Reducing GC-MS Artifacts in Sample-rich Matrices
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GC-MS Artifact Reduction Background and Objectives
Gas Chromatography-Mass Spectrometry (GC-MS) has evolved as a cornerstone analytical technique in various scientific disciplines since its inception in the mid-20th century. The integration of chromatographic separation with mass spectrometric detection has revolutionized the identification and quantification of volatile and semi-volatile compounds across pharmaceutical, environmental, forensic, and food safety applications. However, the persistent challenge of artifacts in GC-MS analysis has become increasingly significant as applications expand into more complex matrices.
Artifacts in GC-MS can originate from multiple sources including sample preparation procedures, column bleed, septum bleed, contamination from vials or solvents, and matrix-induced effects. These unwanted signals can lead to false positives, obscure target analytes, complicate data interpretation, and ultimately compromise analytical reliability. The challenge becomes particularly acute when analyzing sample-rich matrices such as biological fluids, environmental samples, or food products where matrix components can interact with both the analytical system and target compounds.
Recent technological advancements have attempted to address these challenges through improved instrument design, enhanced data processing algorithms, and refined sample preparation techniques. However, a comprehensive and systematic approach to artifact reduction specifically tailored for complex matrices remains an evolving field with significant opportunities for innovation.
The primary objective of this technical research is to comprehensively evaluate current strategies for reducing GC-MS artifacts in sample-rich matrices and identify promising directions for future development. Specifically, we aim to assess the effectiveness of advanced sample preparation techniques, modern column technologies, innovative instrument parameters, and emerging data processing approaches in minimizing artifact formation and interference.
Additionally, this research seeks to establish a framework for systematically identifying the sources of artifacts in different types of complex matrices and developing matrix-specific mitigation strategies. By understanding the fundamental mechanisms of artifact generation, we can move beyond generic solutions toward targeted approaches that address the unique challenges presented by each matrix type.
The ultimate goal is to enhance the reliability, sensitivity, and specificity of GC-MS analysis in complex matrices, thereby expanding its applicability across scientific disciplines and improving the quality of analytical results. This research will provide valuable insights for analytical chemists, instrument manufacturers, and end-users seeking to optimize their GC-MS methodologies for challenging sample types.
Artifacts in GC-MS can originate from multiple sources including sample preparation procedures, column bleed, septum bleed, contamination from vials or solvents, and matrix-induced effects. These unwanted signals can lead to false positives, obscure target analytes, complicate data interpretation, and ultimately compromise analytical reliability. The challenge becomes particularly acute when analyzing sample-rich matrices such as biological fluids, environmental samples, or food products where matrix components can interact with both the analytical system and target compounds.
Recent technological advancements have attempted to address these challenges through improved instrument design, enhanced data processing algorithms, and refined sample preparation techniques. However, a comprehensive and systematic approach to artifact reduction specifically tailored for complex matrices remains an evolving field with significant opportunities for innovation.
The primary objective of this technical research is to comprehensively evaluate current strategies for reducing GC-MS artifacts in sample-rich matrices and identify promising directions for future development. Specifically, we aim to assess the effectiveness of advanced sample preparation techniques, modern column technologies, innovative instrument parameters, and emerging data processing approaches in minimizing artifact formation and interference.
Additionally, this research seeks to establish a framework for systematically identifying the sources of artifacts in different types of complex matrices and developing matrix-specific mitigation strategies. By understanding the fundamental mechanisms of artifact generation, we can move beyond generic solutions toward targeted approaches that address the unique challenges presented by each matrix type.
The ultimate goal is to enhance the reliability, sensitivity, and specificity of GC-MS analysis in complex matrices, thereby expanding its applicability across scientific disciplines and improving the quality of analytical results. This research will provide valuable insights for analytical chemists, instrument manufacturers, and end-users seeking to optimize their GC-MS methodologies for challenging sample types.
Market Analysis for Advanced Analytical Chemistry Solutions
The analytical chemistry market is experiencing robust growth, driven by increasing demand for advanced analytical techniques across various industries. The global analytical instrumentation market was valued at approximately $85 billion in 2022, with GC-MS systems representing a significant segment valued at $4.2 billion. This market is projected to grow at a CAGR of 5.8% through 2028, reflecting the essential role these technologies play in modern research and quality control processes.
The demand for solutions specifically addressing artifact reduction in GC-MS analysis of complex matrices is particularly strong in pharmaceutical, environmental, food safety, and forensic sectors. In pharmaceuticals, which accounts for 28% of the analytical chemistry market, there is increasing pressure to eliminate analytical interferences that can compromise drug development and quality control processes. Environmental testing laboratories, representing 22% of the market, require more reliable detection of trace contaminants in complex environmental samples without matrix interference.
Food safety testing, growing at 7.2% annually, presents a substantial opportunity as regulatory requirements become more stringent globally. Manufacturers are seeking analytical solutions that can accurately detect contaminants in diverse food matrices without false positives from artifacts. Similarly, the forensic toxicology segment, though smaller at $1.8 billion, demands extremely high accuracy in complex biological samples where artifacts can lead to serious legal consequences.
Regional analysis reveals North America holds the largest market share (38%) for advanced analytical chemistry solutions, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth at 8.3% annually, driven by expanding pharmaceutical manufacturing, environmental concerns, and food export quality requirements in countries like China, India, and South Korea.
Customer segmentation shows that large pharmaceutical companies and contract research organizations are willing to pay premium prices for solutions that eliminate artifacts and increase analytical reliability. Academic and government laboratories, while more price-sensitive, represent a substantial volume market, particularly for consumables and software solutions that can be implemented on existing instrumentation.
Competitive analysis indicates that while major analytical instrument manufacturers offer general solutions, there remains a significant gap for specialized products specifically targeting artifact reduction in complex matrices. This presents an opportunity for both established players and innovative startups to develop targeted solutions addressing this specific pain point, with potential for premium pricing and rapid market adoption if demonstrable improvements in analytical accuracy can be achieved.
The demand for solutions specifically addressing artifact reduction in GC-MS analysis of complex matrices is particularly strong in pharmaceutical, environmental, food safety, and forensic sectors. In pharmaceuticals, which accounts for 28% of the analytical chemistry market, there is increasing pressure to eliminate analytical interferences that can compromise drug development and quality control processes. Environmental testing laboratories, representing 22% of the market, require more reliable detection of trace contaminants in complex environmental samples without matrix interference.
Food safety testing, growing at 7.2% annually, presents a substantial opportunity as regulatory requirements become more stringent globally. Manufacturers are seeking analytical solutions that can accurately detect contaminants in diverse food matrices without false positives from artifacts. Similarly, the forensic toxicology segment, though smaller at $1.8 billion, demands extremely high accuracy in complex biological samples where artifacts can lead to serious legal consequences.
Regional analysis reveals North America holds the largest market share (38%) for advanced analytical chemistry solutions, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth at 8.3% annually, driven by expanding pharmaceutical manufacturing, environmental concerns, and food export quality requirements in countries like China, India, and South Korea.
Customer segmentation shows that large pharmaceutical companies and contract research organizations are willing to pay premium prices for solutions that eliminate artifacts and increase analytical reliability. Academic and government laboratories, while more price-sensitive, represent a substantial volume market, particularly for consumables and software solutions that can be implemented on existing instrumentation.
Competitive analysis indicates that while major analytical instrument manufacturers offer general solutions, there remains a significant gap for specialized products specifically targeting artifact reduction in complex matrices. This presents an opportunity for both established players and innovative startups to develop targeted solutions addressing this specific pain point, with potential for premium pricing and rapid market adoption if demonstrable improvements in analytical accuracy can be achieved.
Current Challenges in Complex Matrix Analysis
Complex matrices present significant analytical challenges in GC-MS analysis, particularly when dealing with biological samples, environmental specimens, or food products. These matrices contain numerous interfering compounds that can generate artifacts, compromising the accuracy and reliability of analytical results. The primary challenge lies in distinguishing between genuine analytes and matrix-induced artifacts, which often manifest as ghost peaks, baseline distortions, or co-eluting compounds.
Sample preparation techniques, while designed to minimize interferences, frequently introduce additional artifacts. Solvent impurities, degradation products from derivatization reagents, and contamination from laboratory consumables all contribute to the analytical noise. Even state-of-the-art sample cleanup procedures like solid-phase extraction (SPE) or QuEChERS methodology cannot completely eliminate these interferences in highly complex matrices.
Instrument-related challenges further complicate the analysis. Thermal degradation within the GC inlet can transform analytes into artifacts that were not present in the original sample. Active sites in the GC flow path, particularly in aged or improperly maintained systems, catalyze unwanted reactions that generate secondary compounds. Column bleed, especially at elevated temperatures, introduces siloxane-based artifacts that can mask low-concentration analytes of interest.
Data processing presents another layer of complexity. Current automated deconvolution algorithms struggle to differentiate between true signals and matrix-induced artifacts when spectral overlap is significant. False positives and negatives frequently occur in untargeted screening approaches, requiring extensive manual verification that diminishes analytical throughput and efficiency.
The quantitative impact of these artifacts is particularly problematic in regulatory and clinical applications. Matrix effects can suppress or enhance analyte responses, leading to inaccurate quantification even when using internal standards. Method validation becomes exceptionally challenging as matrix effects vary significantly between different sample sources, necessitating matrix-matched calibrations that are often impractical for routine analysis.
Emerging high-resolution techniques, while offering improved selectivity, introduce their own artifacts through more complex data processing algorithms. The higher sensitivity of these instruments paradoxically reveals more potential interferences, creating a signal-to-noise paradox where improved detection capabilities lead to more complex data interpretation challenges.
These multifaceted challenges necessitate innovative approaches that address both the chemical and computational aspects of artifact reduction in complex matrices, as current methodologies provide only partial solutions to this persistent analytical problem.
Sample preparation techniques, while designed to minimize interferences, frequently introduce additional artifacts. Solvent impurities, degradation products from derivatization reagents, and contamination from laboratory consumables all contribute to the analytical noise. Even state-of-the-art sample cleanup procedures like solid-phase extraction (SPE) or QuEChERS methodology cannot completely eliminate these interferences in highly complex matrices.
Instrument-related challenges further complicate the analysis. Thermal degradation within the GC inlet can transform analytes into artifacts that were not present in the original sample. Active sites in the GC flow path, particularly in aged or improperly maintained systems, catalyze unwanted reactions that generate secondary compounds. Column bleed, especially at elevated temperatures, introduces siloxane-based artifacts that can mask low-concentration analytes of interest.
Data processing presents another layer of complexity. Current automated deconvolution algorithms struggle to differentiate between true signals and matrix-induced artifacts when spectral overlap is significant. False positives and negatives frequently occur in untargeted screening approaches, requiring extensive manual verification that diminishes analytical throughput and efficiency.
The quantitative impact of these artifacts is particularly problematic in regulatory and clinical applications. Matrix effects can suppress or enhance analyte responses, leading to inaccurate quantification even when using internal standards. Method validation becomes exceptionally challenging as matrix effects vary significantly between different sample sources, necessitating matrix-matched calibrations that are often impractical for routine analysis.
Emerging high-resolution techniques, while offering improved selectivity, introduce their own artifacts through more complex data processing algorithms. The higher sensitivity of these instruments paradoxically reveals more potential interferences, creating a signal-to-noise paradox where improved detection capabilities lead to more complex data interpretation challenges.
These multifaceted challenges necessitate innovative approaches that address both the chemical and computational aspects of artifact reduction in complex matrices, as current methodologies provide only partial solutions to this persistent analytical problem.
Established Methodologies for Artifact Minimization
01 Identification and reduction of GC-MS artifacts in analytical methods
GC-MS analysis can produce artifacts that interfere with accurate compound identification and quantification. These artifacts may arise from sample preparation, column degradation, or instrument conditions. Methods to identify and reduce these artifacts include optimized temperature programming, proper column selection, and regular system maintenance. Advanced data processing algorithms can also help distinguish between true analytes and artifacts, improving the reliability of analytical results.- Identification and elimination of GC-MS artifacts: Various methods and systems are developed to identify and eliminate artifacts in GC-MS analysis. These artifacts can arise from sample preparation, column bleeding, or instrument contamination. Advanced algorithms and software solutions can detect these artifacts by analyzing mass spectral patterns and retention time anomalies, allowing for their removal from analytical results to improve data accuracy and reliability.
- Column-related artifacts in GC-MS analysis: Stationary phase degradation in GC columns can produce artifacts that interfere with accurate compound identification. These artifacts typically appear as siloxane compounds or column bleed products that increase at higher temperatures. Specialized column technologies and conditioning procedures are developed to minimize these artifacts, including thermal stabilization treatments and novel bonding chemistries that reduce column bleeding during analysis.
- Sample preparation techniques to reduce artifacts: Improved sample preparation methods are designed to minimize the formation of artifacts during GC-MS analysis. These include optimized extraction procedures, derivatization techniques that reduce side reactions, and clean-up protocols that remove interfering compounds. Selective adsorbents and filtration systems can be employed to purify samples before injection, significantly reducing the occurrence of artifacts in the final analysis.
- Instrument design modifications to minimize artifacts: Specialized GC-MS instrument designs incorporate features to reduce artifact formation during analysis. These include improved inlet systems that minimize thermal degradation, enhanced vacuum systems that reduce background contamination, and optimized ion source configurations that prevent unwanted reactions. Advanced detector technologies with higher sensitivity and selectivity help distinguish between true analytes and artifacts.
- Software solutions for artifact detection and correction: Sophisticated software algorithms are developed to automatically identify and correct for artifacts in GC-MS data. These systems employ pattern recognition, statistical analysis, and machine learning techniques to distinguish between true analyte signals and various types of artifacts. Reference libraries of known artifacts aid in their identification, while deconvolution algorithms separate overlapping peaks and remove background noise, resulting in cleaner, more accurate analytical results.
02 Thermal decomposition artifacts in GC-MS analysis
Thermal decomposition during GC-MS analysis can create artifacts that complicate interpretation of results. These artifacts form when compounds break down due to high temperatures in the injection port or column. Strategies to minimize thermal decomposition artifacts include using lower injection temperatures, employing on-column injection techniques, optimizing carrier gas flow rates, and selecting appropriate column stationary phases with thermal stability for heat-sensitive compounds.Expand Specific Solutions03 Derivatization-related artifacts in GC-MS
Chemical derivatization used to enhance volatility of compounds for GC-MS analysis can introduce artifacts. These artifacts may result from incomplete reactions, side reactions, or degradation of derivatizing agents. Careful selection of derivatization reagents, optimization of reaction conditions, and use of internal standards can help identify and account for derivatization-related artifacts. Proper storage of derivatized samples and prompt analysis after preparation also reduces artifact formation.Expand Specific Solutions04 Column bleed and septum artifacts in GC-MS systems
Column bleed and septum artifacts are common issues in GC-MS analysis. Column bleed occurs when the stationary phase degrades at high temperatures, producing characteristic siloxane patterns in mass spectra. Septum bleed results from compounds released from the injection port septum. These artifacts can be minimized through proper column conditioning, using low-bleed columns, regular septum replacement, and implementing background subtraction in data processing to improve detection of trace compounds.Expand Specific Solutions05 Software solutions for artifact identification and removal in GC-MS data
Advanced software solutions have been developed to identify and remove artifacts from GC-MS data. These systems employ algorithms that can distinguish between true analyte signals and common artifacts based on their mass spectral patterns and retention behavior. Machine learning approaches can be trained to recognize artifact patterns across multiple samples. Automated background subtraction, deconvolution techniques, and library matching with artifact databases help analysts produce cleaner data for more accurate compound identification and quantification.Expand Specific Solutions
Leading Manufacturers and Research Institutions in GC-MS Technology
The GC-MS artifact reduction market in sample-rich matrices is currently in a growth phase, with increasing demand driven by analytical precision requirements across pharmaceutical, environmental, and clinical sectors. The market is characterized by established players like Agilent Technologies, Shimadzu, and Waters Technology leading innovation, while specialized companies such as LI-COR and Shanghai Luming Biotechnology are developing niche solutions. Technical maturity varies significantly, with major corporations like Siemens Healthineers, Philips, and Canon Medical Systems integrating advanced artifact reduction capabilities into comprehensive analytical platforms. Academic institutions (University of Tokyo, Southern Medical University) and research organizations (Mayo Foundation) are contributing fundamental research to address matrix interference challenges, suggesting the technology still has significant development potential despite commercial availability.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed comprehensive solutions for reducing GC-MS artifacts in sample-rich matrices through their Inert Flow Path technology. This approach focuses on creating an inert sample path from injection to detection, minimizing unwanted interactions between analytes and flow path surfaces. Their deactivated inlet liners feature specialized surface treatments that reduce active sites where sample decomposition can occur. Agilent's J&W Ultra Inert GC columns incorporate proprietary deactivation chemistry that significantly reduces column bleed and artifact formation, particularly important when analyzing complex biological or environmental samples[1]. Additionally, their Retention Gap Technology employs uncoated precolumns to trap non-volatile contaminants before they reach the analytical column, preventing matrix-induced peak distortion and column degradation[2]. Agilent has also pioneered advanced sample preparation techniques including QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) methodology optimized specifically for reducing matrix effects in complex samples prior to GC-MS analysis[3].
Strengths: Comprehensive end-to-end solution addressing multiple artifact sources simultaneously; proprietary deactivation technologies with proven performance across diverse sample types; integrated hardware and software solutions for automated artifact identification and removal. Weaknesses: Premium pricing compared to generic alternatives; some solutions require specialized training; system-specific consumables may limit flexibility for labs using multiple instrument platforms.
Waters Technology Corp.
Technical Solution: Waters Technology has developed the ACQUITY Premier Solution specifically targeting the reduction of GC-MS artifacts in complex matrices. This technology incorporates their patented MaxPeak High-Performance Surface (HPS) technology, which creates an innovative hybrid organic-inorganic surface that significantly reduces unwanted interactions between sensitive analytes and metal surfaces in the flow path[1]. For GC-MS applications, Waters has implemented QuanRecovery sample preparation technologies that minimize sample loss and contamination during preparation stages. Their approach includes specialized QuanRecovery vials and plates with hybrid surface technologies that prevent analyte adsorption, particularly beneficial for phosphorylated compounds, metal-binding proteins, and other challenging analytes in complex matrices[2]. Waters' GC-MS artifact reduction strategy also incorporates advanced column technologies with optimized stationary phases designed to minimize column bleed and artifact formation even at high temperatures. Their time-of-flight MS systems feature novel ion optics designs that reduce background noise and enhance signal-to-noise ratios, allowing analysts to distinguish true analyte signals from matrix-derived artifacts[3].
Strengths: Proprietary surface technologies specifically designed to address metal-sensitive compound analysis; comprehensive solution addressing both sample preparation and instrumental analysis; demonstrated effectiveness for particularly challenging analyte classes like phosphopeptides and oligonucleotides. Weaknesses: Higher initial investment compared to conventional systems; specialized consumables required to maintain optimal performance; technology benefits may be less pronounced for routine applications not involving problematic analyte classes.
Critical Patents and Literature on Matrix Effect Mitigation
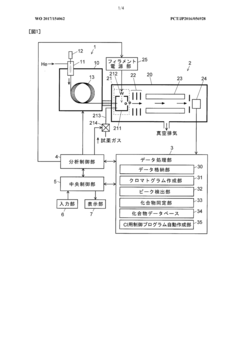
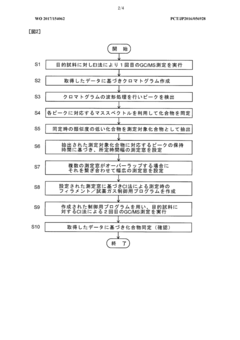
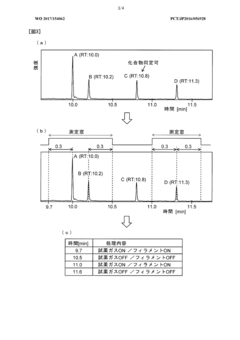
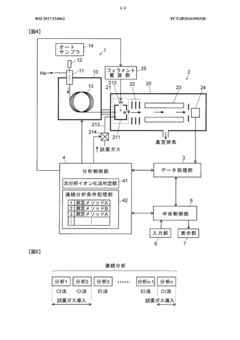
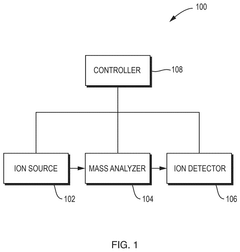
Gas chromatograph mass spectrometer
PatentWO2017154062A1
Innovation
- A GC-MS system with an ion source capable of switching between electron ionization (EI) and chemical ionization (CI) methods, where the reagent gas supply and thermionic electron generation are controlled to minimize reagent gas ion adhesion by only generating ions during specific time ranges corresponding to detected compounds, thereby maintaining high sensitivity and accuracy.
Carrier gas ion scavenger to reduce peak tailing and reactions
PatentPendingUS20250123250A1
Innovation
- A system and method for GC-MS using a carrier gas other than helium, which includes a scavenger gas with a lower ionization energy than the carrier gas, such as methane or a mixture of methane and ammonia, to reduce peak tailing and reactions by undergoing charge exchange with carrier gas ions in the ionization chamber.
Validation and Quality Control Protocols
Validation and quality control protocols are essential components in the reduction of GC-MS artifacts in sample-rich matrices. These protocols ensure that analytical results are reliable, reproducible, and free from systematic errors that could lead to misinterpretation of data. The implementation of robust validation procedures begins with method validation, which should include assessments of linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ), and matrix effects specifically tailored to complex sample matrices.
System suitability tests must be performed regularly to verify that the GC-MS system is operating within acceptable parameters. These tests typically include evaluations of peak resolution, tailing factor, theoretical plate count, and retention time reproducibility. For artifact reduction specifically, system suitability should also incorporate checks for column bleed, septum bleed, and ghost peaks using standard reference materials that mimic the complexity of the target matrices.
Quality control samples should be strategically incorporated throughout analytical batches. These include method blanks to identify background contamination, matrix-matched calibration standards to account for matrix effects, and certified reference materials to verify accuracy. For sample-rich matrices, the preparation and analysis of matrix-specific QC samples are particularly important, as they can reveal artifacts that might not be apparent in simpler matrices.
Statistical process control charts represent a valuable tool for monitoring system performance over time. By tracking key parameters such as response factors, retention times, and background signals, analysts can identify trends that might indicate developing problems before they significantly impact data quality. Control limits should be established based on historical data specific to the sample types being analyzed.
Proficiency testing and inter-laboratory comparisons provide external validation of a laboratory's ability to accurately identify and quantify analytes while minimizing artifacts. Participation in such programs is especially valuable when working with challenging sample matrices, as they expose methodological weaknesses that might not be apparent through internal validation alone.
Documentation and traceability form the foundation of effective quality control. All procedures, from sample preparation to data processing, should be thoroughly documented in standard operating procedures (SOPs). These SOPs should include specific steps for identifying common artifacts in the particular sample matrices being analyzed and protocols for addressing them when they occur.
Regular instrument maintenance schedules tailored to the analysis of complex matrices can significantly reduce artifact formation. This includes more frequent replacement of liners, septa, and columns than might be necessary for simpler samples, as well as specialized cleaning procedures for sample introduction components that come into contact with matrix-rich samples.
System suitability tests must be performed regularly to verify that the GC-MS system is operating within acceptable parameters. These tests typically include evaluations of peak resolution, tailing factor, theoretical plate count, and retention time reproducibility. For artifact reduction specifically, system suitability should also incorporate checks for column bleed, septum bleed, and ghost peaks using standard reference materials that mimic the complexity of the target matrices.
Quality control samples should be strategically incorporated throughout analytical batches. These include method blanks to identify background contamination, matrix-matched calibration standards to account for matrix effects, and certified reference materials to verify accuracy. For sample-rich matrices, the preparation and analysis of matrix-specific QC samples are particularly important, as they can reveal artifacts that might not be apparent in simpler matrices.
Statistical process control charts represent a valuable tool for monitoring system performance over time. By tracking key parameters such as response factors, retention times, and background signals, analysts can identify trends that might indicate developing problems before they significantly impact data quality. Control limits should be established based on historical data specific to the sample types being analyzed.
Proficiency testing and inter-laboratory comparisons provide external validation of a laboratory's ability to accurately identify and quantify analytes while minimizing artifacts. Participation in such programs is especially valuable when working with challenging sample matrices, as they expose methodological weaknesses that might not be apparent through internal validation alone.
Documentation and traceability form the foundation of effective quality control. All procedures, from sample preparation to data processing, should be thoroughly documented in standard operating procedures (SOPs). These SOPs should include specific steps for identifying common artifacts in the particular sample matrices being analyzed and protocols for addressing them when they occur.
Regular instrument maintenance schedules tailored to the analysis of complex matrices can significantly reduce artifact formation. This includes more frequent replacement of liners, septa, and columns than might be necessary for simpler samples, as well as specialized cleaning procedures for sample introduction components that come into contact with matrix-rich samples.
Regulatory Compliance and Method Standardization
Regulatory compliance in GC-MS analysis represents a critical framework that ensures analytical methods for reducing artifacts in sample-rich matrices meet established standards across different jurisdictions. Organizations such as the FDA, EPA, and international bodies like ISO have established specific guidelines that laboratories must follow when developing and validating GC-MS methods for complex sample matrices.
The FDA's Good Laboratory Practice (GLP) regulations provide comprehensive requirements for method validation, including specificity, accuracy, precision, and robustness parameters that directly address artifact reduction strategies. Similarly, the EPA Method 8270 for semi-volatile organic compounds outlines specific procedures for minimizing matrix interference and artifact formation during GC-MS analysis of environmental samples.
International harmonization efforts have led to the development of standardized approaches through organizations like the International Conference on Harmonisation (ICH). The ICH Q2(R1) guideline on validation of analytical procedures provides a structured framework that can be applied to GC-MS method development for complex matrices, ensuring consistent artifact identification and reduction strategies across global laboratories.
Method standardization initiatives specifically targeting artifact reduction in GC-MS have emerged through collaborative efforts between industry consortia and regulatory bodies. These include the development of standard operating procedures (SOPs) for sample preparation, derivatization protocols, and instrument parameter optimization that minimize common artifacts such as siloxanes, phthalates, and thermal decomposition products.
Proficiency testing programs and interlaboratory comparisons have become essential components of regulatory compliance, allowing laboratories to benchmark their artifact reduction capabilities against peers. Organizations like AOAC International and USP have established performance standards that include specific metrics for evaluating a laboratory's ability to minimize matrix-induced artifacts in GC-MS analysis.
The implementation of quality management systems compliant with ISO/IEC 17025 provides a comprehensive framework for ensuring the reliability of GC-MS results through systematic artifact identification and reduction. This standard emphasizes method validation, uncertainty estimation, and continuous improvement processes that are particularly valuable when dealing with challenging sample matrices.
Emerging regulatory trends indicate a move toward risk-based approaches to method validation, where artifact reduction strategies are prioritized based on their potential impact on analytical outcomes and decision-making processes. This approach allows laboratories to focus resources on addressing the most critical artifacts while maintaining overall method performance within acceptable regulatory parameters.
The FDA's Good Laboratory Practice (GLP) regulations provide comprehensive requirements for method validation, including specificity, accuracy, precision, and robustness parameters that directly address artifact reduction strategies. Similarly, the EPA Method 8270 for semi-volatile organic compounds outlines specific procedures for minimizing matrix interference and artifact formation during GC-MS analysis of environmental samples.
International harmonization efforts have led to the development of standardized approaches through organizations like the International Conference on Harmonisation (ICH). The ICH Q2(R1) guideline on validation of analytical procedures provides a structured framework that can be applied to GC-MS method development for complex matrices, ensuring consistent artifact identification and reduction strategies across global laboratories.
Method standardization initiatives specifically targeting artifact reduction in GC-MS have emerged through collaborative efforts between industry consortia and regulatory bodies. These include the development of standard operating procedures (SOPs) for sample preparation, derivatization protocols, and instrument parameter optimization that minimize common artifacts such as siloxanes, phthalates, and thermal decomposition products.
Proficiency testing programs and interlaboratory comparisons have become essential components of regulatory compliance, allowing laboratories to benchmark their artifact reduction capabilities against peers. Organizations like AOAC International and USP have established performance standards that include specific metrics for evaluating a laboratory's ability to minimize matrix-induced artifacts in GC-MS analysis.
The implementation of quality management systems compliant with ISO/IEC 17025 provides a comprehensive framework for ensuring the reliability of GC-MS results through systematic artifact identification and reduction. This standard emphasizes method validation, uncertainty estimation, and continuous improvement processes that are particularly valuable when dealing with challenging sample matrices.
Emerging regulatory trends indicate a move toward risk-based approaches to method validation, where artifact reduction strategies are prioritized based on their potential impact on analytical outcomes and decision-making processes. This approach allows laboratories to focus resources on addressing the most critical artifacts while maintaining overall method performance within acceptable regulatory parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!