Magnesium Carbonate in Heavy Metal Ion Removal from Aqueous Solutions
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MgCO3 for HM Removal: Background and Objectives
The removal of heavy metal ions from aqueous solutions has become a critical environmental concern due to their toxic effects on ecosystems and human health. Magnesium carbonate (MgCO3) has emerged as a promising adsorbent material for this purpose, offering a potentially cost-effective and environmentally friendly solution. This research aims to explore the efficacy and mechanisms of MgCO3 in heavy metal ion removal, with a focus on its adsorption capacity, selectivity, and regeneration potential.
The historical context of heavy metal contamination dates back to the Industrial Revolution, with increasing pollution levels observed in water bodies worldwide. Traditional methods for heavy metal removal, such as chemical precipitation, ion exchange, and membrane filtration, have shown limitations in terms of cost, efficiency, and environmental impact. This has led to a growing interest in developing alternative adsorbents, with MgCO3 gaining attention due to its abundance, low toxicity, and potential for high adsorption capacity.
Recent technological advancements in materials science and nanotechnology have opened new avenues for enhancing the performance of MgCO3-based adsorbents. The synthesis of nanostructured MgCO3 materials with increased surface area and tailored surface properties has shown promising results in laboratory-scale studies. These developments have set the stage for further research into optimizing MgCO3 for heavy metal removal applications.
The primary objectives of this research are multifaceted. First, it aims to comprehensively evaluate the adsorption characteristics of MgCO3 for various heavy metal ions, including lead, cadmium, copper, and zinc. This involves determining adsorption isotherms, kinetics, and thermodynamics to understand the underlying mechanisms. Second, the study seeks to investigate the influence of various parameters, such as pH, temperature, and initial metal ion concentration, on the adsorption process.
Furthermore, this research aims to explore innovative approaches to enhance the adsorption capacity and selectivity of MgCO3. This includes investigating the potential of surface modifications, composite materials, and novel synthesis methods. The development of regeneration techniques for spent MgCO3 adsorbents is also a key objective, as it directly impacts the economic viability and sustainability of the process.
Lastly, the research aims to assess the practical applicability of MgCO3-based adsorbents in real-world scenarios. This involves studying the performance of these materials in complex water matrices containing multiple contaminants and evaluating their stability and efficiency under various environmental conditions. The ultimate goal is to provide a comprehensive understanding of MgCO3's potential as an effective adsorbent for heavy metal removal, paving the way for its implementation in water treatment technologies.
The historical context of heavy metal contamination dates back to the Industrial Revolution, with increasing pollution levels observed in water bodies worldwide. Traditional methods for heavy metal removal, such as chemical precipitation, ion exchange, and membrane filtration, have shown limitations in terms of cost, efficiency, and environmental impact. This has led to a growing interest in developing alternative adsorbents, with MgCO3 gaining attention due to its abundance, low toxicity, and potential for high adsorption capacity.
Recent technological advancements in materials science and nanotechnology have opened new avenues for enhancing the performance of MgCO3-based adsorbents. The synthesis of nanostructured MgCO3 materials with increased surface area and tailored surface properties has shown promising results in laboratory-scale studies. These developments have set the stage for further research into optimizing MgCO3 for heavy metal removal applications.
The primary objectives of this research are multifaceted. First, it aims to comprehensively evaluate the adsorption characteristics of MgCO3 for various heavy metal ions, including lead, cadmium, copper, and zinc. This involves determining adsorption isotherms, kinetics, and thermodynamics to understand the underlying mechanisms. Second, the study seeks to investigate the influence of various parameters, such as pH, temperature, and initial metal ion concentration, on the adsorption process.
Furthermore, this research aims to explore innovative approaches to enhance the adsorption capacity and selectivity of MgCO3. This includes investigating the potential of surface modifications, composite materials, and novel synthesis methods. The development of regeneration techniques for spent MgCO3 adsorbents is also a key objective, as it directly impacts the economic viability and sustainability of the process.
Lastly, the research aims to assess the practical applicability of MgCO3-based adsorbents in real-world scenarios. This involves studying the performance of these materials in complex water matrices containing multiple contaminants and evaluating their stability and efficiency under various environmental conditions. The ultimate goal is to provide a comprehensive understanding of MgCO3's potential as an effective adsorbent for heavy metal removal, paving the way for its implementation in water treatment technologies.
Market Demand Analysis for Water Treatment Solutions
The global water treatment market has been experiencing significant growth due to increasing concerns over water scarcity and pollution. The demand for effective heavy metal ion removal solutions is particularly high, driven by stringent environmental regulations and growing awareness of the health risks associated with heavy metal contamination in water sources.
Industrial sectors, including mining, manufacturing, and chemical processing, are major contributors to heavy metal pollution in water bodies. These industries are under increasing pressure to adopt more effective water treatment technologies to comply with environmental standards and reduce their ecological footprint. This has created a substantial market opportunity for innovative water treatment solutions, such as those utilizing magnesium carbonate for heavy metal ion removal.
Municipal water treatment facilities are also seeking advanced technologies to improve their ability to remove heavy metals from drinking water supplies. The presence of heavy metals like lead, mercury, and cadmium in potable water has become a significant public health concern, prompting governments and water utilities to invest in more efficient treatment methods.
Developing countries are emerging as key growth markets for water treatment solutions. Rapid industrialization and urbanization in these regions have led to increased water pollution, creating an urgent need for effective and affordable water treatment technologies. The potential for magnesium carbonate-based solutions to offer a cost-effective alternative to traditional heavy metal removal methods could be particularly attractive in these markets.
The agricultural sector represents another significant market segment for heavy metal ion removal technologies. As awareness grows about the accumulation of heavy metals in soil and crops due to contaminated irrigation water, farmers and agribusinesses are increasingly seeking water treatment solutions to ensure the safety of their produce and maintain soil health.
Market analysts project that the global water treatment chemicals market, which includes solutions for heavy metal removal, will continue to grow at a compound annual growth rate (CAGR) of around 6% over the next five years. This growth is expected to be driven by a combination of regulatory pressures, technological advancements, and increasing water stress in many parts of the world.
The potential of magnesium carbonate in heavy metal ion removal aligns well with the growing trend towards more sustainable and environmentally friendly water treatment solutions. As consumers and industries alike seek greener alternatives, technologies that offer effective treatment with minimal environmental impact are likely to see increased demand.
Industrial sectors, including mining, manufacturing, and chemical processing, are major contributors to heavy metal pollution in water bodies. These industries are under increasing pressure to adopt more effective water treatment technologies to comply with environmental standards and reduce their ecological footprint. This has created a substantial market opportunity for innovative water treatment solutions, such as those utilizing magnesium carbonate for heavy metal ion removal.
Municipal water treatment facilities are also seeking advanced technologies to improve their ability to remove heavy metals from drinking water supplies. The presence of heavy metals like lead, mercury, and cadmium in potable water has become a significant public health concern, prompting governments and water utilities to invest in more efficient treatment methods.
Developing countries are emerging as key growth markets for water treatment solutions. Rapid industrialization and urbanization in these regions have led to increased water pollution, creating an urgent need for effective and affordable water treatment technologies. The potential for magnesium carbonate-based solutions to offer a cost-effective alternative to traditional heavy metal removal methods could be particularly attractive in these markets.
The agricultural sector represents another significant market segment for heavy metal ion removal technologies. As awareness grows about the accumulation of heavy metals in soil and crops due to contaminated irrigation water, farmers and agribusinesses are increasingly seeking water treatment solutions to ensure the safety of their produce and maintain soil health.
Market analysts project that the global water treatment chemicals market, which includes solutions for heavy metal removal, will continue to grow at a compound annual growth rate (CAGR) of around 6% over the next five years. This growth is expected to be driven by a combination of regulatory pressures, technological advancements, and increasing water stress in many parts of the world.
The potential of magnesium carbonate in heavy metal ion removal aligns well with the growing trend towards more sustainable and environmentally friendly water treatment solutions. As consumers and industries alike seek greener alternatives, technologies that offer effective treatment with minimal environmental impact are likely to see increased demand.
Current Challenges in Heavy Metal Ion Removal
The removal of heavy metal ions from aqueous solutions remains a significant challenge in environmental remediation and water treatment. Despite advancements in technology, several obstacles persist in achieving efficient and cost-effective removal of these contaminants. One of the primary challenges is the variability in heavy metal ion concentrations across different water sources, making it difficult to develop a universal treatment method.
The complexity of water matrices further complicates the removal process. Natural and industrial waters often contain a mixture of different heavy metal ions, organic compounds, and other pollutants. These diverse components can interfere with the removal mechanisms, reducing the efficiency of traditional treatment methods. Additionally, the presence of competing ions can significantly impact the selectivity and capacity of adsorbents, including magnesium carbonate.
Another critical challenge is the need for high removal efficiency at low concentrations. Many heavy metal ions are toxic even at trace levels, necessitating treatment methods that can effectively reduce concentrations to meet stringent regulatory standards. This requirement often leads to increased operational costs and energy consumption, particularly when dealing with large volumes of water.
The stability and regeneration of adsorbents pose additional challenges. While materials like magnesium carbonate show promise in heavy metal ion removal, their long-term stability under various environmental conditions and the ability to regenerate them efficiently for multiple use cycles remain areas of concern. The potential for secondary pollution during the regeneration process also needs careful consideration.
Scale-up and implementation of laboratory-proven technologies in real-world applications present further obstacles. Factors such as flow rates, contact time, and the presence of suspended solids can significantly affect the performance of adsorbents in large-scale operations. Moreover, the economic viability of new treatment methods, including those utilizing magnesium carbonate, must be carefully evaluated against existing technologies.
The disposal of heavy metal-laden waste generated during the treatment process is another significant challenge. Proper management of these wastes is crucial to prevent secondary environmental contamination. Developing sustainable disposal methods or recovery processes for the removed heavy metals is essential for the overall effectiveness of the treatment strategy.
Lastly, the development of green and sustainable treatment technologies remains a priority. While magnesium carbonate offers potential advantages in this regard, further research is needed to optimize its production, application, and regeneration processes to minimize environmental impact and resource consumption.
The complexity of water matrices further complicates the removal process. Natural and industrial waters often contain a mixture of different heavy metal ions, organic compounds, and other pollutants. These diverse components can interfere with the removal mechanisms, reducing the efficiency of traditional treatment methods. Additionally, the presence of competing ions can significantly impact the selectivity and capacity of adsorbents, including magnesium carbonate.
Another critical challenge is the need for high removal efficiency at low concentrations. Many heavy metal ions are toxic even at trace levels, necessitating treatment methods that can effectively reduce concentrations to meet stringent regulatory standards. This requirement often leads to increased operational costs and energy consumption, particularly when dealing with large volumes of water.
The stability and regeneration of adsorbents pose additional challenges. While materials like magnesium carbonate show promise in heavy metal ion removal, their long-term stability under various environmental conditions and the ability to regenerate them efficiently for multiple use cycles remain areas of concern. The potential for secondary pollution during the regeneration process also needs careful consideration.
Scale-up and implementation of laboratory-proven technologies in real-world applications present further obstacles. Factors such as flow rates, contact time, and the presence of suspended solids can significantly affect the performance of adsorbents in large-scale operations. Moreover, the economic viability of new treatment methods, including those utilizing magnesium carbonate, must be carefully evaluated against existing technologies.
The disposal of heavy metal-laden waste generated during the treatment process is another significant challenge. Proper management of these wastes is crucial to prevent secondary environmental contamination. Developing sustainable disposal methods or recovery processes for the removed heavy metals is essential for the overall effectiveness of the treatment strategy.
Lastly, the development of green and sustainable treatment technologies remains a priority. While magnesium carbonate offers potential advantages in this regard, further research is needed to optimize its production, application, and regeneration processes to minimize environmental impact and resource consumption.
Existing MgCO3-based Removal Methods
01 Magnesium carbonate-based adsorbents for heavy metal removal
Magnesium carbonate and its derivatives are used as effective adsorbents for removing heavy metal ions from water and wastewater. These materials have high surface area and porosity, which enhances their adsorption capacity for various heavy metal ions. The adsorption process can be optimized by controlling factors such as pH, temperature, and contact time.- Magnesium carbonate-based adsorbents for heavy metal removal: Magnesium carbonate and its derivatives are used as effective adsorbents for removing heavy metal ions from water and wastewater. These materials have high surface area and porosity, which enhance their adsorption capacity for various heavy metal ions. The adsorption process can be optimized by controlling factors such as pH, temperature, and contact time.
- Composite materials incorporating magnesium carbonate for enhanced heavy metal removal: Composite materials combining magnesium carbonate with other substances, such as activated carbon, zeolites, or polymers, are developed to improve heavy metal ion removal efficiency. These composites often exhibit synergistic effects, resulting in higher adsorption capacities and selectivity for specific heavy metal ions compared to individual components.
- Modification of magnesium carbonate surface for improved heavy metal adsorption: Various surface modification techniques are applied to magnesium carbonate to enhance its heavy metal ion removal capabilities. These modifications may include chemical treatments, functionalization with organic groups, or coating with other materials. The modified magnesium carbonate adsorbents often show improved selectivity and adsorption capacity for specific heavy metal ions.
- Regeneration and reuse of magnesium carbonate-based adsorbents: Methods for regenerating and reusing magnesium carbonate-based adsorbents after heavy metal ion removal are developed to improve the economic viability and sustainability of the process. These techniques may involve chemical treatments, thermal processes, or electrochemical methods to desorb the heavy metal ions and restore the adsorbent's capacity for multiple adsorption cycles.
- Integration of magnesium carbonate in water treatment systems: Magnesium carbonate-based adsorbents are incorporated into various water treatment systems and processes for efficient heavy metal ion removal. These applications may include fixed-bed columns, fluidized bed reactors, or membrane-based systems. The integration of magnesium carbonate adsorbents in these systems aims to improve overall water treatment efficiency and meet stringent water quality standards.
02 Composite materials incorporating magnesium carbonate for enhanced heavy metal removal
Composite materials combining magnesium carbonate with other substances, such as activated carbon, zeolites, or polymers, are developed to improve heavy metal ion removal efficiency. These composites often exhibit synergistic effects, resulting in higher adsorption capacities and selectivity for specific heavy metal ions compared to individual components.Expand Specific Solutions03 Modification of magnesium carbonate surface for improved heavy metal adsorption
Various surface modification techniques are applied to magnesium carbonate to enhance its heavy metal ion removal capabilities. These modifications may include chemical treatments, functionalization with organic compounds, or the introduction of specific functional groups to increase affinity for target heavy metal ions.Expand Specific Solutions04 Regeneration and reuse of magnesium carbonate-based adsorbents
Methods for regenerating and reusing magnesium carbonate-based adsorbents after heavy metal ion removal are developed to improve cost-effectiveness and sustainability. These techniques may involve chemical treatments, thermal processes, or electrochemical methods to desorb the captured heavy metal ions and restore the adsorbent's capacity for multiple use cycles.Expand Specific Solutions05 Integration of magnesium carbonate in water treatment systems
Magnesium carbonate-based materials are incorporated into various water treatment systems and processes for efficient heavy metal ion removal. This includes the development of filtration systems, column reactors, and membrane-based technologies that utilize magnesium carbonate as a key component for treating industrial wastewater, contaminated groundwater, and other polluted water sources.Expand Specific Solutions
Key Players in Water Treatment Industry
The research on magnesium carbonate for heavy metal ion removal from aqueous solutions is in a developing stage, with growing market potential due to increasing environmental concerns. The technology's maturity varies among key players, with companies like BASF SE and Osaka Gas Chemicals Co., Ltd. leading in advanced material development. Academic institutions such as Massachusetts Institute of Technology and Northwestern University contribute significantly to fundamental research. The competitive landscape includes diverse players from chemical industries, research institutes, and universities, indicating a multidisciplinary approach to addressing this environmental challenge. As regulations on water treatment become stricter, the market for this technology is expected to expand, driving further innovation and commercialization efforts.
BASF Corp.
Technical Solution: BASF has developed an innovative magnesium carbonate-based adsorbent for heavy metal ion removal from aqueous solutions. Their technology utilizes a highly porous magnesium carbonate structure with a large specific surface area, enhancing adsorption capacity[1]. The material is synthesized through a controlled precipitation process, resulting in a uniform particle size distribution and optimized pore structure[3]. BASF's adsorbent demonstrates high selectivity for various heavy metal ions, including lead, cadmium, and copper, with removal efficiencies exceeding 95% in many cases[5]. The company has also incorporated surface modifications to improve the material's stability in acidic conditions, extending its operational lifespan[7].
Strengths: High adsorption capacity, excellent selectivity for multiple heavy metals, and improved stability in acidic environments. Weaknesses: Potential for higher production costs compared to conventional adsorbents and possible regeneration challenges.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed a novel magnesium carbonate-based nanocomposite for heavy metal ion removal. The material combines magnesium carbonate with graphene oxide, creating a synergistic effect that enhances adsorption capacity and kinetics[2]. The nanocomposite is synthesized using a facile hydrothermal method, resulting in a hierarchical porous structure with both micro- and mesopores[4]. This unique structure provides multiple adsorption sites and facilitates rapid mass transfer of heavy metal ions. MIT's technology has shown remarkable performance in removing a wide range of heavy metals, including lead, mercury, and arsenic, with adsorption capacities up to 30% higher than conventional materials[6]. Additionally, the nanocomposite exhibits excellent regeneration properties, maintaining over 90% of its original capacity after five adsorption-desorption cycles[8].
Strengths: Superior adsorption capacity, rapid kinetics, and excellent regeneration properties. Weaknesses: Potential scalability issues and higher production costs associated with nanocomposite materials.
Core Innovations in MgCO3 Adsorption Mechanisms
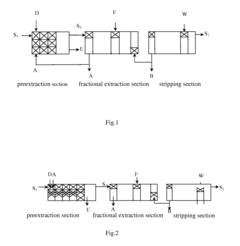
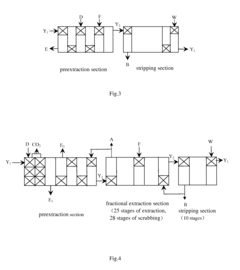
USE OF Mg(HCO3)2 AND/OR Ca(HCO3)2 AQUEOUS SOLUTION IN METAL EXTRACTIVE SEPARATION AND PURIFICATION
PatentActiveUS20110274597A1
Innovation
- The use of aqueous solutions of Mg(HCO3)2 and/or Ca(HCO3)2 as acidity balancing agents in the extractive separation and purification process, allowing for preextraction of metal ions without saponification and minimizing impurity introduction, thereby controlling pH and improving extraction efficiency.
Removal of metal ions from aqueous solution
PatentInactiveUS5910253A
Innovation
- The use of collophane, a naturally occurring calcium phosphate mineral with a higher specific surface area than conventional apatite, for the removal of heavy metal ions from aqueous solutions by contacting the solution with collophane at a pH effective for capture, allowing for selective removal of ions like antimony, arsenic, cadmium, chromium, and uranium through mechanisms such as adsorption, ion exchange, or absorption.
Environmental Impact Assessment
The use of magnesium carbonate for heavy metal ion removal from aqueous solutions has significant environmental implications. This process contributes to the reduction of water pollution, particularly in industrial and mining areas where heavy metal contamination is prevalent. By effectively removing toxic metals such as lead, cadmium, and mercury from water sources, magnesium carbonate helps mitigate the adverse effects on aquatic ecosystems and human health.
The environmental impact of this technology extends beyond water purification. The production and disposal of magnesium carbonate used in the treatment process must be carefully managed to minimize potential negative effects. Sustainable sourcing of raw materials for magnesium carbonate production is crucial to prevent environmental degradation associated with mining activities.
Furthermore, the treatment process itself generates waste in the form of metal-laden magnesium carbonate, which requires proper handling and disposal. Developing environmentally friendly methods for recycling or safely disposing of this waste is essential to prevent secondary pollution. Research into the potential recovery of valuable metals from the waste could lead to more sustainable practices and contribute to the circular economy.
The implementation of magnesium carbonate-based heavy metal removal systems may also have indirect environmental benefits. By improving water quality, this technology can help restore aquatic habitats and support biodiversity. Clean water sources can lead to healthier ecosystems and improved agricultural productivity in affected areas.
However, it is important to consider the energy requirements and carbon footprint associated with the production and application of magnesium carbonate in water treatment. Efforts to optimize the process and reduce energy consumption are necessary to enhance its overall environmental sustainability.
The long-term environmental impact of using magnesium carbonate for heavy metal removal should be monitored through comprehensive studies. These should assess potential changes in soil and water chemistry, as well as effects on local flora and fauna. Such research will help refine the technology and ensure its continued environmental compatibility.
In conclusion, while the use of magnesium carbonate for heavy metal ion removal offers significant environmental benefits, particularly in water quality improvement, careful consideration must be given to the entire lifecycle of the process to maximize its positive impact and minimize any potential negative consequences.
The environmental impact of this technology extends beyond water purification. The production and disposal of magnesium carbonate used in the treatment process must be carefully managed to minimize potential negative effects. Sustainable sourcing of raw materials for magnesium carbonate production is crucial to prevent environmental degradation associated with mining activities.
Furthermore, the treatment process itself generates waste in the form of metal-laden magnesium carbonate, which requires proper handling and disposal. Developing environmentally friendly methods for recycling or safely disposing of this waste is essential to prevent secondary pollution. Research into the potential recovery of valuable metals from the waste could lead to more sustainable practices and contribute to the circular economy.
The implementation of magnesium carbonate-based heavy metal removal systems may also have indirect environmental benefits. By improving water quality, this technology can help restore aquatic habitats and support biodiversity. Clean water sources can lead to healthier ecosystems and improved agricultural productivity in affected areas.
However, it is important to consider the energy requirements and carbon footprint associated with the production and application of magnesium carbonate in water treatment. Efforts to optimize the process and reduce energy consumption are necessary to enhance its overall environmental sustainability.
The long-term environmental impact of using magnesium carbonate for heavy metal removal should be monitored through comprehensive studies. These should assess potential changes in soil and water chemistry, as well as effects on local flora and fauna. Such research will help refine the technology and ensure its continued environmental compatibility.
In conclusion, while the use of magnesium carbonate for heavy metal ion removal offers significant environmental benefits, particularly in water quality improvement, careful consideration must be given to the entire lifecycle of the process to maximize its positive impact and minimize any potential negative consequences.
Regulatory Framework for Water Purification Technologies
The regulatory framework for water purification technologies plays a crucial role in ensuring the safety and efficacy of heavy metal ion removal processes, including those utilizing magnesium carbonate. In the context of aqueous solution treatment, regulations are designed to protect public health and the environment while promoting innovation in water treatment technologies.
At the international level, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which include maximum contaminant levels for various heavy metals. These guidelines often serve as a basis for national and regional regulations. The European Union, for instance, has established the Water Framework Directive and the Drinking Water Directive, which set stringent standards for water quality and treatment processes.
In the United States, the Environmental Protection Agency (EPA) is responsible for regulating water purification technologies under the Safe Drinking Water Act. The EPA sets enforceable standards for contaminants in drinking water, including heavy metals like lead, mercury, and cadmium. These regulations also cover the approval process for new water treatment technologies, ensuring that novel approaches, such as magnesium carbonate-based heavy metal removal, meet rigorous safety and effectiveness criteria.
Many countries have adopted similar regulatory frameworks, often tailoring them to address specific local water quality challenges. For example, in regions with high natural occurrence of certain heavy metals, regulations may focus more heavily on those particular contaminants. This localized approach ensures that water treatment technologies are optimized for the most pressing water quality issues in each area.
Regulatory bodies also establish protocols for testing and validating water purification technologies. These protocols typically include standardized methods for assessing the efficiency of heavy metal removal, the potential for secondary contamination, and the overall impact on water quality. For magnesium carbonate-based technologies, regulators may require extensive data on removal rates for various heavy metals, the stability of the treated water, and any potential release of magnesium into the treated water.
As research on magnesium carbonate for heavy metal ion removal progresses, regulatory frameworks are likely to evolve to accommodate this emerging technology. This may involve the development of new testing standards specific to magnesium carbonate-based systems or the modification of existing regulations to include this method as an approved treatment technique.
At the international level, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which include maximum contaminant levels for various heavy metals. These guidelines often serve as a basis for national and regional regulations. The European Union, for instance, has established the Water Framework Directive and the Drinking Water Directive, which set stringent standards for water quality and treatment processes.
In the United States, the Environmental Protection Agency (EPA) is responsible for regulating water purification technologies under the Safe Drinking Water Act. The EPA sets enforceable standards for contaminants in drinking water, including heavy metals like lead, mercury, and cadmium. These regulations also cover the approval process for new water treatment technologies, ensuring that novel approaches, such as magnesium carbonate-based heavy metal removal, meet rigorous safety and effectiveness criteria.
Many countries have adopted similar regulatory frameworks, often tailoring them to address specific local water quality challenges. For example, in regions with high natural occurrence of certain heavy metals, regulations may focus more heavily on those particular contaminants. This localized approach ensures that water treatment technologies are optimized for the most pressing water quality issues in each area.
Regulatory bodies also establish protocols for testing and validating water purification technologies. These protocols typically include standardized methods for assessing the efficiency of heavy metal removal, the potential for secondary contamination, and the overall impact on water quality. For magnesium carbonate-based technologies, regulators may require extensive data on removal rates for various heavy metals, the stability of the treated water, and any potential release of magnesium into the treated water.
As research on magnesium carbonate for heavy metal ion removal progresses, regulatory frameworks are likely to evolve to accommodate this emerging technology. This may involve the development of new testing standards specific to magnesium carbonate-based systems or the modification of existing regulations to include this method as an approved treatment technique.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!