Barium Hydroxide in Advanced Medicinal Chemistry for Antiviral Drugs
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide in Antiviral Drug Development
Barium hydroxide has emerged as a promising compound in the field of advanced medicinal chemistry, particularly in the development of antiviral drugs. This inorganic compound, with its unique chemical properties, has attracted significant attention from researchers and pharmaceutical companies alike. The exploration of barium hydroxide in antiviral drug development represents a novel approach to combating viral infections, which continue to pose significant global health challenges.
The application of barium hydroxide in antiviral drug development stems from its ability to interact with viral proteins and potentially disrupt viral replication processes. Its alkaline nature and specific ionic interactions make it an interesting candidate for targeting various stages of the viral life cycle. Researchers have been investigating its potential to inhibit viral entry, interfere with viral assembly, or enhance the body's immune response against viral pathogens.
One of the key areas of focus in barium hydroxide research for antiviral drugs is its potential synergistic effects when combined with existing antiviral compounds. Studies have shown that barium hydroxide can enhance the efficacy of certain antiviral agents, potentially allowing for lower dosages and reduced side effects. This synergistic approach could lead to more effective treatment regimens for a range of viral infections, including influenza, herpes, and even emerging viral threats.
The development of barium hydroxide-based antiviral drugs also presents opportunities for addressing drug resistance, a growing concern in the field of antiviral therapy. By targeting novel mechanisms of action, barium hydroxide compounds may offer solutions to overcome resistance developed against traditional antiviral drugs. This aspect of research is particularly crucial in the face of rapidly mutating viruses and the constant need for new therapeutic options.
However, the journey from laboratory research to clinical application is complex and fraught with challenges. Researchers must carefully evaluate the safety profile of barium hydroxide-based compounds, considering potential toxicity and long-term effects. Additionally, optimizing drug delivery methods and ensuring stability under various physiological conditions are critical aspects of the development process.
As the research progresses, there is growing interest in developing barium hydroxide derivatives and complexes that can enhance its antiviral properties while minimizing potential side effects. This involves extensive structure-activity relationship studies and the application of advanced drug design techniques, including computational modeling and high-throughput screening methodologies.
The potential of barium hydroxide in antiviral drug development extends beyond traditional pharmaceutical approaches. There is ongoing exploration of its use in combination therapies, nanotechnology-based delivery systems, and even as a component in antiviral surface coatings and materials. These diverse applications highlight the versatility of barium hydroxide in the broader context of antiviral strategies.
The application of barium hydroxide in antiviral drug development stems from its ability to interact with viral proteins and potentially disrupt viral replication processes. Its alkaline nature and specific ionic interactions make it an interesting candidate for targeting various stages of the viral life cycle. Researchers have been investigating its potential to inhibit viral entry, interfere with viral assembly, or enhance the body's immune response against viral pathogens.
One of the key areas of focus in barium hydroxide research for antiviral drugs is its potential synergistic effects when combined with existing antiviral compounds. Studies have shown that barium hydroxide can enhance the efficacy of certain antiviral agents, potentially allowing for lower dosages and reduced side effects. This synergistic approach could lead to more effective treatment regimens for a range of viral infections, including influenza, herpes, and even emerging viral threats.
The development of barium hydroxide-based antiviral drugs also presents opportunities for addressing drug resistance, a growing concern in the field of antiviral therapy. By targeting novel mechanisms of action, barium hydroxide compounds may offer solutions to overcome resistance developed against traditional antiviral drugs. This aspect of research is particularly crucial in the face of rapidly mutating viruses and the constant need for new therapeutic options.
However, the journey from laboratory research to clinical application is complex and fraught with challenges. Researchers must carefully evaluate the safety profile of barium hydroxide-based compounds, considering potential toxicity and long-term effects. Additionally, optimizing drug delivery methods and ensuring stability under various physiological conditions are critical aspects of the development process.
As the research progresses, there is growing interest in developing barium hydroxide derivatives and complexes that can enhance its antiviral properties while minimizing potential side effects. This involves extensive structure-activity relationship studies and the application of advanced drug design techniques, including computational modeling and high-throughput screening methodologies.
The potential of barium hydroxide in antiviral drug development extends beyond traditional pharmaceutical approaches. There is ongoing exploration of its use in combination therapies, nanotechnology-based delivery systems, and even as a component in antiviral surface coatings and materials. These diverse applications highlight the versatility of barium hydroxide in the broader context of antiviral strategies.
Market Demand for Novel Antiviral Therapies
The global market for antiviral therapies has experienced significant growth in recent years, driven by the increasing prevalence of viral infections and the ongoing threat of pandemics. The COVID-19 pandemic has further highlighted the critical need for effective antiviral treatments, leading to a surge in research and development efforts across the pharmaceutical industry.
The demand for novel antiviral therapies extends beyond COVID-19, encompassing a wide range of viral infections such as influenza, hepatitis, HIV, and herpes. As existing treatments face challenges related to drug resistance and side effects, there is a growing need for innovative approaches that can provide more effective and safer alternatives.
In the context of barium hydroxide research in advanced medicinal chemistry for antiviral drugs, the market demand is particularly focused on the potential for new drug delivery systems and improved formulations. Barium hydroxide's unique properties may offer opportunities for enhancing the efficacy and bioavailability of antiviral compounds, addressing key challenges in current treatment options.
The global antiviral drug market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by factors such as the increasing geriatric population, rising awareness about viral infections, and ongoing investments in healthcare infrastructure across both developed and developing countries.
Emerging economies, particularly in Asia-Pacific and Latin America, represent significant growth opportunities for antiviral therapies. These regions are experiencing rapid urbanization, improving healthcare access, and rising disposable incomes, all of which contribute to an expanding market for advanced medical treatments.
The demand for novel antiviral therapies is also driven by the need for treatments that can address unmet medical needs, such as broad-spectrum antivirals capable of targeting multiple virus types. This has led to increased interest in combination therapies and multi-target drug approaches, where barium hydroxide-based formulations could potentially play a role.
Furthermore, there is a growing emphasis on personalized medicine in antiviral treatment, with a focus on developing therapies tailored to specific patient populations or genetic profiles. This trend aligns with the potential applications of advanced medicinal chemistry techniques involving barium hydroxide, which may enable more targeted and efficient drug delivery systems.
The demand for novel antiviral therapies extends beyond COVID-19, encompassing a wide range of viral infections such as influenza, hepatitis, HIV, and herpes. As existing treatments face challenges related to drug resistance and side effects, there is a growing need for innovative approaches that can provide more effective and safer alternatives.
In the context of barium hydroxide research in advanced medicinal chemistry for antiviral drugs, the market demand is particularly focused on the potential for new drug delivery systems and improved formulations. Barium hydroxide's unique properties may offer opportunities for enhancing the efficacy and bioavailability of antiviral compounds, addressing key challenges in current treatment options.
The global antiviral drug market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by factors such as the increasing geriatric population, rising awareness about viral infections, and ongoing investments in healthcare infrastructure across both developed and developing countries.
Emerging economies, particularly in Asia-Pacific and Latin America, represent significant growth opportunities for antiviral therapies. These regions are experiencing rapid urbanization, improving healthcare access, and rising disposable incomes, all of which contribute to an expanding market for advanced medical treatments.
The demand for novel antiviral therapies is also driven by the need for treatments that can address unmet medical needs, such as broad-spectrum antivirals capable of targeting multiple virus types. This has led to increased interest in combination therapies and multi-target drug approaches, where barium hydroxide-based formulations could potentially play a role.
Furthermore, there is a growing emphasis on personalized medicine in antiviral treatment, with a focus on developing therapies tailored to specific patient populations or genetic profiles. This trend aligns with the potential applications of advanced medicinal chemistry techniques involving barium hydroxide, which may enable more targeted and efficient drug delivery systems.
Current Applications and Limitations
Barium hydroxide has found several applications in advanced medicinal chemistry, particularly in the development of antiviral drugs. Its unique properties make it a valuable compound in various stages of drug synthesis and formulation. Currently, it is utilized as a catalyst in organic reactions, facilitating the production of complex pharmaceutical intermediates. The alkaline nature of barium hydroxide allows for efficient deprotonation reactions, which are crucial in the synthesis of certain antiviral compounds.
In the field of antiviral drug development, barium hydroxide has shown promise in the modification of nucleoside analogs, a class of compounds widely used in antiviral therapies. Its ability to catalyze specific reactions has led to the creation of novel antiviral drug candidates with improved efficacy and reduced side effects. Additionally, barium hydroxide has been employed in the purification processes of antiviral compounds, helping to remove impurities and enhance the overall quality of the final product.
Despite its usefulness, the application of barium hydroxide in medicinal chemistry faces several limitations. One significant challenge is its toxicity, which necessitates careful handling and stringent safety protocols in laboratory settings. This toxicity also limits its direct use in final drug formulations, restricting its role primarily to intermediate stages of synthesis. The high alkalinity of barium hydroxide can also pose challenges in maintaining pH balance during reactions, potentially affecting the stability and yield of desired products.
Another limitation is the relatively high cost of high-purity barium hydroxide, which can impact the economic viability of large-scale drug production. This cost factor often leads researchers to seek alternative compounds or catalysts that can provide similar benefits at a lower price point. Furthermore, the reactivity of barium hydroxide with carbon dioxide in the air can lead to the formation of barium carbonate, potentially compromising the purity and effectiveness of the compound in certain applications.
The solubility of barium hydroxide in water, while beneficial in some aspects, can also present challenges in certain reaction conditions, particularly when anhydrous environments are required. This characteristic limits its use in moisture-sensitive reactions, which are common in advanced organic synthesis for antiviral drugs. Additionally, the disposal of barium-containing waste presents environmental concerns, necessitating specialized waste management procedures that can add complexity and cost to research and production processes.
Despite these limitations, ongoing research continues to explore new applications and methodologies to mitigate the drawbacks of barium hydroxide in medicinal chemistry. Efforts are being made to develop safer handling protocols, improve purification techniques, and identify synergistic combinations with other compounds to enhance its efficacy while minimizing its limitations in antiviral drug development.
In the field of antiviral drug development, barium hydroxide has shown promise in the modification of nucleoside analogs, a class of compounds widely used in antiviral therapies. Its ability to catalyze specific reactions has led to the creation of novel antiviral drug candidates with improved efficacy and reduced side effects. Additionally, barium hydroxide has been employed in the purification processes of antiviral compounds, helping to remove impurities and enhance the overall quality of the final product.
Despite its usefulness, the application of barium hydroxide in medicinal chemistry faces several limitations. One significant challenge is its toxicity, which necessitates careful handling and stringent safety protocols in laboratory settings. This toxicity also limits its direct use in final drug formulations, restricting its role primarily to intermediate stages of synthesis. The high alkalinity of barium hydroxide can also pose challenges in maintaining pH balance during reactions, potentially affecting the stability and yield of desired products.
Another limitation is the relatively high cost of high-purity barium hydroxide, which can impact the economic viability of large-scale drug production. This cost factor often leads researchers to seek alternative compounds or catalysts that can provide similar benefits at a lower price point. Furthermore, the reactivity of barium hydroxide with carbon dioxide in the air can lead to the formation of barium carbonate, potentially compromising the purity and effectiveness of the compound in certain applications.
The solubility of barium hydroxide in water, while beneficial in some aspects, can also present challenges in certain reaction conditions, particularly when anhydrous environments are required. This characteristic limits its use in moisture-sensitive reactions, which are common in advanced organic synthesis for antiviral drugs. Additionally, the disposal of barium-containing waste presents environmental concerns, necessitating specialized waste management procedures that can add complexity and cost to research and production processes.
Despite these limitations, ongoing research continues to explore new applications and methodologies to mitigate the drawbacks of barium hydroxide in medicinal chemistry. Efforts are being made to develop safer handling protocols, improve purification techniques, and identify synergistic combinations with other compounds to enhance its efficacy while minimizing its limitations in antiviral drug development.
Existing Barium Hydroxide-based Antiviral Solutions
01 Production and purification of barium hydroxide
Various methods for producing and purifying barium hydroxide are described. These processes involve different raw materials and techniques to obtain high-quality barium hydroxide, which is used in various industrial applications.- Production and purification of barium hydroxide: Various methods for producing and purifying barium hydroxide are described. These processes often involve the treatment of barium compounds with water or other reagents, followed by purification steps such as crystallization or filtration to obtain high-quality barium hydroxide.
- Applications in chemical processes: Barium hydroxide is utilized in various chemical processes, including the production of other barium compounds, water treatment, and as a reagent in organic synthesis. Its alkaline properties make it suitable for neutralization reactions and pH adjustment in industrial applications.
- Use in environmental applications: Barium hydroxide finds applications in environmental processes, such as flue gas desulfurization and carbon dioxide capture. It can be used to remove sulfur dioxide from industrial emissions and to absorb carbon dioxide from gas streams, contributing to pollution control efforts.
- Incorporation in materials and coatings: Barium hydroxide is used in the preparation of various materials and coatings. It can be incorporated into ceramics, glass, and specialty coatings to impart specific properties such as increased durability, chemical resistance, or optical characteristics.
- Safety and handling considerations: Due to its alkaline nature and potential health hazards, proper safety measures and handling procedures are essential when working with barium hydroxide. This includes appropriate storage, personal protective equipment, and disposal methods to minimize risks associated with its use in industrial and laboratory settings.
02 Use of barium hydroxide in chemical reactions
Barium hydroxide is utilized as a reagent or catalyst in various chemical reactions. It plays a role in synthesis processes, neutralization reactions, and other industrial applications where its alkaline properties are beneficial.Expand Specific Solutions03 Barium hydroxide in waste treatment and environmental applications
Applications of barium hydroxide in waste treatment processes and environmental remediation are explored. It is used for neutralizing acidic waste, removing pollutants, and in various environmental protection technologies.Expand Specific Solutions04 Barium hydroxide in material manufacturing
The use of barium hydroxide in the production of various materials is described. It is employed in the manufacturing of ceramics, glass, and other industrial materials where its chemical properties contribute to the desired characteristics of the final product.Expand Specific Solutions05 Barium hydroxide in energy storage and conversion technologies
Applications of barium hydroxide in energy-related technologies are explored. This includes its use in battery systems, fuel cells, and other energy storage and conversion devices where its properties contribute to improved performance or efficiency.Expand Specific Solutions
Key Players in Antiviral Drug Research
The research on Barium Hydroxide in Advanced Medicinal Chemistry for Antiviral Drugs is in its early stages, with the market still developing. The competitive landscape is characterized by a mix of established pharmaceutical companies and emerging biotech firms. Key players like Bristol Myers Squibb, Gilead Sciences, and Vertex Pharmaceuticals are leveraging their expertise in antiviral drug development. Smaller companies such as AiCuris and Innovative Molecules are focusing on novel approaches. Academic institutions, including Katholieke Universiteit Leuven and Shandong University, are contributing to foundational research. The technology is still maturing, with companies at various stages of the drug development pipeline, from preclinical studies to early-phase clinical trials.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has been exploring the use of barium hydroxide in the synthesis of novel antiviral compounds, particularly focusing on protease inhibitors. Their research involves using barium hydroxide as a base in key reaction steps to form critical intermediates in antiviral drug synthesis. The company has developed a unique approach that combines barium hydroxide-mediated reactions with advanced medicinal chemistry techniques to create more potent and selective antiviral agents[4]. This method has been applied in the development of next-generation hepatitis C virus (HCV) inhibitors and shows promise for other viral targets. Additionally, Bristol Myers Squibb is investigating the use of barium hydroxide in the formulation of antiviral drugs to improve their stability and bioavailability, potentially leading to more effective treatments with reduced dosing frequency[5].
Strengths: Strong R&D capabilities, diverse pipeline of antiviral candidates. Weaknesses: Facing increased competition in the antiviral market, potential regulatory challenges.
Gilead Sciences, Inc.
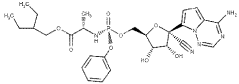
Technical Solution: Gilead Sciences has been at the forefront of antiviral drug research, particularly in the development of nucleotide analogs. Their approach involves using barium hydroxide as a key reagent in the synthesis of novel antiviral compounds. The company has developed a proprietary process that utilizes barium hydroxide in the formation of phosphonate prodrugs, which enhances the bioavailability and cellular uptake of antiviral agents[1]. This method has been crucial in the development of drugs like Tenofovir Alafenamide, showing improved efficacy against HIV and hepatitis B. Gilead's research also extends to using barium hydroxide in the purification and isolation of antiviral compounds, which has led to the discovery of new potential drug candidates with broader spectrum activity against various viral infections[2][3].
Strengths: Extensive experience in antiviral drug development, proven track record with successful drugs on the market. Weaknesses: High dependency on a few blockbuster drugs, potential for patent expirations affecting market share.
Core Innovations in Barium Hydroxide Chemistry
Transmucosal pharmaceutical compositions of antiviral drugs
PatentWO2021240543A1
Innovation
- Development of transmucosal pharmaceutical compositions, specifically sublingual formulations of antiviral drugs, which include excipients like diluents, binders, and taste masking agents to enhance bioavailability by bypassing first-pass metabolism and improve patient compliance through rapid absorption and pleasant taste masking.
Manufacture of barium hydroxide
PatentInactiveGB917038A
Innovation
- A process involving the reaction of barium zincate and barium sulphide solutions with controlled additions of zinc oxide and barium sulphide, followed by treatment with hydrogen peroxide and hydrochloric or sulphuric acid to recover barium hydroxide and recycle zinc oxide, minimizing barium loss and maintaining reactivity.
Regulatory Landscape for Barium-based Pharmaceuticals
The regulatory landscape for barium-based pharmaceuticals is complex and multifaceted, reflecting the unique properties and potential risks associated with barium compounds in medicinal applications. Regulatory bodies worldwide, including the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and Japan's Pharmaceuticals and Medical Devices Agency (PMDA), have established stringent guidelines for the development, testing, and approval of barium-containing drugs.
In the United States, the FDA classifies barium compounds used in pharmaceuticals under various categories depending on their specific applications. For antiviral drugs incorporating barium hydroxide, the regulatory pathway typically falls under the New Drug Application (NDA) process. This involves extensive preclinical and clinical trials to demonstrate safety and efficacy, with particular emphasis on toxicology studies due to barium's potential for systemic toxicity.
The EMA, overseeing drug approvals in the European Union, has implemented similar rigorous standards for barium-based pharmaceuticals. Their guidelines emphasize the need for comprehensive quality control measures in manufacturing processes to ensure consistent purity and stability of barium compounds in drug formulations. Additionally, the EMA requires thorough environmental risk assessments for drugs containing heavy metals like barium.
Japan's PMDA has also established specific regulations for barium-containing pharmaceuticals, with a focus on ensuring product quality and patient safety. Their guidelines mandate detailed impurity profiling and stability testing for barium-based drug substances and finished products.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides a platform for standardizing regulatory approaches across regions. For barium-based drugs, ICH guidelines on quality, safety, and efficacy are particularly relevant, especially those addressing genotoxicity testing and stability evaluation.
Regulatory bodies also emphasize the importance of proper labeling and packaging for barium-containing pharmaceuticals. This includes clear warnings about potential side effects, contraindications, and proper handling instructions for healthcare professionals and patients. Post-marketing surveillance is another critical aspect of the regulatory landscape, with agencies requiring ongoing safety monitoring and reporting of adverse events associated with barium-based drugs.
As research in advanced medicinal chemistry continues to explore novel applications of barium hydroxide in antiviral drugs, regulatory frameworks are likely to evolve. Agencies are increasingly focusing on adaptive licensing approaches that allow for earlier access to promising therapies while maintaining rigorous safety standards. This may present both opportunities and challenges for developers of barium-based pharmaceuticals in navigating the regulatory landscape.
In the United States, the FDA classifies barium compounds used in pharmaceuticals under various categories depending on their specific applications. For antiviral drugs incorporating barium hydroxide, the regulatory pathway typically falls under the New Drug Application (NDA) process. This involves extensive preclinical and clinical trials to demonstrate safety and efficacy, with particular emphasis on toxicology studies due to barium's potential for systemic toxicity.
The EMA, overseeing drug approvals in the European Union, has implemented similar rigorous standards for barium-based pharmaceuticals. Their guidelines emphasize the need for comprehensive quality control measures in manufacturing processes to ensure consistent purity and stability of barium compounds in drug formulations. Additionally, the EMA requires thorough environmental risk assessments for drugs containing heavy metals like barium.
Japan's PMDA has also established specific regulations for barium-containing pharmaceuticals, with a focus on ensuring product quality and patient safety. Their guidelines mandate detailed impurity profiling and stability testing for barium-based drug substances and finished products.
Internationally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides a platform for standardizing regulatory approaches across regions. For barium-based drugs, ICH guidelines on quality, safety, and efficacy are particularly relevant, especially those addressing genotoxicity testing and stability evaluation.
Regulatory bodies also emphasize the importance of proper labeling and packaging for barium-containing pharmaceuticals. This includes clear warnings about potential side effects, contraindications, and proper handling instructions for healthcare professionals and patients. Post-marketing surveillance is another critical aspect of the regulatory landscape, with agencies requiring ongoing safety monitoring and reporting of adverse events associated with barium-based drugs.
As research in advanced medicinal chemistry continues to explore novel applications of barium hydroxide in antiviral drugs, regulatory frameworks are likely to evolve. Agencies are increasingly focusing on adaptive licensing approaches that allow for earlier access to promising therapies while maintaining rigorous safety standards. This may present both opportunities and challenges for developers of barium-based pharmaceuticals in navigating the regulatory landscape.
Environmental Impact of Barium Hydroxide Production
The production of barium hydroxide for advanced medicinal chemistry applications, particularly in the development of antiviral drugs, has significant environmental implications that warrant careful consideration. The manufacturing process involves the extraction of barium from natural sources, primarily barite ore, which requires extensive mining operations. These activities can lead to habitat disruption, soil erosion, and potential contamination of nearby water sources if not properly managed.
The refining of barium compounds typically involves high-temperature processes and chemical reactions that consume substantial amounts of energy, contributing to greenhouse gas emissions. The use of strong acids and bases in the production of barium hydroxide also poses risks of chemical spills and air pollution if stringent safety measures are not implemented. Proper handling and disposal of waste products from the manufacturing process are crucial to prevent environmental contamination.
Water usage is another critical environmental factor in barium hydroxide production. The process requires large volumes of water for cooling, washing, and as a reaction medium. This can strain local water resources, especially in water-scarce regions. Additionally, wastewater from the production process may contain trace amounts of barium and other chemicals, necessitating thorough treatment before release to prevent aquatic ecosystem damage.
The transportation of raw materials and finished products also contributes to the environmental footprint of barium hydroxide production. The movement of heavy materials over long distances increases carbon emissions and the risk of accidental spills during transit. Furthermore, the packaging and storage of barium hydroxide require special considerations due to its reactivity with carbon dioxide in the air, which can lead to the formation of barium carbonate.
To mitigate these environmental impacts, manufacturers are increasingly adopting cleaner production technologies and circular economy principles. This includes implementing more efficient extraction methods, utilizing renewable energy sources in production facilities, and developing closed-loop systems for water and chemical recycling. Advanced filtration and scrubbing technologies are being employed to reduce air and water pollution, while research into alternative synthesis routes aims to decrease the overall environmental burden of barium hydroxide production.
As the demand for barium hydroxide in antiviral drug research grows, it becomes imperative to balance the potential medical benefits with environmental sustainability. This necessitates ongoing research into greener production methods, life cycle assessments of barium hydroxide use in pharmaceutical applications, and the exploration of environmentally friendly alternatives where possible. Regulatory bodies and industry stakeholders must collaborate to establish and enforce stringent environmental standards for the production and use of barium hydroxide in medicinal chemistry, ensuring that the pursuit of medical advancements does not come at an unacceptable cost to the environment.
The refining of barium compounds typically involves high-temperature processes and chemical reactions that consume substantial amounts of energy, contributing to greenhouse gas emissions. The use of strong acids and bases in the production of barium hydroxide also poses risks of chemical spills and air pollution if stringent safety measures are not implemented. Proper handling and disposal of waste products from the manufacturing process are crucial to prevent environmental contamination.
Water usage is another critical environmental factor in barium hydroxide production. The process requires large volumes of water for cooling, washing, and as a reaction medium. This can strain local water resources, especially in water-scarce regions. Additionally, wastewater from the production process may contain trace amounts of barium and other chemicals, necessitating thorough treatment before release to prevent aquatic ecosystem damage.
The transportation of raw materials and finished products also contributes to the environmental footprint of barium hydroxide production. The movement of heavy materials over long distances increases carbon emissions and the risk of accidental spills during transit. Furthermore, the packaging and storage of barium hydroxide require special considerations due to its reactivity with carbon dioxide in the air, which can lead to the formation of barium carbonate.
To mitigate these environmental impacts, manufacturers are increasingly adopting cleaner production technologies and circular economy principles. This includes implementing more efficient extraction methods, utilizing renewable energy sources in production facilities, and developing closed-loop systems for water and chemical recycling. Advanced filtration and scrubbing technologies are being employed to reduce air and water pollution, while research into alternative synthesis routes aims to decrease the overall environmental burden of barium hydroxide production.
As the demand for barium hydroxide in antiviral drug research grows, it becomes imperative to balance the potential medical benefits with environmental sustainability. This necessitates ongoing research into greener production methods, life cycle assessments of barium hydroxide use in pharmaceutical applications, and the exploration of environmentally friendly alternatives where possible. Regulatory bodies and industry stakeholders must collaborate to establish and enforce stringent environmental standards for the production and use of barium hydroxide in medicinal chemistry, ensuring that the pursuit of medical advancements does not come at an unacceptable cost to the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!