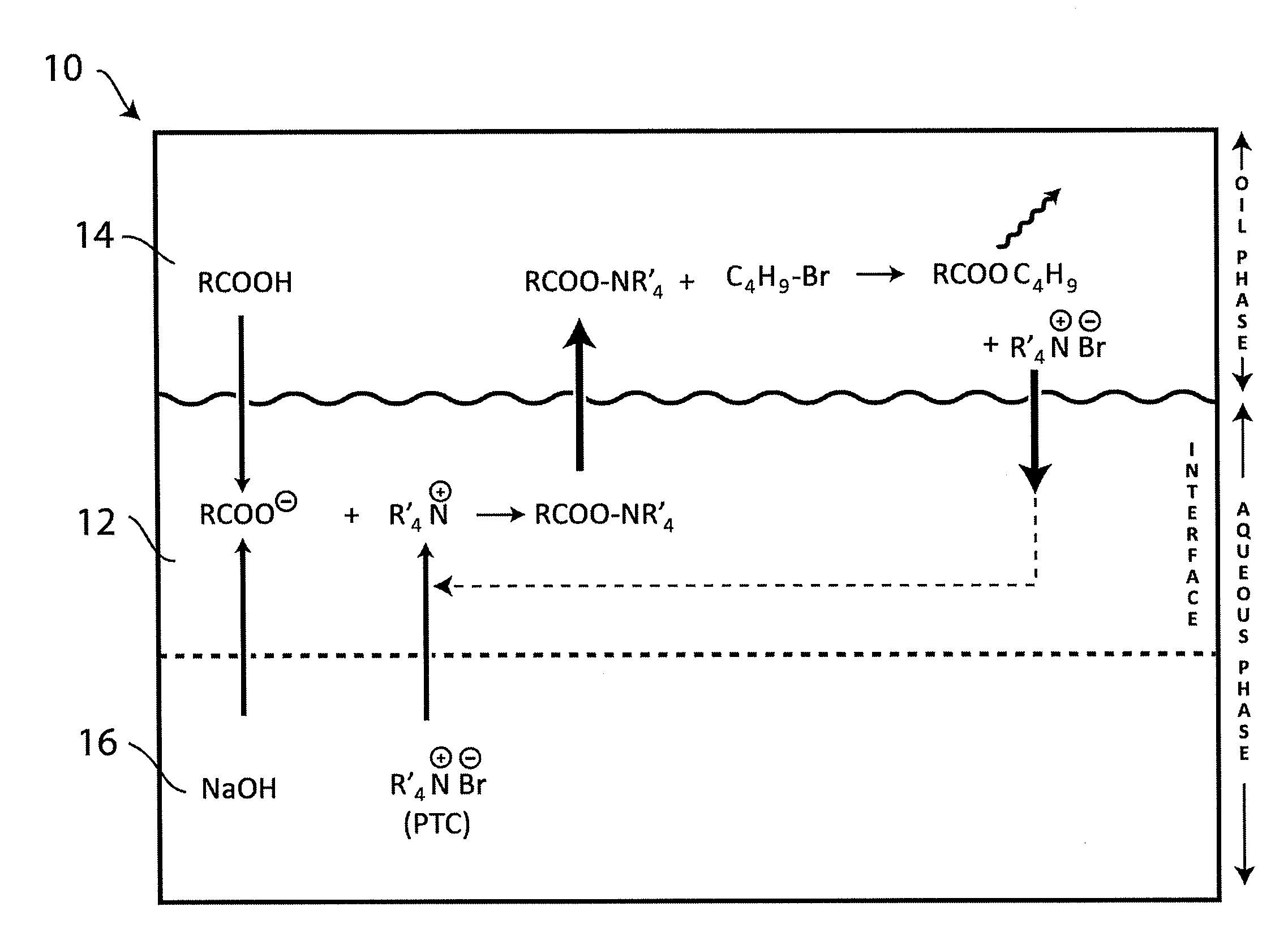
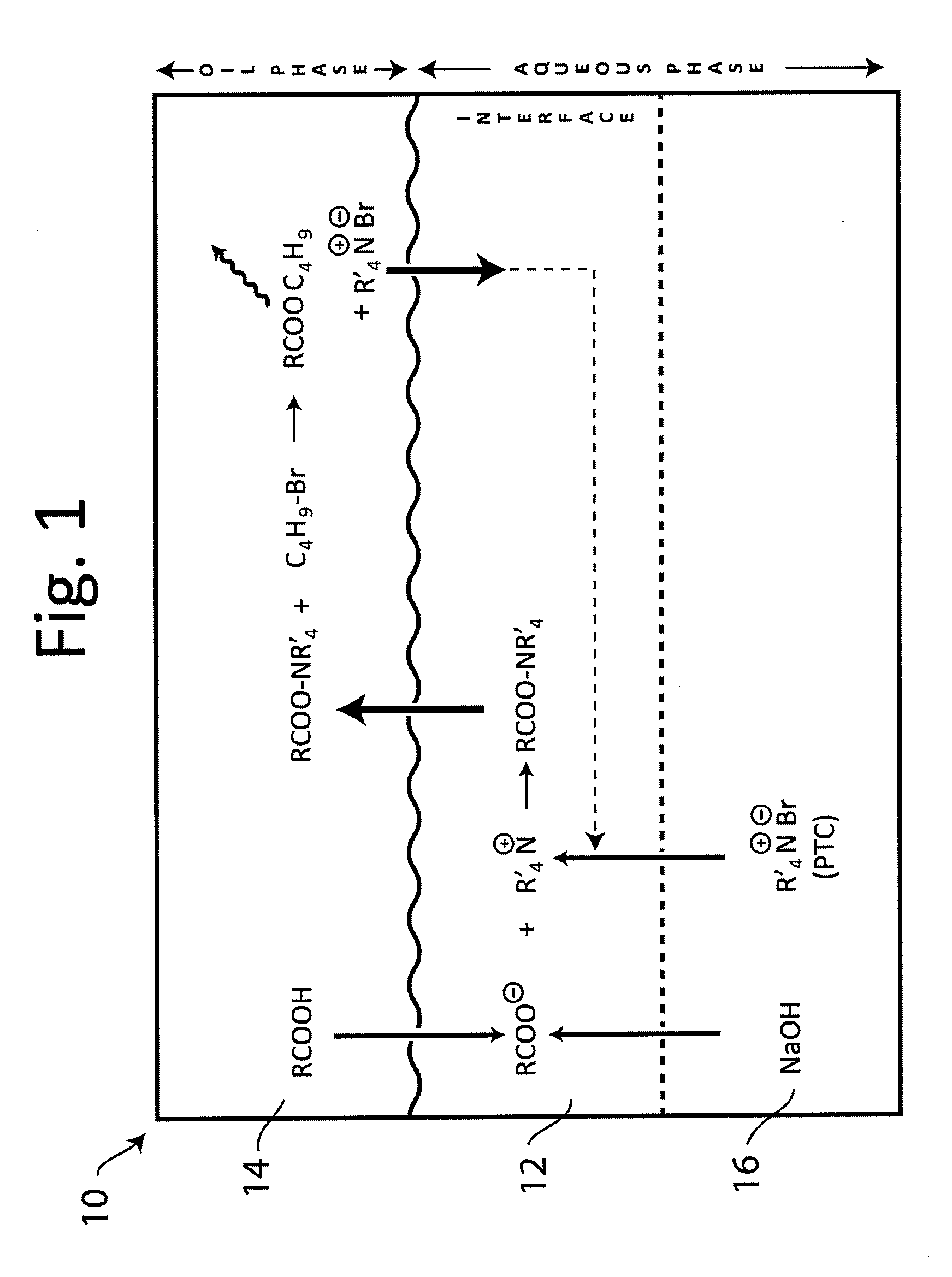
Ammonium Hydroxide as a Phase Transfer Catalyst in Green Chemistry Processes
JUL 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NH4OH as PTC: Background and Objectives
Ammonium hydroxide (NH4OH) has emerged as a promising phase transfer catalyst (PTC) in green chemistry processes, offering a sustainable alternative to traditional catalysts. The development of this technology stems from the growing need for environmentally friendly and efficient chemical processes in various industries. Over the past few decades, researchers have been exploring the potential of NH4OH as a PTC due to its unique properties and compatibility with green chemistry principles.
The evolution of NH4OH as a PTC can be traced back to the broader field of phase transfer catalysis, which gained prominence in the 1960s. Traditional PTCs, such as quaternary ammonium salts, have been widely used to facilitate reactions between immiscible phases. However, the increasing focus on sustainability and environmental concerns has driven the search for greener alternatives, leading to the exploration of NH4OH as a potential candidate.
The primary objective of research in this area is to harness the catalytic properties of NH4OH to enhance reaction rates, improve yields, and reduce the environmental impact of chemical processes. By utilizing NH4OH as a PTC, researchers aim to develop more efficient and sustainable synthetic routes for various organic compounds, particularly in biphasic systems where reactants are present in different phases.
One of the key advantages of NH4OH as a PTC is its dual nature, acting both as a base and a phase transfer agent. This unique characteristic allows it to facilitate the transfer of reactive species across phase boundaries while simultaneously promoting base-catalyzed reactions. Additionally, NH4OH is readily available, cost-effective, and relatively safe to handle, making it an attractive option for industrial applications.
The research objectives in this field encompass several aspects, including understanding the mechanism of NH4OH-mediated phase transfer catalysis, optimizing reaction conditions, and expanding the scope of reactions that can benefit from this catalytic system. Researchers are also focused on comparing the efficiency and environmental impact of NH4OH-based processes with traditional PTC systems to establish its viability as a green alternative.
Furthermore, the development of NH4OH as a PTC aligns with the broader goals of green chemistry, such as reducing waste generation, minimizing energy consumption, and utilizing renewable resources. By employing NH4OH in chemical processes, researchers aim to contribute to the overall sustainability of the chemical industry and address growing environmental concerns.
As the field progresses, researchers are exploring the potential synergies between NH4OH and other green chemistry techniques, such as the use of renewable solvents or microwave-assisted reactions. These combined approaches hold promise for further enhancing the efficiency and sustainability of chemical processes, paving the way for more environmentally friendly industrial practices.
The evolution of NH4OH as a PTC can be traced back to the broader field of phase transfer catalysis, which gained prominence in the 1960s. Traditional PTCs, such as quaternary ammonium salts, have been widely used to facilitate reactions between immiscible phases. However, the increasing focus on sustainability and environmental concerns has driven the search for greener alternatives, leading to the exploration of NH4OH as a potential candidate.
The primary objective of research in this area is to harness the catalytic properties of NH4OH to enhance reaction rates, improve yields, and reduce the environmental impact of chemical processes. By utilizing NH4OH as a PTC, researchers aim to develop more efficient and sustainable synthetic routes for various organic compounds, particularly in biphasic systems where reactants are present in different phases.
One of the key advantages of NH4OH as a PTC is its dual nature, acting both as a base and a phase transfer agent. This unique characteristic allows it to facilitate the transfer of reactive species across phase boundaries while simultaneously promoting base-catalyzed reactions. Additionally, NH4OH is readily available, cost-effective, and relatively safe to handle, making it an attractive option for industrial applications.
The research objectives in this field encompass several aspects, including understanding the mechanism of NH4OH-mediated phase transfer catalysis, optimizing reaction conditions, and expanding the scope of reactions that can benefit from this catalytic system. Researchers are also focused on comparing the efficiency and environmental impact of NH4OH-based processes with traditional PTC systems to establish its viability as a green alternative.
Furthermore, the development of NH4OH as a PTC aligns with the broader goals of green chemistry, such as reducing waste generation, minimizing energy consumption, and utilizing renewable resources. By employing NH4OH in chemical processes, researchers aim to contribute to the overall sustainability of the chemical industry and address growing environmental concerns.
As the field progresses, researchers are exploring the potential synergies between NH4OH and other green chemistry techniques, such as the use of renewable solvents or microwave-assisted reactions. These combined approaches hold promise for further enhancing the efficiency and sustainability of chemical processes, paving the way for more environmentally friendly industrial practices.
Green Chemistry Market Analysis
The green chemistry market has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations. The global green chemistry market size was valued at approximately $100 billion in 2020 and is projected to reach $165 billion by 2026, growing at a CAGR of around 8% during the forecast period. This growth is primarily attributed to the rising demand for sustainable products and processes across various industries, including pharmaceuticals, agriculture, and consumer goods.
The use of ammonium hydroxide as a phase transfer catalyst in green chemistry processes represents a promising area within this expanding market. Phase transfer catalysis has gained attention in green chemistry due to its ability to facilitate reactions between immiscible phases, reducing the need for harmful organic solvents and improving reaction efficiency. Ammonium hydroxide, being a relatively benign and readily available compound, aligns well with green chemistry principles.
In the pharmaceutical sector, which accounts for a significant portion of the green chemistry market, there is a growing interest in using ammonium hydroxide as a phase transfer catalyst for various organic syntheses. This approach can potentially reduce the environmental impact of drug manufacturing processes while maintaining or improving product yields. The agricultural chemicals segment is another key area where ammonium hydroxide-based phase transfer catalysis could find applications, particularly in the development of eco-friendly pesticides and fertilizers.
Consumer goods manufacturers are also exploring green chemistry solutions to meet the increasing consumer demand for sustainable products. The use of ammonium hydroxide in phase transfer catalysis could contribute to the development of greener household cleaning products, personal care items, and other consumer goods. This trend is supported by the growing awareness of environmental issues among consumers and their willingness to pay a premium for eco-friendly products.
Geographically, North America and Europe currently dominate the green chemistry market, with the United States and Germany being key players. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing environmental regulations, and growing adoption of sustainable practices in countries like China and India. The research on ammonium hydroxide as a phase transfer catalyst in green chemistry processes is likely to find significant opportunities in these emerging markets.
Despite the promising outlook, challenges remain in the widespread adoption of green chemistry practices, including the use of ammonium hydroxide as a phase transfer catalyst. These challenges include the need for further research and development to optimize processes, potential scalability issues, and the initial costs associated with transitioning to new chemical processes. However, as governments worldwide continue to implement stricter environmental regulations and provide incentives for sustainable practices, the market for green chemistry solutions, including those involving ammonium hydroxide, is expected to expand further.
The use of ammonium hydroxide as a phase transfer catalyst in green chemistry processes represents a promising area within this expanding market. Phase transfer catalysis has gained attention in green chemistry due to its ability to facilitate reactions between immiscible phases, reducing the need for harmful organic solvents and improving reaction efficiency. Ammonium hydroxide, being a relatively benign and readily available compound, aligns well with green chemistry principles.
In the pharmaceutical sector, which accounts for a significant portion of the green chemistry market, there is a growing interest in using ammonium hydroxide as a phase transfer catalyst for various organic syntheses. This approach can potentially reduce the environmental impact of drug manufacturing processes while maintaining or improving product yields. The agricultural chemicals segment is another key area where ammonium hydroxide-based phase transfer catalysis could find applications, particularly in the development of eco-friendly pesticides and fertilizers.
Consumer goods manufacturers are also exploring green chemistry solutions to meet the increasing consumer demand for sustainable products. The use of ammonium hydroxide in phase transfer catalysis could contribute to the development of greener household cleaning products, personal care items, and other consumer goods. This trend is supported by the growing awareness of environmental issues among consumers and their willingness to pay a premium for eco-friendly products.
Geographically, North America and Europe currently dominate the green chemistry market, with the United States and Germany being key players. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing environmental regulations, and growing adoption of sustainable practices in countries like China and India. The research on ammonium hydroxide as a phase transfer catalyst in green chemistry processes is likely to find significant opportunities in these emerging markets.
Despite the promising outlook, challenges remain in the widespread adoption of green chemistry practices, including the use of ammonium hydroxide as a phase transfer catalyst. These challenges include the need for further research and development to optimize processes, potential scalability issues, and the initial costs associated with transitioning to new chemical processes. However, as governments worldwide continue to implement stricter environmental regulations and provide incentives for sustainable practices, the market for green chemistry solutions, including those involving ammonium hydroxide, is expected to expand further.
Current Status and Challenges in PTC Research
Phase Transfer Catalysis (PTC) has emerged as a powerful tool in green chemistry processes, with ammonium hydroxide gaining attention as a potential catalyst. The current status of PTC research involving ammonium hydroxide is characterized by a growing body of literature and experimental data, yet significant challenges remain.
Recent studies have demonstrated the efficacy of ammonium hydroxide as a phase transfer catalyst in various organic reactions, particularly in biphasic systems. Its ability to facilitate the transfer of reactants between immiscible phases has shown promise in enhancing reaction rates and yields while reducing the need for harmful organic solvents. This aligns well with the principles of green chemistry, promoting safer and more environmentally friendly synthetic processes.
However, the widespread adoption of ammonium hydroxide as a PTC faces several hurdles. One major challenge is the limited understanding of the precise mechanism by which it functions in different reaction systems. While general principles of PTC are well-established, the specific interactions and intermediates involved when using ammonium hydroxide are not fully elucidated, hindering optimization efforts.
Another significant obstacle is the relatively narrow scope of reactions where ammonium hydroxide has been successfully employed as a PTC. Current research primarily focuses on nucleophilic substitution reactions and some oxidation processes. Expanding its application to a broader range of organic transformations remains a key challenge for researchers in the field.
The stability and recyclability of ammonium hydroxide in PTC systems also present ongoing challenges. Unlike some traditional phase transfer catalysts, ammonium hydroxide can be more susceptible to degradation under certain reaction conditions, potentially limiting its reusability and overall efficiency in industrial applications.
Furthermore, the development of efficient separation and recovery methods for ammonium hydroxide from reaction mixtures is an area that requires additional research. This aspect is crucial for the economic viability and environmental sustainability of processes employing this catalyst.
Scalability is another critical issue facing the current state of research. While promising results have been obtained at laboratory scales, translating these findings to industrial-scale processes presents significant engineering and economic challenges. Factors such as heat transfer, mixing efficiency, and reactor design need to be carefully considered and optimized for large-scale applications.
Despite these challenges, the potential benefits of using ammonium hydroxide as a PTC in green chemistry processes continue to drive research efforts. Ongoing studies are focusing on addressing these limitations through various approaches, including the development of novel reaction conditions, the use of supportive additives, and the exploration of synergistic effects with other catalytic systems.
Recent studies have demonstrated the efficacy of ammonium hydroxide as a phase transfer catalyst in various organic reactions, particularly in biphasic systems. Its ability to facilitate the transfer of reactants between immiscible phases has shown promise in enhancing reaction rates and yields while reducing the need for harmful organic solvents. This aligns well with the principles of green chemistry, promoting safer and more environmentally friendly synthetic processes.
However, the widespread adoption of ammonium hydroxide as a PTC faces several hurdles. One major challenge is the limited understanding of the precise mechanism by which it functions in different reaction systems. While general principles of PTC are well-established, the specific interactions and intermediates involved when using ammonium hydroxide are not fully elucidated, hindering optimization efforts.
Another significant obstacle is the relatively narrow scope of reactions where ammonium hydroxide has been successfully employed as a PTC. Current research primarily focuses on nucleophilic substitution reactions and some oxidation processes. Expanding its application to a broader range of organic transformations remains a key challenge for researchers in the field.
The stability and recyclability of ammonium hydroxide in PTC systems also present ongoing challenges. Unlike some traditional phase transfer catalysts, ammonium hydroxide can be more susceptible to degradation under certain reaction conditions, potentially limiting its reusability and overall efficiency in industrial applications.
Furthermore, the development of efficient separation and recovery methods for ammonium hydroxide from reaction mixtures is an area that requires additional research. This aspect is crucial for the economic viability and environmental sustainability of processes employing this catalyst.
Scalability is another critical issue facing the current state of research. While promising results have been obtained at laboratory scales, translating these findings to industrial-scale processes presents significant engineering and economic challenges. Factors such as heat transfer, mixing efficiency, and reactor design need to be carefully considered and optimized for large-scale applications.
Despite these challenges, the potential benefits of using ammonium hydroxide as a PTC in green chemistry processes continue to drive research efforts. Ongoing studies are focusing on addressing these limitations through various approaches, including the development of novel reaction conditions, the use of supportive additives, and the exploration of synergistic effects with other catalytic systems.
Existing NH4OH-based PTC Solutions
01 Ammonium hydroxide as a catalyst in chemical reactions
Ammonium hydroxide is used as a catalyst in various chemical reactions, enhancing reaction rates and efficiency. Its basic nature and ability to donate protons make it effective in catalyzing certain types of reactions, particularly those involving organic compounds.- Ammonium hydroxide as a catalyst in chemical reactions: Ammonium hydroxide is utilized as an effective catalyst in various chemical reactions, enhancing reaction rates and yields. Its catalytic properties are particularly useful in organic synthesis, polymerization processes, and the production of certain industrial chemicals.
- Improving catalytic efficiency of ammonium hydroxide: Research focuses on enhancing the catalytic efficiency of ammonium hydroxide through various methods, such as optimizing concentration, temperature, and pressure conditions. Additionally, the use of support materials or co-catalysts can significantly improve its performance in specific reactions.
- Applications in environmental processes: Ammonium hydroxide's catalytic properties are utilized in environmental applications, including wastewater treatment, air pollution control, and the degradation of organic pollutants. Its efficiency in these processes contributes to more sustainable and eco-friendly industrial practices.
- Role in energy-related technologies: The catalytic efficiency of ammonium hydroxide is explored in energy-related technologies, such as fuel cell applications, hydrogen production, and the synthesis of alternative fuels. Its use in these areas aims to improve energy efficiency and promote cleaner energy solutions.
- Synergistic effects with other catalysts: Research investigates the synergistic effects of combining ammonium hydroxide with other catalysts or additives to enhance overall catalytic efficiency. These combinations can lead to improved reaction kinetics, selectivity, and yield in various chemical processes.
02 Catalytic efficiency in ammonia production
Ammonium hydroxide plays a role in improving the catalytic efficiency of ammonia production processes. It can be used to optimize reaction conditions, enhance catalyst performance, and increase overall yield in industrial ammonia synthesis.Expand Specific Solutions03 Application in environmental catalysis
Ammonium hydroxide is utilized in environmental catalysis applications, such as flue gas treatment and emission control. Its catalytic properties help in the reduction of harmful pollutants and improve the efficiency of pollution control systems.Expand Specific Solutions04 Enhancing catalytic efficiency in organic synthesis
In organic synthesis, ammonium hydroxide is employed to enhance catalytic efficiency. It can act as a promoter or co-catalyst in various reactions, improving yields and selectivity in the production of organic compounds.Expand Specific Solutions05 Optimization of catalyst systems using ammonium hydroxide
Ammonium hydroxide is used to optimize catalyst systems in various industrial processes. It can modify catalyst properties, improve stability, and enhance overall catalytic performance, leading to increased efficiency and productivity.Expand Specific Solutions
Key Players in Green Chemistry Industry
The research on ammonium hydroxide as a phase transfer catalyst in green chemistry processes is in an emerging stage, with growing interest due to its potential environmental benefits. The market size is expanding as industries seek sustainable alternatives, though it remains relatively niche. Technological maturity varies among key players, with companies like Otsuka Pharmaceutical, Daiichi Sankyo, and Nippon Soda leading in research and development. Academic institutions such as Beijing University of Chemical Technology and the National University of Singapore are also contributing significantly to advancing this field. As the technology progresses, collaboration between industry and academia is likely to accelerate its adoption and commercialization.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has been actively researching the use of ammonium hydroxide as a phase transfer catalyst in green chemistry processes. Their approach involves utilizing ammonium hydroxide in a biphasic system to facilitate the transfer of reactants between immiscible organic and aqueous phases. This method has shown promising results in various organic transformations, including alkylation and oxidation reactions[1]. CSIR has developed a novel process that employs ammonium hydroxide as both a catalyst and a phase transfer agent, significantly reducing the need for organic solvents and improving reaction yields[2]. Their research has also focused on optimizing reaction conditions, such as temperature and concentration, to enhance the catalytic efficiency of ammonium hydroxide in green chemistry applications[3].
Strengths: Expertise in green chemistry, access to advanced research facilities, and a multidisciplinary approach. Weaknesses: Potential challenges in scaling up laboratory processes for industrial applications.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has made significant strides in researching ammonium hydroxide as a phase transfer catalyst for green chemistry processes. Their approach focuses on developing novel reaction systems that leverage the unique properties of ammonium hydroxide to promote environmentally friendly chemical transformations. The university's research team has successfully demonstrated the use of ammonium hydroxide in catalyzing various organic reactions, including nucleophilic substitutions and eliminations, with improved yields and selectivity compared to traditional methods[4]. They have also explored the synergistic effects of combining ammonium hydroxide with other green solvents, such as ionic liquids, to further enhance the efficiency of phase transfer catalysis[5]. Additionally, the university has been investigating the potential of ammonium hydroxide in promoting asymmetric reactions, opening up new possibilities for sustainable synthesis of chiral compounds[6].
Strengths: Strong expertise in chemical engineering and catalysis, state-of-the-art research facilities. Weaknesses: Potential limitations in commercialization experience and industry partnerships.
Innovations in NH4OH Catalytic Mechanisms
Treatment of hydrocarbons containing acids
PatentInactiveUS20100155304A1
Innovation
- A method involving contact between hydrocarbons and an aqueous liquid containing an alkaline compound and a phase transfer catalyst, along with an alkylating agent, to convert naphthenic acids into non-corrosive oil-soluble esters, allowing for their separation from the hydrocarbon phase, which can be applied at mild temperatures and atmospheric pressure, reducing equipment corrosion and emulsion formation.
Method of producing aminophenol compounds
PatentInactiveEP1727782A1
Innovation
- A method involving the reaction of a cyclohexanedione compound with an amine compound under neutral or basic conditions, eliminating the need for expensive catalysts and simplifying the process, allowing for high yield and purity production of aminophenol compounds.
Environmental Impact Assessment
The use of ammonium hydroxide as a phase transfer catalyst in green chemistry processes necessitates a comprehensive environmental impact assessment. This evaluation is crucial to understand the potential effects of this catalyst on various environmental components and to ensure its alignment with sustainable practices.
Ammonium hydroxide, when used as a phase transfer catalyst, offers several environmental benefits. It is a relatively benign compound compared to many traditional catalysts, with lower toxicity and reduced environmental persistence. The catalyst's ability to facilitate reactions between immiscible phases can lead to increased reaction efficiency, potentially reducing the overall energy consumption and waste generation in chemical processes.
However, the environmental impact of ammonium hydroxide is not negligible. Its release into aquatic ecosystems can lead to increased ammonia levels, which can be toxic to fish and other aquatic organisms. The compound can also contribute to eutrophication, a process that causes excessive algal growth and oxygen depletion in water bodies. Therefore, proper handling and disposal protocols are essential to mitigate these risks.
In terms of air quality, the use of ammonium hydroxide as a catalyst may result in ammonia emissions. While these emissions are generally less harmful than those associated with many traditional catalysts, they can still contribute to the formation of particulate matter and impact local air quality. Proper ventilation and emission control systems should be implemented to minimize these effects.
The lifecycle assessment of ammonium hydroxide as a phase transfer catalyst reveals a mixed environmental profile. On one hand, its production requires less energy and resources compared to many complex organic catalysts, potentially reducing the overall carbon footprint of chemical processes. On the other hand, the production of ammonia, a precursor to ammonium hydroxide, is energy-intensive and often relies on fossil fuels, which can offset some of the environmental gains.
Soil impact is another consideration in the environmental assessment. While ammonium hydroxide is less likely to persist in soil compared to many organic catalysts, its improper disposal can lead to soil alkalinization. This can affect soil microbial communities and plant growth. However, when used properly and disposed of correctly, the soil impact is generally minimal.
In the context of green chemistry, the use of ammonium hydroxide as a phase transfer catalyst aligns with several principles, such as atom economy and the use of safer solvents and auxiliaries. Its potential to enable reactions in aqueous media can reduce the reliance on organic solvents, further enhancing its environmental credentials.
To fully realize the environmental benefits of ammonium hydroxide as a phase transfer catalyst, it is crucial to implement robust waste management and recycling strategies. Recovering and reusing the catalyst can significantly reduce its environmental footprint and improve the overall sustainability of chemical processes.
Ammonium hydroxide, when used as a phase transfer catalyst, offers several environmental benefits. It is a relatively benign compound compared to many traditional catalysts, with lower toxicity and reduced environmental persistence. The catalyst's ability to facilitate reactions between immiscible phases can lead to increased reaction efficiency, potentially reducing the overall energy consumption and waste generation in chemical processes.
However, the environmental impact of ammonium hydroxide is not negligible. Its release into aquatic ecosystems can lead to increased ammonia levels, which can be toxic to fish and other aquatic organisms. The compound can also contribute to eutrophication, a process that causes excessive algal growth and oxygen depletion in water bodies. Therefore, proper handling and disposal protocols are essential to mitigate these risks.
In terms of air quality, the use of ammonium hydroxide as a catalyst may result in ammonia emissions. While these emissions are generally less harmful than those associated with many traditional catalysts, they can still contribute to the formation of particulate matter and impact local air quality. Proper ventilation and emission control systems should be implemented to minimize these effects.
The lifecycle assessment of ammonium hydroxide as a phase transfer catalyst reveals a mixed environmental profile. On one hand, its production requires less energy and resources compared to many complex organic catalysts, potentially reducing the overall carbon footprint of chemical processes. On the other hand, the production of ammonia, a precursor to ammonium hydroxide, is energy-intensive and often relies on fossil fuels, which can offset some of the environmental gains.
Soil impact is another consideration in the environmental assessment. While ammonium hydroxide is less likely to persist in soil compared to many organic catalysts, its improper disposal can lead to soil alkalinization. This can affect soil microbial communities and plant growth. However, when used properly and disposed of correctly, the soil impact is generally minimal.
In the context of green chemistry, the use of ammonium hydroxide as a phase transfer catalyst aligns with several principles, such as atom economy and the use of safer solvents and auxiliaries. Its potential to enable reactions in aqueous media can reduce the reliance on organic solvents, further enhancing its environmental credentials.
To fully realize the environmental benefits of ammonium hydroxide as a phase transfer catalyst, it is crucial to implement robust waste management and recycling strategies. Recovering and reusing the catalyst can significantly reduce its environmental footprint and improve the overall sustainability of chemical processes.
Scalability and Industrial Applications
The scalability and industrial applications of ammonium hydroxide as a phase transfer catalyst in green chemistry processes present significant opportunities for sustainable manufacturing. As industries seek to reduce environmental impact and improve efficiency, the use of ammonium hydroxide in large-scale operations has gained traction. Its ability to facilitate reactions between immiscible phases makes it particularly valuable in industrial settings where traditional catalysts may be less effective or environmentally problematic.
In terms of scalability, ammonium hydroxide demonstrates promising characteristics. Its relatively low cost and wide availability make it an attractive option for large-scale production. Moreover, its high solubility in water allows for easy separation and recovery, which is crucial for continuous industrial processes. This recyclability aspect not only reduces waste but also improves the economic viability of using ammonium hydroxide as a phase transfer catalyst in industrial applications.
Several industries have already begun to incorporate ammonium hydroxide-based phase transfer catalysis into their processes. The pharmaceutical sector, for instance, has found applications in the synthesis of complex organic compounds, where traditional methods often require harsh conditions or generate significant waste. By utilizing ammonium hydroxide, pharmaceutical manufacturers can achieve higher yields and purity levels while minimizing environmental impact.
The polymer industry is another area where ammonium hydroxide shows promise as a phase transfer catalyst. In the production of specialty polymers and advanced materials, it can facilitate reactions between organic and inorganic components, leading to novel materials with enhanced properties. This has implications for the development of high-performance plastics, coatings, and composite materials used in various sectors, including automotive and aerospace.
Furthermore, the use of ammonium hydroxide as a phase transfer catalyst aligns well with the principles of green chemistry. Its low toxicity and biodegradability make it a safer alternative to many traditional catalysts. This aspect is particularly important as regulatory bodies worldwide continue to tighten environmental standards for industrial processes.
However, challenges remain in fully realizing the potential of ammonium hydroxide in large-scale applications. Optimizing reaction conditions, improving catalyst efficiency, and developing robust recycling systems are areas that require ongoing research and development. Additionally, the integration of ammonium hydroxide-based processes into existing industrial infrastructure may require significant investment and process modifications.
As research progresses, it is likely that we will see an expansion of ammonium hydroxide's role in green chemistry processes across various industries. Its potential to enable more sustainable manufacturing practices while maintaining or improving product quality positions it as a key player in the transition towards greener industrial chemistry.
In terms of scalability, ammonium hydroxide demonstrates promising characteristics. Its relatively low cost and wide availability make it an attractive option for large-scale production. Moreover, its high solubility in water allows for easy separation and recovery, which is crucial for continuous industrial processes. This recyclability aspect not only reduces waste but also improves the economic viability of using ammonium hydroxide as a phase transfer catalyst in industrial applications.
Several industries have already begun to incorporate ammonium hydroxide-based phase transfer catalysis into their processes. The pharmaceutical sector, for instance, has found applications in the synthesis of complex organic compounds, where traditional methods often require harsh conditions or generate significant waste. By utilizing ammonium hydroxide, pharmaceutical manufacturers can achieve higher yields and purity levels while minimizing environmental impact.
The polymer industry is another area where ammonium hydroxide shows promise as a phase transfer catalyst. In the production of specialty polymers and advanced materials, it can facilitate reactions between organic and inorganic components, leading to novel materials with enhanced properties. This has implications for the development of high-performance plastics, coatings, and composite materials used in various sectors, including automotive and aerospace.
Furthermore, the use of ammonium hydroxide as a phase transfer catalyst aligns well with the principles of green chemistry. Its low toxicity and biodegradability make it a safer alternative to many traditional catalysts. This aspect is particularly important as regulatory bodies worldwide continue to tighten environmental standards for industrial processes.
However, challenges remain in fully realizing the potential of ammonium hydroxide in large-scale applications. Optimizing reaction conditions, improving catalyst efficiency, and developing robust recycling systems are areas that require ongoing research and development. Additionally, the integration of ammonium hydroxide-based processes into existing industrial infrastructure may require significant investment and process modifications.
As research progresses, it is likely that we will see an expansion of ammonium hydroxide's role in green chemistry processes across various industries. Its potential to enable more sustainable manufacturing practices while maintaining or improving product quality positions it as a key player in the transition towards greener industrial chemistry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!