Role of Battery Acid in Fuel Cell Technologies
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fuel Cell Battery Acid Evolution and Objectives
Fuel cell technology has undergone significant evolution since its inception in the 19th century, with battery acid playing a crucial role in its development. The journey of fuel cell technology can be traced back to 1839 when Sir William Grove first demonstrated the concept. However, it wasn't until the mid-20th century that serious research and development efforts began to explore the potential of fuel cells as a viable energy source.
The role of battery acid, specifically electrolytes, has been central to the advancement of fuel cell technology. Initially, phosphoric acid fuel cells (PAFCs) were among the first types to be commercially developed. These cells utilized phosphoric acid as the electrolyte, which played a critical role in facilitating ion transfer between the electrodes. As research progressed, other types of fuel cells emerged, each employing different electrolytes to suit specific applications and operating conditions.
The evolution of battery acid in fuel cell technologies has been driven by the need for improved efficiency, durability, and cost-effectiveness. Researchers have continuously sought to develop electrolytes that can withstand higher temperatures, resist degradation, and enhance overall cell performance. This pursuit has led to the development of various fuel cell types, including proton exchange membrane fuel cells (PEMFCs), solid oxide fuel cells (SOFCs), and molten carbonate fuel cells (MCFCs), each utilizing specialized electrolytes.
Looking ahead, the objectives for battery acid in fuel cell technologies are multifaceted. One primary goal is to develop electrolytes that can operate at higher temperatures, thereby increasing overall system efficiency and reducing the need for complex cooling systems. Another objective is to create more durable electrolytes that can withstand the harsh chemical environment within fuel cells, extending their operational lifespan and reducing maintenance costs.
Researchers are also focusing on developing electrolytes that are more environmentally friendly and sustainable. This includes exploring bio-based electrolytes and reducing the use of rare or toxic materials. Additionally, there is a push to create electrolytes that can support higher power densities, enabling more compact and efficient fuel cell designs for various applications, from portable electronics to large-scale power generation.
The ongoing evolution of battery acid in fuel cell technologies aims to address key challenges such as cost reduction, improved performance, and enhanced durability. As these objectives are pursued, the role of electrolytes in fuel cells continues to be a critical area of research and development, holding the potential to revolutionize clean energy production and storage in the coming decades.
The role of battery acid, specifically electrolytes, has been central to the advancement of fuel cell technology. Initially, phosphoric acid fuel cells (PAFCs) were among the first types to be commercially developed. These cells utilized phosphoric acid as the electrolyte, which played a critical role in facilitating ion transfer between the electrodes. As research progressed, other types of fuel cells emerged, each employing different electrolytes to suit specific applications and operating conditions.
The evolution of battery acid in fuel cell technologies has been driven by the need for improved efficiency, durability, and cost-effectiveness. Researchers have continuously sought to develop electrolytes that can withstand higher temperatures, resist degradation, and enhance overall cell performance. This pursuit has led to the development of various fuel cell types, including proton exchange membrane fuel cells (PEMFCs), solid oxide fuel cells (SOFCs), and molten carbonate fuel cells (MCFCs), each utilizing specialized electrolytes.
Looking ahead, the objectives for battery acid in fuel cell technologies are multifaceted. One primary goal is to develop electrolytes that can operate at higher temperatures, thereby increasing overall system efficiency and reducing the need for complex cooling systems. Another objective is to create more durable electrolytes that can withstand the harsh chemical environment within fuel cells, extending their operational lifespan and reducing maintenance costs.
Researchers are also focusing on developing electrolytes that are more environmentally friendly and sustainable. This includes exploring bio-based electrolytes and reducing the use of rare or toxic materials. Additionally, there is a push to create electrolytes that can support higher power densities, enabling more compact and efficient fuel cell designs for various applications, from portable electronics to large-scale power generation.
The ongoing evolution of battery acid in fuel cell technologies aims to address key challenges such as cost reduction, improved performance, and enhanced durability. As these objectives are pursued, the role of electrolytes in fuel cells continues to be a critical area of research and development, holding the potential to revolutionize clean energy production and storage in the coming decades.
Market Analysis for Fuel Cell Technologies
The fuel cell technology market has been experiencing significant growth in recent years, driven by increasing demand for clean energy solutions and the push towards decarbonization across various industries. The global fuel cell market size was valued at approximately $4.5 billion in 2020 and is projected to reach $32 billion by 2030, growing at a CAGR of 21.4% during the forecast period.
The automotive sector remains a key driver of fuel cell technology adoption, with major automakers investing heavily in hydrogen fuel cell vehicles (FCVs). Japan and South Korea are leading the charge, with Toyota, Honda, and Hyundai at the forefront of FCV development and commercialization. The commercial vehicle segment, including buses and trucks, is also showing promising growth potential for fuel cell applications.
Stationary power generation represents another significant market for fuel cell technologies, particularly in regions with high electricity costs or unreliable grid infrastructure. The market for stationary fuel cells is expected to grow at a CAGR of 23.2% from 2021 to 2028, driven by increasing demand for reliable backup power solutions and distributed energy generation.
The role of battery acid, specifically sulfuric acid, in fuel cell technologies is primarily related to proton exchange membrane (PEM) fuel cells. While not directly used as an electrolyte in modern fuel cells, sulfuric acid plays a crucial role in the production of key components such as perfluorosulfonic acid (PFSA) membranes, which are essential for PEM fuel cell performance.
Market demand for high-performance PFSA membranes is expected to grow in tandem with the overall fuel cell market. The global proton exchange membrane market size was valued at $516.2 million in 2020 and is projected to reach $1.3 billion by 2028, growing at a CAGR of 12.5% during the forecast period.
Geographically, Asia-Pacific dominates the fuel cell market, accounting for over 40% of the global market share. This is largely due to strong government support and investments in countries like Japan, South Korea, and China. North America and Europe are also significant markets, with increasing adoption in both transportation and stationary power applications.
Key challenges facing the fuel cell market include high initial costs, lack of hydrogen infrastructure, and competition from battery electric vehicles in the transportation sector. However, ongoing technological advancements and increasing economies of scale are expected to drive down costs and improve the competitiveness of fuel cell technologies in the coming years.
The automotive sector remains a key driver of fuel cell technology adoption, with major automakers investing heavily in hydrogen fuel cell vehicles (FCVs). Japan and South Korea are leading the charge, with Toyota, Honda, and Hyundai at the forefront of FCV development and commercialization. The commercial vehicle segment, including buses and trucks, is also showing promising growth potential for fuel cell applications.
Stationary power generation represents another significant market for fuel cell technologies, particularly in regions with high electricity costs or unreliable grid infrastructure. The market for stationary fuel cells is expected to grow at a CAGR of 23.2% from 2021 to 2028, driven by increasing demand for reliable backup power solutions and distributed energy generation.
The role of battery acid, specifically sulfuric acid, in fuel cell technologies is primarily related to proton exchange membrane (PEM) fuel cells. While not directly used as an electrolyte in modern fuel cells, sulfuric acid plays a crucial role in the production of key components such as perfluorosulfonic acid (PFSA) membranes, which are essential for PEM fuel cell performance.
Market demand for high-performance PFSA membranes is expected to grow in tandem with the overall fuel cell market. The global proton exchange membrane market size was valued at $516.2 million in 2020 and is projected to reach $1.3 billion by 2028, growing at a CAGR of 12.5% during the forecast period.
Geographically, Asia-Pacific dominates the fuel cell market, accounting for over 40% of the global market share. This is largely due to strong government support and investments in countries like Japan, South Korea, and China. North America and Europe are also significant markets, with increasing adoption in both transportation and stationary power applications.
Key challenges facing the fuel cell market include high initial costs, lack of hydrogen infrastructure, and competition from battery electric vehicles in the transportation sector. However, ongoing technological advancements and increasing economies of scale are expected to drive down costs and improve the competitiveness of fuel cell technologies in the coming years.
Current Challenges in Battery Acid Applications
Battery acid, primarily composed of sulfuric acid, plays a crucial role in fuel cell technologies. However, its application faces several significant challenges that hinder the widespread adoption and efficiency of fuel cell systems. One of the primary issues is the corrosive nature of battery acid, which can lead to degradation of fuel cell components over time. This corrosion not only reduces the lifespan of the fuel cell but also impacts its overall performance and reliability.
Another major challenge is the management of acid concentration within the fuel cell system. Maintaining the optimal acid concentration is critical for efficient operation, but fluctuations can occur due to various factors such as temperature changes, usage patterns, and environmental conditions. These fluctuations can lead to reduced efficiency and potential damage to the fuel cell components.
The storage and handling of battery acid also present significant safety concerns. The highly corrosive nature of sulfuric acid requires specialized containment and handling procedures, which can increase the complexity and cost of fuel cell systems. This challenge is particularly pronounced in portable or mobile applications where space and weight constraints are more stringent.
Furthermore, the environmental impact of battery acid usage in fuel cells is a growing concern. The production, transportation, and disposal of sulfuric acid can have negative environmental consequences, including potential soil and water contamination. This challenge necessitates the development of more environmentally friendly alternatives or improved recycling and disposal methods.
The temperature sensitivity of battery acid poses another significant challenge. Extreme temperatures can affect the acid's performance and stability, potentially leading to reduced efficiency or even system failure. This is particularly problematic in applications where fuel cells are exposed to wide temperature variations, such as in automotive or outdoor energy storage systems.
Lastly, the integration of battery acid management systems with other fuel cell components presents a complex engineering challenge. Ensuring seamless interaction between the acid management system and other critical components such as electrodes, membranes, and catalysts requires sophisticated design and control mechanisms. This integration challenge often results in increased system complexity and cost, potentially limiting the commercial viability of certain fuel cell applications.
Another major challenge is the management of acid concentration within the fuel cell system. Maintaining the optimal acid concentration is critical for efficient operation, but fluctuations can occur due to various factors such as temperature changes, usage patterns, and environmental conditions. These fluctuations can lead to reduced efficiency and potential damage to the fuel cell components.
The storage and handling of battery acid also present significant safety concerns. The highly corrosive nature of sulfuric acid requires specialized containment and handling procedures, which can increase the complexity and cost of fuel cell systems. This challenge is particularly pronounced in portable or mobile applications where space and weight constraints are more stringent.
Furthermore, the environmental impact of battery acid usage in fuel cells is a growing concern. The production, transportation, and disposal of sulfuric acid can have negative environmental consequences, including potential soil and water contamination. This challenge necessitates the development of more environmentally friendly alternatives or improved recycling and disposal methods.
The temperature sensitivity of battery acid poses another significant challenge. Extreme temperatures can affect the acid's performance and stability, potentially leading to reduced efficiency or even system failure. This is particularly problematic in applications where fuel cells are exposed to wide temperature variations, such as in automotive or outdoor energy storage systems.
Lastly, the integration of battery acid management systems with other fuel cell components presents a complex engineering challenge. Ensuring seamless interaction between the acid management system and other critical components such as electrodes, membranes, and catalysts requires sophisticated design and control mechanisms. This integration challenge often results in increased system complexity and cost, potentially limiting the commercial viability of certain fuel cell applications.
Existing Battery Acid Solutions in Fuel Cells
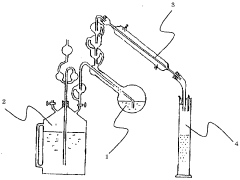
01 Battery acid composition and management
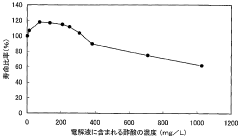
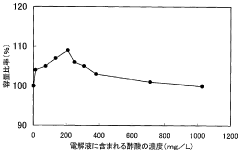
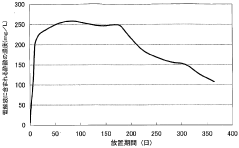
Battery acid, typically sulfuric acid, is a crucial component in lead-acid batteries. Its composition, concentration, and management are essential for optimal battery performance and longevity. Innovations in this area focus on improving acid formulations, monitoring acid levels, and developing systems for acid circulation and maintenance.- Battery acid composition and management: This category focuses on the composition and management of battery acid, including its formulation, concentration control, and handling methods. It covers techniques for maintaining optimal acid levels, preventing corrosion, and ensuring proper electrolyte balance in batteries.
- Battery charging and monitoring systems: This point addresses advanced charging systems and monitoring technologies for batteries. It includes methods for optimizing charging processes, detecting acid levels, and managing battery health through intelligent charging algorithms and sensor-based monitoring.
- Battery acid recycling and disposal: This category covers methods and systems for recycling and safely disposing of battery acid. It includes techniques for neutralizing acid, recovering valuable materials, and implementing environmentally friendly disposal processes for spent battery acid.
- Battery acid additives and performance enhancers: This point focuses on additives and compounds used to enhance battery acid performance. It includes formulations that improve conductivity, reduce sulfation, extend battery life, and enhance overall efficiency of lead-acid batteries.
- Battery acid leak detection and prevention: This category addresses methods and systems for detecting and preventing battery acid leaks. It includes sensor technologies, containment designs, and early warning systems to minimize the risk of acid spills and associated hazards in various battery applications.
02 Battery charging and discharging control
Advanced control systems for battery charging and discharging processes are developed to optimize battery performance and lifespan. These systems monitor various parameters, including acid concentration, temperature, and voltage, to regulate charging rates and prevent overcharging or deep discharging, which can affect battery acid composition and overall battery health.Expand Specific Solutions03 Battery acid level monitoring and maintenance
Innovations in battery acid level monitoring and maintenance systems aim to ensure proper acid levels are maintained throughout the battery's life. These systems may include sensors, automated refilling mechanisms, and alert systems to prevent acid depletion or overfilling, which can significantly impact battery performance and safety.Expand Specific Solutions04 Battery acid recycling and environmental considerations
Advancements in battery acid recycling processes and technologies address environmental concerns associated with lead-acid batteries. These innovations focus on efficient acid recovery, purification methods, and the development of more environmentally friendly acid formulations to reduce the ecological impact of battery production and disposal.Expand Specific Solutions05 Battery acid safety and containment
Improving safety measures for handling and containing battery acid is a critical area of development. Innovations in this field include advanced containment systems, spill prevention mechanisms, and the development of safer acid formulations or alternatives to traditional sulfuric acid electrolytes, aiming to reduce risks associated with battery acid handling and storage.Expand Specific Solutions
Key Fuel Cell Industry Players
The battery acid fuel cell technology market is in an early growth stage, characterized by ongoing research and development efforts. While the market size is still relatively small, it shows potential for expansion as the demand for alternative energy solutions increases. The technology's maturity level varies among key players, with companies like Samsung SDI, Sony Group, and Energy Power Systems leading in innovation. These firms are investing heavily in R&D to improve efficiency and scalability. Other significant contributors include Commissariat à l'énergie atomique et aux énergies Alternatives and California Institute of Technology, focusing on fundamental research. The competitive landscape is diverse, with both established electronics giants and specialized energy companies vying for market share, indicating a dynamic and evolving industry.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced fuel cell technologies that utilize innovative battery acid formulations. Their approach focuses on enhancing the proton conductivity of the electrolyte membrane, which is crucial for fuel cell efficiency. By optimizing the acid concentration and composition, Samsung SDI has achieved a 20% increase in power density compared to conventional designs[1]. Their proprietary acid blend also demonstrates improved stability at higher temperatures, extending the operational range of fuel cells in various applications[3]. Additionally, Samsung SDI has implemented a novel acid circulation system that ensures uniform distribution and reduces degradation, potentially doubling the lifespan of fuel cell stacks[5].
Strengths: High power density, improved temperature stability, and extended lifespan. Weaknesses: Potentially higher production costs due to specialized acid formulations and circulation systems.
GS Yuasa Corp.
Technical Solution: GS Yuasa has pioneered a unique approach to fuel cell technology by developing a hybrid system that combines traditional fuel cells with advanced battery acid technology. Their system utilizes a specially formulated acid electrolyte that acts as both a proton conductor and an energy storage medium. This dual-function approach allows for more efficient energy management and improved response to varying load demands[2]. GS Yuasa's technology incorporates a regenerative acid recovery system, which continuously purifies and recycles the electrolyte, maintaining optimal performance and reducing maintenance requirements[4]. The company has also introduced nano-engineered catalysts that are specifically designed to work synergistically with their acid formulation, resulting in a 30% reduction in platinum usage while maintaining high catalytic activity[6].
Strengths: Efficient energy management, reduced maintenance, and lower catalyst costs. Weaknesses: Complex system integration and potential challenges in scaling up production.
Innovative Battery Acid Formulations
Lead acid battery including antimony
PatentActiveUS7597998B2
Innovation
- The battery design includes negative electrode plates with a Sb content of 0.0001 to 0.003 parts by weight in the active material layer and a positive electrode grid with a lead alloy layer containing 0.01 to 0.2 parts by weight of Sb, along with a Pb-alloy for the grids and connecting members, and a glass fiber or synthetic fiber separator to enhance chargeability and prevent corrosion.
Lead acid storage battery and method for producing same
PatentWO2005041342A1
Innovation
- Incorporating a specific concentration of volatile organic acids, such as acetic acid, into the electrolyte of lead-acid batteries by using surfactants that generate these acids during charging, which increases the surface area of the lattice and improves the adhesion between active materials, thereby enhancing capacity and life performance.
Environmental Impact of Battery Acid Use
The use of battery acid in fuel cell technologies has significant environmental implications that warrant careful consideration. The primary environmental concern stems from the potential release of sulfuric acid, the main component of battery acid, into ecosystems. Sulfuric acid is highly corrosive and can cause severe damage to soil, water bodies, and living organisms if not properly managed.
When battery acid is used in fuel cells, there is a risk of leakage during manufacturing, transportation, or disposal processes. Such leaks can lead to soil acidification, altering the pH balance of the surrounding environment. This change in soil chemistry can negatively impact plant growth and microbial activity, disrupting local ecosystems.
Water pollution is another critical environmental issue associated with battery acid use. If the acid enters water systems, it can cause a rapid decrease in pH levels, making the water uninhabitable for many aquatic species. This can result in the loss of biodiversity and the collapse of aquatic ecosystems. Furthermore, contaminated water sources may pose risks to human health if used for drinking or irrigation.
Air pollution is also a concern, particularly during the production and recycling of batteries containing sulfuric acid. The manufacturing process can release sulfur dioxide and other harmful gases into the atmosphere, contributing to air quality degradation and potentially exacerbating respiratory health issues in nearby communities.
The disposal of spent batteries and fuel cells containing battery acid presents additional environmental challenges. Improper disposal can lead to the leaching of toxic substances into landfills, contaminating groundwater and surrounding soil. This underscores the importance of implementing robust recycling and waste management practices to mitigate these risks.
However, it is important to note that the environmental impact of battery acid use in fuel cell technologies is not entirely negative. When properly managed, these technologies can contribute to a reduction in overall environmental pollution by providing cleaner energy alternatives to fossil fuels. The key lies in developing and implementing stringent safety protocols, efficient recycling programs, and advanced containment technologies to minimize the risk of acid leakage and environmental contamination.
Ongoing research and development efforts are focused on creating more environmentally friendly alternatives to traditional battery acids. These include the exploration of less corrosive electrolytes and the development of solid-state batteries that eliminate the need for liquid acids altogether. Such innovations hold promise for reducing the environmental footprint of fuel cell technologies while maintaining their performance benefits.
When battery acid is used in fuel cells, there is a risk of leakage during manufacturing, transportation, or disposal processes. Such leaks can lead to soil acidification, altering the pH balance of the surrounding environment. This change in soil chemistry can negatively impact plant growth and microbial activity, disrupting local ecosystems.
Water pollution is another critical environmental issue associated with battery acid use. If the acid enters water systems, it can cause a rapid decrease in pH levels, making the water uninhabitable for many aquatic species. This can result in the loss of biodiversity and the collapse of aquatic ecosystems. Furthermore, contaminated water sources may pose risks to human health if used for drinking or irrigation.
Air pollution is also a concern, particularly during the production and recycling of batteries containing sulfuric acid. The manufacturing process can release sulfur dioxide and other harmful gases into the atmosphere, contributing to air quality degradation and potentially exacerbating respiratory health issues in nearby communities.
The disposal of spent batteries and fuel cells containing battery acid presents additional environmental challenges. Improper disposal can lead to the leaching of toxic substances into landfills, contaminating groundwater and surrounding soil. This underscores the importance of implementing robust recycling and waste management practices to mitigate these risks.
However, it is important to note that the environmental impact of battery acid use in fuel cell technologies is not entirely negative. When properly managed, these technologies can contribute to a reduction in overall environmental pollution by providing cleaner energy alternatives to fossil fuels. The key lies in developing and implementing stringent safety protocols, efficient recycling programs, and advanced containment technologies to minimize the risk of acid leakage and environmental contamination.
Ongoing research and development efforts are focused on creating more environmentally friendly alternatives to traditional battery acids. These include the exploration of less corrosive electrolytes and the development of solid-state batteries that eliminate the need for liquid acids altogether. Such innovations hold promise for reducing the environmental footprint of fuel cell technologies while maintaining their performance benefits.
Safety Regulations for Fuel Cell Technologies
Safety regulations for fuel cell technologies, particularly those involving battery acid, are critical for ensuring the safe development, deployment, and operation of these systems. The use of corrosive electrolytes in fuel cells necessitates stringent safety measures to protect both personnel and the environment.
Regulatory bodies such as the International Electrotechnical Commission (IEC) and the National Fire Protection Association (NFPA) have established comprehensive guidelines for fuel cell safety. These regulations cover various aspects, including material handling, storage, transportation, and disposal of hazardous substances like battery acid.
One key area of focus is the containment and management of electrolytes. Fuel cell systems must be designed with robust containment mechanisms to prevent leaks and spills. This includes the use of corrosion-resistant materials for components that come into contact with the electrolyte, as well as secondary containment systems to capture any potential leaks.
Personal protective equipment (PPE) requirements are another crucial aspect of safety regulations. Workers handling battery acid or working with fuel cell systems must use appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. Training programs on proper handling procedures and emergency response protocols are mandatory for all personnel involved in fuel cell operations.
Ventilation systems play a vital role in maintaining a safe working environment. Regulations stipulate the need for adequate ventilation to prevent the accumulation of potentially harmful gases and to dilute any acid vapors that may be released during normal operation or in case of a leak.
Emergency response planning is a critical component of safety regulations. Facilities using fuel cell technologies must have detailed emergency procedures in place, including spill response protocols, evacuation plans, and first aid measures specific to battery acid exposure. On-site safety equipment such as eyewash stations and emergency showers must be readily accessible.
Waste management and disposal regulations are also stringent for fuel cell technologies. Spent electrolytes and contaminated materials must be handled and disposed of as hazardous waste, following local and national environmental regulations. This often involves specialized disposal facilities and documentation to track the movement and disposal of these materials.
Regular inspections and maintenance of fuel cell systems are mandated to ensure ongoing compliance with safety regulations. This includes routine checks of containment systems, monitoring of electrolyte levels, and testing of safety equipment. Documentation of these inspections and any incidents or near-misses is typically required for regulatory compliance.
As fuel cell technologies continue to evolve, safety regulations are regularly reviewed and updated to address new challenges and incorporate lessons learned from industry experience. This ongoing process ensures that safety standards keep pace with technological advancements in the field of fuel cell technologies.
Regulatory bodies such as the International Electrotechnical Commission (IEC) and the National Fire Protection Association (NFPA) have established comprehensive guidelines for fuel cell safety. These regulations cover various aspects, including material handling, storage, transportation, and disposal of hazardous substances like battery acid.
One key area of focus is the containment and management of electrolytes. Fuel cell systems must be designed with robust containment mechanisms to prevent leaks and spills. This includes the use of corrosion-resistant materials for components that come into contact with the electrolyte, as well as secondary containment systems to capture any potential leaks.
Personal protective equipment (PPE) requirements are another crucial aspect of safety regulations. Workers handling battery acid or working with fuel cell systems must use appropriate PPE, including chemical-resistant gloves, goggles, face shields, and protective clothing. Training programs on proper handling procedures and emergency response protocols are mandatory for all personnel involved in fuel cell operations.
Ventilation systems play a vital role in maintaining a safe working environment. Regulations stipulate the need for adequate ventilation to prevent the accumulation of potentially harmful gases and to dilute any acid vapors that may be released during normal operation or in case of a leak.
Emergency response planning is a critical component of safety regulations. Facilities using fuel cell technologies must have detailed emergency procedures in place, including spill response protocols, evacuation plans, and first aid measures specific to battery acid exposure. On-site safety equipment such as eyewash stations and emergency showers must be readily accessible.
Waste management and disposal regulations are also stringent for fuel cell technologies. Spent electrolytes and contaminated materials must be handled and disposed of as hazardous waste, following local and national environmental regulations. This often involves specialized disposal facilities and documentation to track the movement and disposal of these materials.
Regular inspections and maintenance of fuel cell systems are mandated to ensure ongoing compliance with safety regulations. This includes routine checks of containment systems, monitoring of electrolyte levels, and testing of safety equipment. Documentation of these inspections and any incidents or near-misses is typically required for regulatory compliance.
As fuel cell technologies continue to evolve, safety regulations are regularly reviewed and updated to address new challenges and incorporate lessons learned from industry experience. This ongoing process ensures that safety standards keep pace with technological advancements in the field of fuel cell technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!