Role Of Coordination Environment In SAC Activity
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SAC Catalysis Background and Objectives
Single-atom catalysts (SACs) have emerged as a revolutionary frontier in heterogeneous catalysis over the past decade, representing a paradigm shift in catalyst design. These catalysts feature isolated metal atoms dispersed on support materials, maximizing atomic efficiency and offering unique catalytic properties distinct from traditional nanoparticle catalysts. The coordination environment surrounding these single metal atoms plays a pivotal role in determining their catalytic performance, stability, and selectivity.
The evolution of SAC technology can be traced back to early theoretical predictions in the 1990s, followed by breakthrough experimental validations in the early 2000s. However, it was not until 2011 when Zhang and colleagues published their seminal work on Pt1/FeOx catalysts that the field gained significant momentum. Since then, research interest has grown exponentially, with publications increasing by approximately 300% between 2015 and 2020.
Current technological trends in SAC research focus on precise control of the coordination environment, including the nature of coordinating atoms (O, N, S, etc.), coordination number, geometric configuration, and electronic properties. These factors collectively determine the binding strength of reactants, activation barriers, and reaction pathways. Recent advances in characterization techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ/operando methods have enabled unprecedented insights into the structure-activity relationships of SACs.
The primary objective of research in this domain is to establish fundamental understanding of how the coordination environment influences catalytic activity at the atomic level. This includes elucidating electronic structure modifications, charge transfer mechanisms, and orbital interactions between the metal center and its surrounding ligands. Additionally, researchers aim to develop rational design principles for tailoring coordination environments to specific reactions, thereby enabling predictive catalyst synthesis rather than empirical approaches.
Another critical goal is to bridge the materials gap between idealized model systems studied in laboratories and practical catalysts for industrial applications. This involves addressing challenges related to stability under realistic reaction conditions, scalable synthesis methods, and integration with existing industrial processes. The development of computational models that can accurately predict the influence of coordination environments on catalytic performance represents another important objective, potentially accelerating discovery through high-throughput virtual screening.
Ultimately, research in SAC coordination environments aims to unlock unprecedented catalytic performance for sustainable chemical transformations, including energy conversion, environmental remediation, and fine chemical synthesis, while minimizing the use of precious metals and maximizing atom efficiency.
The evolution of SAC technology can be traced back to early theoretical predictions in the 1990s, followed by breakthrough experimental validations in the early 2000s. However, it was not until 2011 when Zhang and colleagues published their seminal work on Pt1/FeOx catalysts that the field gained significant momentum. Since then, research interest has grown exponentially, with publications increasing by approximately 300% between 2015 and 2020.
Current technological trends in SAC research focus on precise control of the coordination environment, including the nature of coordinating atoms (O, N, S, etc.), coordination number, geometric configuration, and electronic properties. These factors collectively determine the binding strength of reactants, activation barriers, and reaction pathways. Recent advances in characterization techniques such as aberration-corrected electron microscopy, X-ray absorption spectroscopy, and in-situ/operando methods have enabled unprecedented insights into the structure-activity relationships of SACs.
The primary objective of research in this domain is to establish fundamental understanding of how the coordination environment influences catalytic activity at the atomic level. This includes elucidating electronic structure modifications, charge transfer mechanisms, and orbital interactions between the metal center and its surrounding ligands. Additionally, researchers aim to develop rational design principles for tailoring coordination environments to specific reactions, thereby enabling predictive catalyst synthesis rather than empirical approaches.
Another critical goal is to bridge the materials gap between idealized model systems studied in laboratories and practical catalysts for industrial applications. This involves addressing challenges related to stability under realistic reaction conditions, scalable synthesis methods, and integration with existing industrial processes. The development of computational models that can accurately predict the influence of coordination environments on catalytic performance represents another important objective, potentially accelerating discovery through high-throughput virtual screening.
Ultimately, research in SAC coordination environments aims to unlock unprecedented catalytic performance for sustainable chemical transformations, including energy conversion, environmental remediation, and fine chemical synthesis, while minimizing the use of precious metals and maximizing atom efficiency.
Market Applications of Single-Atom Catalysts
Single-atom catalysts (SACs) have emerged as a revolutionary class of materials with diverse market applications across multiple industries. The unique coordination environment of isolated metal atoms on support materials enables unprecedented catalytic performance, driving adoption in several high-value sectors.
In the energy industry, SACs are transforming fuel cell technology through enhanced oxygen reduction reactions. Companies like Toyota and Hyundai are exploring SAC integration in their hydrogen fuel cell vehicles to improve efficiency and reduce platinum group metal usage. The coordination environment of metal atoms in these catalysts significantly impacts their stability and activity under operational conditions, potentially extending fuel cell lifespans while reducing costs.
Environmental applications represent another major market segment, with SACs showing exceptional performance in pollution control systems. Their precisely tuned coordination environments enable selective catalytic reduction of nitrogen oxides and oxidation of carbon monoxide at lower temperatures than conventional catalysts. This property makes them particularly valuable for automotive emission control systems and industrial exhaust treatment, where regulatory standards continue to tighten globally.
The petrochemical industry has identified SACs as potential game-changers for selective hydrogenation and oxidation processes. Companies including BASF and Dow Chemical are investigating SAC implementation in existing production lines to improve product selectivity and reduce energy consumption. The ability to fine-tune the coordination environment of single metal atoms allows for unprecedented control over reaction pathways, potentially revolutionizing chemical manufacturing economics.
Pharmaceutical manufacturing represents an emerging application area where SACs' selectivity enables more efficient synthesis routes for complex molecules. The coordination environment's influence on catalytic activity allows for milder reaction conditions and fewer side products, addressing key challenges in green chemistry implementation. Several major pharmaceutical companies have initiated research programs exploring SAC integration into their manufacturing processes.
The electronics industry is exploring SACs for next-generation semiconductor manufacturing, where precisely controlled surface reactions are essential. The unique coordination environments of SACs enable atomic-level precision in etching and deposition processes, potentially addressing critical challenges in sub-5nm fabrication nodes.
Agricultural applications are also emerging, with SACs showing promise in fertilizer production through more efficient nitrogen fixation processes. By optimizing the coordination environment of single metal atoms, researchers have demonstrated ammonia synthesis under milder conditions than the traditional Haber-Bosch process, potentially reducing the carbon footprint of global fertilizer production.
In the energy industry, SACs are transforming fuel cell technology through enhanced oxygen reduction reactions. Companies like Toyota and Hyundai are exploring SAC integration in their hydrogen fuel cell vehicles to improve efficiency and reduce platinum group metal usage. The coordination environment of metal atoms in these catalysts significantly impacts their stability and activity under operational conditions, potentially extending fuel cell lifespans while reducing costs.
Environmental applications represent another major market segment, with SACs showing exceptional performance in pollution control systems. Their precisely tuned coordination environments enable selective catalytic reduction of nitrogen oxides and oxidation of carbon monoxide at lower temperatures than conventional catalysts. This property makes them particularly valuable for automotive emission control systems and industrial exhaust treatment, where regulatory standards continue to tighten globally.
The petrochemical industry has identified SACs as potential game-changers for selective hydrogenation and oxidation processes. Companies including BASF and Dow Chemical are investigating SAC implementation in existing production lines to improve product selectivity and reduce energy consumption. The ability to fine-tune the coordination environment of single metal atoms allows for unprecedented control over reaction pathways, potentially revolutionizing chemical manufacturing economics.
Pharmaceutical manufacturing represents an emerging application area where SACs' selectivity enables more efficient synthesis routes for complex molecules. The coordination environment's influence on catalytic activity allows for milder reaction conditions and fewer side products, addressing key challenges in green chemistry implementation. Several major pharmaceutical companies have initiated research programs exploring SAC integration into their manufacturing processes.
The electronics industry is exploring SACs for next-generation semiconductor manufacturing, where precisely controlled surface reactions are essential. The unique coordination environments of SACs enable atomic-level precision in etching and deposition processes, potentially addressing critical challenges in sub-5nm fabrication nodes.
Agricultural applications are also emerging, with SACs showing promise in fertilizer production through more efficient nitrogen fixation processes. By optimizing the coordination environment of single metal atoms, researchers have demonstrated ammonia synthesis under milder conditions than the traditional Haber-Bosch process, potentially reducing the carbon footprint of global fertilizer production.
Current Coordination Environment Challenges
Single-atom catalysts (SACs) represent a frontier in heterogeneous catalysis, offering maximum atom efficiency and unique catalytic properties. However, the coordination environment surrounding these isolated metal atoms presents significant challenges that currently limit their widespread application and performance optimization.
The primary challenge lies in achieving precise control over the coordination structure. Unlike traditional catalysts where metal clusters provide multiple binding sites, SACs feature individual metal atoms with limited coordination possibilities. This constraint makes it difficult to engineer specific electronic structures and binding geometries necessary for targeted catalytic reactions. Researchers struggle to systematically manipulate coordination numbers, bond distances, and geometric arrangements around the central metal atom.
Environmental stability poses another critical challenge. The coordination environment in SACs often exhibits dynamic behavior under reaction conditions, with ligands potentially undergoing exchange, restructuring, or degradation. This instability can lead to unpredictable catalytic performance and accelerated deactivation. Particularly in harsh reaction environments involving high temperatures or corrosive reagents, maintaining the integrity of the coordination sphere becomes exceptionally difficult.
Characterization limitations further complicate progress in this field. Current analytical techniques provide insufficient resolution to fully elucidate the three-dimensional coordination environment of single atoms during catalytic processes. While advanced microscopy and spectroscopy methods like aberration-corrected STEM, XAFS, and in-situ XPS offer valuable insights, they still cannot capture the complete dynamic picture of coordination changes occurring during reactions, especially at the atomic level under operating conditions.
The heterogeneity of coordination sites represents another significant obstacle. Even in carefully synthesized SACs, variations in the local environment surrounding individual metal atoms create a distribution of coordination structures rather than a uniform arrangement. This heterogeneity complicates mechanistic studies and rational catalyst design, as catalytic performance becomes an average of diverse active sites with varying coordination environments.
Scalable synthesis methods that can produce SACs with precisely controlled coordination environments remain elusive. Current approaches often yield materials with a mixture of coordination states or suffer from poor reproducibility. The challenge intensifies when attempting to create specific coordination environments tailored for particular reaction pathways, as synthetic protocols lack the precision needed to selectively form desired metal-ligand arrangements.
The primary challenge lies in achieving precise control over the coordination structure. Unlike traditional catalysts where metal clusters provide multiple binding sites, SACs feature individual metal atoms with limited coordination possibilities. This constraint makes it difficult to engineer specific electronic structures and binding geometries necessary for targeted catalytic reactions. Researchers struggle to systematically manipulate coordination numbers, bond distances, and geometric arrangements around the central metal atom.
Environmental stability poses another critical challenge. The coordination environment in SACs often exhibits dynamic behavior under reaction conditions, with ligands potentially undergoing exchange, restructuring, or degradation. This instability can lead to unpredictable catalytic performance and accelerated deactivation. Particularly in harsh reaction environments involving high temperatures or corrosive reagents, maintaining the integrity of the coordination sphere becomes exceptionally difficult.
Characterization limitations further complicate progress in this field. Current analytical techniques provide insufficient resolution to fully elucidate the three-dimensional coordination environment of single atoms during catalytic processes. While advanced microscopy and spectroscopy methods like aberration-corrected STEM, XAFS, and in-situ XPS offer valuable insights, they still cannot capture the complete dynamic picture of coordination changes occurring during reactions, especially at the atomic level under operating conditions.
The heterogeneity of coordination sites represents another significant obstacle. Even in carefully synthesized SACs, variations in the local environment surrounding individual metal atoms create a distribution of coordination structures rather than a uniform arrangement. This heterogeneity complicates mechanistic studies and rational catalyst design, as catalytic performance becomes an average of diverse active sites with varying coordination environments.
Scalable synthesis methods that can produce SACs with precisely controlled coordination environments remain elusive. Current approaches often yield materials with a mixture of coordination states or suffer from poor reproducibility. The challenge intensifies when attempting to create specific coordination environments tailored for particular reaction pathways, as synthetic protocols lack the precision needed to selectively form desired metal-ligand arrangements.
Current Approaches to Coordination Control
01 Metal-nitrogen coordination in single-atom catalysts
The coordination environment between metal atoms and nitrogen atoms plays a crucial role in single-atom catalyst (SAC) activity. Metal-nitrogen (M-N) coordination structures, particularly M-N4 configurations, provide stable anchoring sites for single metal atoms on carbon supports. This coordination environment influences electron transfer, adsorption properties, and catalytic performance in various reactions including oxygen reduction and CO2 conversion.- Metal-nitrogen coordination in single-atom catalysts: The coordination environment between metal atoms and nitrogen atoms plays a crucial role in single-atom catalyst (SAC) activity. Metal-nitrogen (M-N) coordination structures, particularly M-N4 configurations, provide stable anchoring sites for single metal atoms on carbon supports. This coordination environment influences electron transfer, adsorption properties, and catalytic performance in various reactions including oxygen reduction and CO2 conversion.
- Oxygen coordination effects on catalytic performance: Oxygen-containing functional groups in the coordination environment significantly impact SAC activity. Metal-oxygen coordination can modify the electronic structure of the metal center, affecting charge distribution and catalytic properties. The presence of oxygen in the coordination sphere can enhance stability, selectivity, and activity of single-atom catalysts, particularly in oxidation reactions and electrochemical applications.
- Tuning coordination number for optimized catalytic activity: The coordination number of metal atoms in SACs directly influences their catalytic performance. By precisely controlling the number of coordinating atoms around the metal center, the electronic structure and geometric configuration can be optimized for specific reactions. Lower coordination numbers often expose more active sites, while higher coordination numbers may provide better stability. Synthetic methods that allow precise control over coordination environments are essential for designing high-performance catalysts.
- Support material effects on coordination environment: The choice of support material significantly affects the coordination environment and resulting catalytic activity of SACs. Different supports such as carbon-based materials, metal oxides, and 2D materials provide unique binding sites and electronic interactions with the metal atoms. The support's surface chemistry, porosity, and crystallinity influence the formation and stability of specific coordination structures, thereby determining the catalyst's activity, selectivity, and durability.
- Coordination environment characterization techniques: Advanced characterization techniques are essential for understanding the coordination environment in SACs. X-ray absorption spectroscopy (XAS), including XANES and EXAFS, provides detailed information about the local structure around metal atoms. Scanning transmission electron microscopy (STEM), X-ray photoelectron spectroscopy (XPS), and computational modeling are also crucial for elucidating coordination structures. These techniques help establish structure-activity relationships and guide the rational design of more efficient catalysts.
02 Oxygen coordination effects on catalytic performance
Oxygen-containing functional groups in the coordination environment significantly affect SAC activity. Metal-oxygen coordination can modify the electronic structure of the metal center, altering its catalytic properties. The presence of oxygen in the coordination sphere can enhance stability, promote specific reaction pathways, and improve selectivity in catalytic processes such as hydrogenation and oxidation reactions.Expand Specific Solutions03 Tuning coordination number for optimized activity
The coordination number of metal centers in SACs directly impacts their catalytic activity. By precisely controlling the number of coordinating atoms around the metal center, the electronic properties and geometric configuration can be optimized. Lower coordination numbers often expose more active sites, while specific coordination environments can be tailored to enhance activity for target reactions and improve catalyst efficiency.Expand Specific Solutions04 Heteroatom doping to modify coordination environment
Introducing heteroatoms such as sulfur, phosphorus, or boron into the coordination sphere of single-atom catalysts can significantly alter their electronic properties and catalytic behavior. These heteroatoms create unique coordination environments that can enhance electron transfer, modify binding energies, and improve selectivity in various catalytic reactions. Strategic heteroatom doping provides a versatile approach to fine-tune the coordination environment for specific applications.Expand Specific Solutions05 Dynamic coordination environment under reaction conditions
The coordination environment of single-atom catalysts is not static but undergoes dynamic changes during catalytic reactions. Under operating conditions, coordination structures may transform, ligands may exchange, and oxidation states may shift, all affecting catalytic performance. Understanding these dynamic processes is essential for designing stable and efficient catalysts, as the actual active coordination environment may differ significantly from the as-synthesized state.Expand Specific Solutions
Leading Research Groups and Industry Players
The coordination environment in Single-Atom Catalysts (SAC) activity is currently in a rapidly evolving research phase, with the market expanding as industries recognize its potential for efficient catalytic processes. The technology is approaching early maturity, with significant advancements from key players across academia and industry. Leading research institutions like Dalian Institute of Chemical Physics and Beijing University of Chemical Technology are pioneering fundamental research, while technology giants including Google, Huawei, and Apple are exploring applications in advanced computing and energy systems. Telecommunications leaders such as Ericsson, Nokia, and ZTE are investigating SAC for next-generation communication technologies. Automotive companies like Toyota and DENSO are leveraging this technology for emissions control and fuel cell applications, demonstrating the cross-industry relevance of coordination environment optimization in single-atom catalysis.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has pioneered research on coordination environment engineering in single-atom catalysts (SACs), developing a systematic approach to manipulate metal-support interactions. Their technology involves precise control of coordination numbers, bond distances, and electronic structures through advanced synthesis methods including atomic layer deposition and high-temperature atom trapping. They've demonstrated that tuning the coordination environment with N, O, and S ligands significantly enhances catalytic performance in various reactions including CO oxidation and hydrogen evolution. Their recent work has established quantitative structure-activity relationships correlating coordination geometry with catalytic efficiency, enabling rational design of SACs with optimized metal-support interfaces.
Strengths: Exceptional control over atomic-level coordination structures; comprehensive understanding of structure-activity relationships; advanced characterization capabilities. Weaknesses: Laboratory-scale synthesis methods may face challenges in industrial scaling; requires sophisticated equipment for precise atomic manipulation.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics has developed proprietary technology for manipulating coordination environments in single-atom catalysts through their "coordination sphere engineering" approach. Their methodology involves precise control of first and second coordination spheres around metal centers using tailored supports and ligand structures. They've pioneered the use of in-situ characterization techniques including XAFS and environmental TEM to directly observe coordination changes during catalytic processes. Their research has demonstrated that manipulating the coordination environment can tune the d-band center of metal atoms, thereby optimizing adsorption energies of reactants and improving catalytic performance. Their technology has shown particular success in electrochemical CO2 reduction and nitrogen fixation applications, achieving up to 95% selectivity through coordination environment optimization.
Strengths: World-leading in-situ characterization capabilities; established correlations between coordination structure and catalytic performance; demonstrated industrial relevance. Weaknesses: Complex synthesis procedures may limit large-scale production; highly specialized expertise required for implementation.
Key Mechanisms in SAC Coordination Effects
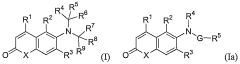
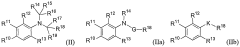
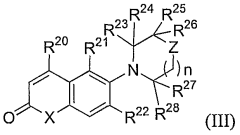
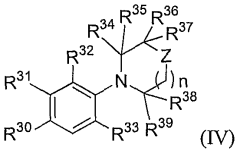
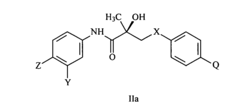
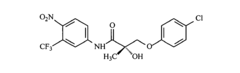
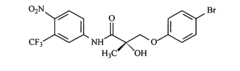
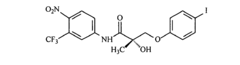
Androgen receptor modulator compounds, compositions and uses thereof
PatentWO2007005887A2
Innovation
- Development of novel compounds with specific structures, such as those represented by Formulas I to VI, which selectively bind to, modulate, or degrade androgen receptors, offering agonist, antagonist, or partial agonist activities to treat various conditions by administering these compounds to modulate androgen receptor activity in cells and tissues.
Selective androgen receptor modulators and methods of use thereof
PatentInactiveHK1156298A
Innovation
- Development of selective androgen receptor modulator (SARM) compounds that act as nonsteroidal ligands for the androgen receptor, providing both androgenic and antiandrogenic activities, useful for male contraception, treating androgen decline-related conditions, and addressing prostate cancer, with improved safety and efficacy profiles.
Sustainability Impact of SAC Technologies
The sustainability impact of Single-Atom Catalyst (SAC) technologies extends far beyond their immediate applications in chemical processes. These catalysts represent a significant advancement in green chemistry, offering unprecedented atom efficiency by utilizing nearly every metal atom as an active catalytic site.
Environmental benefits of SAC technologies are substantial and multifaceted. By enabling reactions at lower temperatures and pressures, these catalysts significantly reduce energy consumption in industrial processes. This translates directly to lower carbon emissions across various sectors, particularly in chemical manufacturing, petroleum refining, and pharmaceutical production. Studies indicate that SAC-based processes can achieve energy savings of 20-40% compared to conventional catalytic systems.
Resource conservation represents another critical sustainability advantage. SAC technologies dramatically reduce the amount of precious metals required for catalytic applications, addressing concerns about resource scarcity. This efficiency is particularly important for platinum group metals, where supply constraints and geopolitical factors create market volatility. The atom-efficient nature of SACs ensures minimal waste generation during catalyst production and application.
From a lifecycle perspective, SAC technologies demonstrate favorable environmental profiles. Their enhanced durability reduces the frequency of catalyst replacement, minimizing waste generation and resource consumption over time. Additionally, many SAC systems exhibit improved selectivity, reducing unwanted by-products and decreasing the environmental footprint associated with separation and waste treatment processes.
Economic sustainability is equally compelling. Despite higher initial development costs, the long-term economic benefits of SAC technologies include reduced operational expenses, decreased raw material requirements, and potential regulatory advantages. As environmental regulations become increasingly stringent worldwide, the cleaner production methods enabled by SACs offer significant compliance benefits and potential competitive advantages.
Social sustainability dimensions should not be overlooked. By enabling greener manufacturing processes, SAC technologies contribute to improved workplace safety and reduced community exposure to hazardous emissions. The development of these advanced catalytic systems also drives innovation and creates high-skilled employment opportunities in research, development, and specialized manufacturing.
Looking forward, the integration of SAC technologies into circular economy frameworks presents exciting possibilities. Their precision at the atomic level makes them ideal candidates for catalyzing chemical transformations in recycling processes, potentially enabling more efficient material recovery and upcycling pathways that are currently not economically viable.
Environmental benefits of SAC technologies are substantial and multifaceted. By enabling reactions at lower temperatures and pressures, these catalysts significantly reduce energy consumption in industrial processes. This translates directly to lower carbon emissions across various sectors, particularly in chemical manufacturing, petroleum refining, and pharmaceutical production. Studies indicate that SAC-based processes can achieve energy savings of 20-40% compared to conventional catalytic systems.
Resource conservation represents another critical sustainability advantage. SAC technologies dramatically reduce the amount of precious metals required for catalytic applications, addressing concerns about resource scarcity. This efficiency is particularly important for platinum group metals, where supply constraints and geopolitical factors create market volatility. The atom-efficient nature of SACs ensures minimal waste generation during catalyst production and application.
From a lifecycle perspective, SAC technologies demonstrate favorable environmental profiles. Their enhanced durability reduces the frequency of catalyst replacement, minimizing waste generation and resource consumption over time. Additionally, many SAC systems exhibit improved selectivity, reducing unwanted by-products and decreasing the environmental footprint associated with separation and waste treatment processes.
Economic sustainability is equally compelling. Despite higher initial development costs, the long-term economic benefits of SAC technologies include reduced operational expenses, decreased raw material requirements, and potential regulatory advantages. As environmental regulations become increasingly stringent worldwide, the cleaner production methods enabled by SACs offer significant compliance benefits and potential competitive advantages.
Social sustainability dimensions should not be overlooked. By enabling greener manufacturing processes, SAC technologies contribute to improved workplace safety and reduced community exposure to hazardous emissions. The development of these advanced catalytic systems also drives innovation and creates high-skilled employment opportunities in research, development, and specialized manufacturing.
Looking forward, the integration of SAC technologies into circular economy frameworks presents exciting possibilities. Their precision at the atomic level makes them ideal candidates for catalyzing chemical transformations in recycling processes, potentially enabling more efficient material recovery and upcycling pathways that are currently not economically viable.
Characterization Methods for Coordination Environments
The characterization of coordination environments in single-atom catalysts (SACs) requires sophisticated analytical techniques to precisely identify the local structure around isolated metal atoms. X-ray absorption spectroscopy (XAS), including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), serves as a primary tool for probing the coordination environment. XANES provides information about oxidation states and electronic structures, while EXAFS reveals bond distances, coordination numbers, and neighboring atom identities with sub-angstrom precision.
Advanced electron microscopy techniques, particularly aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), enable direct visualization of single metal atoms and their distribution on support materials. The Z-contrast imaging capability allows differentiation between metal atoms and support elements, confirming the single-atom dispersion essential for SAC characterization.
Synchrotron-based techniques offer exceptional sensitivity for coordination environment analysis. X-ray photoelectron spectroscopy (XPS) provides information about oxidation states and chemical bonding, while diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) with probe molecules reveals active site accessibility and binding characteristics. These techniques are often combined with in-situ or operando setups to monitor coordination changes under reaction conditions.
Computational methods complement experimental characterization through density functional theory (DFT) calculations and molecular dynamics simulations. These approaches help interpret experimental data and predict coordination structures that may be challenging to observe directly. Machine learning algorithms are increasingly employed to analyze complex spectroscopic datasets, enhancing the accuracy of coordination environment determination.
Solid-state nuclear magnetic resonance (NMR) spectroscopy provides valuable information about the local electronic environment of metal centers, particularly for diamagnetic species. Temperature-programmed techniques (TPR, TPD, TPO) offer insights into the redox properties and stability of coordination environments under varying conditions, which directly influence catalytic performance.
Multi-technique approaches combining several characterization methods have become standard practice for comprehensive coordination environment analysis. The correlation between characterization data and catalytic performance metrics enables the establishment of structure-activity relationships, guiding rational design of more efficient SACs with optimized coordination environments.
Advanced electron microscopy techniques, particularly aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), enable direct visualization of single metal atoms and their distribution on support materials. The Z-contrast imaging capability allows differentiation between metal atoms and support elements, confirming the single-atom dispersion essential for SAC characterization.
Synchrotron-based techniques offer exceptional sensitivity for coordination environment analysis. X-ray photoelectron spectroscopy (XPS) provides information about oxidation states and chemical bonding, while diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) with probe molecules reveals active site accessibility and binding characteristics. These techniques are often combined with in-situ or operando setups to monitor coordination changes under reaction conditions.
Computational methods complement experimental characterization through density functional theory (DFT) calculations and molecular dynamics simulations. These approaches help interpret experimental data and predict coordination structures that may be challenging to observe directly. Machine learning algorithms are increasingly employed to analyze complex spectroscopic datasets, enhancing the accuracy of coordination environment determination.
Solid-state nuclear magnetic resonance (NMR) spectroscopy provides valuable information about the local electronic environment of metal centers, particularly for diamagnetic species. Temperature-programmed techniques (TPR, TPD, TPO) offer insights into the redox properties and stability of coordination environments under varying conditions, which directly influence catalytic performance.
Multi-technique approaches combining several characterization methods have become standard practice for comprehensive coordination environment analysis. The correlation between characterization data and catalytic performance metrics enables the establishment of structure-activity relationships, guiding rational design of more efficient SACs with optimized coordination environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!