activation energies for hydrogen evolution reactions by up to 30% compared to undoped variants.
Resource scarcity considerations are driving innovation toward supports composed of earth-abundant elements. Silicon-based materials and aluminum oxides are increasingly preferred over rare earth element-containing supports. This shift aligns with circular economy principles, where material selection prioritizes long-term resource availability and minimizes dependence on geopolitically sensitive supply chains.
The integration of waste valorization with support material development represents a promising frontier. Industrial by-products like fly ash, red mud, and agricultural residues are being repurposed as precursors for catalyst supports, simultaneously addressing waste management challenges and creating value-added materials for advanced catalytic applications.
Life cycle assessment (LCA) studies reveal that traditional support materials often involve energy-intensive production processes and hazardous chemicals. For instance, the synthesis of metal-organic frameworks (MOFs) typically requires organic solvents that pose environmental risks. Recent innovations have introduced greener synthesis routes, including solvent-free mechanochemical approaches and low-temperature aqueous methods, significantly reducing environmental impact.
Recyclability emerges as another crucial sustainability factor. Ideal support materials should maintain structural integrity through multiple catalytic cycles, allowing for metal atom recovery and redeposition. Advanced materials like graphene-based supports demonstrate exceptional recyclability, with some systems maintaining over 90% catalytic activity after five regeneration cycles.
The toxicity profiles of support materials also warrant careful consideration. Nanoscale materials may present unique environmental and health risks due to their high surface reactivity and potential bioaccumulation. Research indicates that carbon-based supports generally exhibit lower ecotoxicity compared to metal oxide counterparts, though comprehensive long-term studies remain limited.
Energy efficiency in catalyst operation represents another sustainability dimension. Support materials that enable catalytic reactions under milder conditions (lower temperatures and pressures) contribute significantly to reducing the overall energy demands of chemical processes. For example, nitrogen-doped carbon supports have demonstrated the ability to lower activation energies for hydrogen evolution reactions by up to 30% compared to undoped variants.
Resource scarcity considerations are driving innovation toward supports composed of earth-abundant elements. Silicon-based materials and aluminum oxides are increasingly preferred over rare earth element-containing supports. This shift aligns with circular economy principles, where material selection prioritizes long-term resource availability and minimizes dependence on geopolitically sensitive supply chains.
The integration of waste valorization with support material development represents a promising frontier. Industrial by-products like fly ash, red mud, and agricultural residues are being repurposed as precursors for catalyst supports, simultaneously addressing waste management challenges and creating value-added materials for advanced catalytic applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!