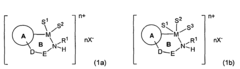
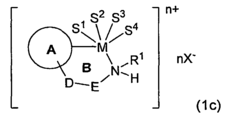
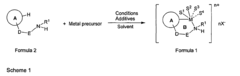
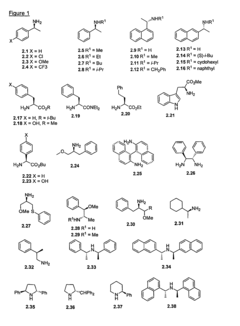
Role of Magnesium Carbonate in Chiral Catalyst Synthesis
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chiral Catalyst Evolution
The evolution of chiral catalysts represents a significant milestone in the field of asymmetric synthesis. This journey began in the mid-20th century with the discovery of the first chiral catalysts, which were primarily based on transition metal complexes. These early catalysts, while groundbreaking, often suffered from low enantioselectivity and limited substrate scope.
The 1970s and 1980s saw rapid advancements in chiral catalyst design, with the development of more sophisticated ligand systems. Researchers focused on creating catalysts with well-defined three-dimensional structures that could effectively differentiate between enantiomeric faces of prochiral substrates. This period witnessed the emergence of C2-symmetric diphosphine ligands, such as DIOP and BINAP, which became instrumental in various asymmetric transformations.
The 1990s marked a paradigm shift in chiral catalyst evolution with the introduction of organocatalysts. These small organic molecules, often derived from natural amino acids or alkaloids, offered a metal-free approach to asymmetric synthesis. Proline-catalyzed aldol reactions and iminium catalysis exemplified this new class of catalysts, expanding the toolbox for enantioselective transformations.
The turn of the millennium brought about a renaissance in transition metal catalysis, with the development of chiral metal-organic frameworks (MOFs) and supported chiral catalysts. These heterogeneous systems combined the advantages of homogeneous catalysts with improved recyclability and ease of separation. Concurrently, the field of biocatalysis gained prominence, with engineered enzymes offering unparalleled selectivity for certain transformations.
In recent years, the focus has shifted towards developing more sustainable and efficient chiral catalysts. This has led to the exploration of earth-abundant metals, such as iron and copper, as alternatives to precious metal catalysts. Additionally, the concept of cooperative catalysis, where multiple catalytic species work in tandem, has gained traction, allowing for more complex transformations with enhanced selectivity.
The role of magnesium carbonate in chiral catalyst synthesis has emerged as an intriguing area of research within this evolutionary timeline. Its potential as a support material or as a component in novel catalyst systems has attracted attention due to its abundance, low cost, and unique properties. Researchers are investigating how magnesium carbonate can influence the structure and performance of chiral catalysts, potentially offering new avenues for catalyst design and optimization.
As we look to the future, the evolution of chiral catalysts continues to be driven by the pursuit of perfect stereoselectivity, broader substrate scope, and improved sustainability. Emerging technologies such as artificial intelligence and high-throughput experimentation are accelerating the discovery and optimization of new catalyst systems, promising to unlock even more powerful tools for asymmetric synthesis in the years to come.
The 1970s and 1980s saw rapid advancements in chiral catalyst design, with the development of more sophisticated ligand systems. Researchers focused on creating catalysts with well-defined three-dimensional structures that could effectively differentiate between enantiomeric faces of prochiral substrates. This period witnessed the emergence of C2-symmetric diphosphine ligands, such as DIOP and BINAP, which became instrumental in various asymmetric transformations.
The 1990s marked a paradigm shift in chiral catalyst evolution with the introduction of organocatalysts. These small organic molecules, often derived from natural amino acids or alkaloids, offered a metal-free approach to asymmetric synthesis. Proline-catalyzed aldol reactions and iminium catalysis exemplified this new class of catalysts, expanding the toolbox for enantioselective transformations.
The turn of the millennium brought about a renaissance in transition metal catalysis, with the development of chiral metal-organic frameworks (MOFs) and supported chiral catalysts. These heterogeneous systems combined the advantages of homogeneous catalysts with improved recyclability and ease of separation. Concurrently, the field of biocatalysis gained prominence, with engineered enzymes offering unparalleled selectivity for certain transformations.
In recent years, the focus has shifted towards developing more sustainable and efficient chiral catalysts. This has led to the exploration of earth-abundant metals, such as iron and copper, as alternatives to precious metal catalysts. Additionally, the concept of cooperative catalysis, where multiple catalytic species work in tandem, has gained traction, allowing for more complex transformations with enhanced selectivity.
The role of magnesium carbonate in chiral catalyst synthesis has emerged as an intriguing area of research within this evolutionary timeline. Its potential as a support material or as a component in novel catalyst systems has attracted attention due to its abundance, low cost, and unique properties. Researchers are investigating how magnesium carbonate can influence the structure and performance of chiral catalysts, potentially offering new avenues for catalyst design and optimization.
As we look to the future, the evolution of chiral catalysts continues to be driven by the pursuit of perfect stereoselectivity, broader substrate scope, and improved sustainability. Emerging technologies such as artificial intelligence and high-throughput experimentation are accelerating the discovery and optimization of new catalyst systems, promising to unlock even more powerful tools for asymmetric synthesis in the years to come.
Market Demand Analysis
The market demand for chiral catalysts, particularly those involving magnesium carbonate in their synthesis, has been steadily growing in recent years. This growth is primarily driven by the increasing need for enantiopure compounds in various industries, especially pharmaceuticals, agrochemicals, and fine chemicals. The pharmaceutical sector, in particular, has been a major contributor to this demand, as chiral drugs often exhibit superior efficacy and fewer side effects compared to their racemic counterparts.
The global chiral technology market, which includes chiral catalysts, is projected to expand significantly in the coming years. This growth is fueled by the rising emphasis on green chemistry practices and the push for more efficient and sustainable manufacturing processes. Magnesium carbonate, as a component in chiral catalyst synthesis, plays a crucial role in this market due to its cost-effectiveness, abundance, and environmentally friendly nature.
In the pharmaceutical industry, the demand for chiral catalysts is particularly strong. With an increasing number of chiral drugs in development pipelines and stricter regulatory requirements for single-enantiomer compounds, pharmaceutical companies are investing heavily in chiral technologies. This trend is expected to continue, driving the demand for innovative chiral catalysts, including those synthesized using magnesium carbonate.
The agrochemical sector is another significant market for chiral catalysts. As the agricultural industry moves towards more targeted and environmentally friendly pesticides and herbicides, the need for enantiopure compounds has grown. This shift has created new opportunities for chiral catalysts, with magnesium carbonate-based synthesis methods gaining attention due to their potential for producing more effective and less harmful agrochemicals.
In the fine chemicals industry, the demand for chiral catalysts is also on the rise. Manufacturers of flavors, fragrances, and specialty chemicals are increasingly seeking ways to produce enantiopure compounds efficiently. The use of magnesium carbonate in chiral catalyst synthesis offers a promising avenue for meeting this demand, as it can lead to more selective and efficient production processes.
The market for chiral catalysts is not limited to these industries alone. Emerging applications in materials science, such as the development of advanced polymers and liquid crystals, are opening up new avenues for chiral catalyst technologies. This diversification of applications is expected to further drive the demand for innovative chiral catalysts, including those involving magnesium carbonate in their synthesis.
Geographically, North America and Europe currently dominate the chiral technology market, but rapid growth is expected in Asia-Pacific regions, particularly in China and India. This regional expansion is driven by the growing pharmaceutical and chemical industries in these countries, coupled with increasing research and development activities in chiral technologies.
The global chiral technology market, which includes chiral catalysts, is projected to expand significantly in the coming years. This growth is fueled by the rising emphasis on green chemistry practices and the push for more efficient and sustainable manufacturing processes. Magnesium carbonate, as a component in chiral catalyst synthesis, plays a crucial role in this market due to its cost-effectiveness, abundance, and environmentally friendly nature.
In the pharmaceutical industry, the demand for chiral catalysts is particularly strong. With an increasing number of chiral drugs in development pipelines and stricter regulatory requirements for single-enantiomer compounds, pharmaceutical companies are investing heavily in chiral technologies. This trend is expected to continue, driving the demand for innovative chiral catalysts, including those synthesized using magnesium carbonate.
The agrochemical sector is another significant market for chiral catalysts. As the agricultural industry moves towards more targeted and environmentally friendly pesticides and herbicides, the need for enantiopure compounds has grown. This shift has created new opportunities for chiral catalysts, with magnesium carbonate-based synthesis methods gaining attention due to their potential for producing more effective and less harmful agrochemicals.
In the fine chemicals industry, the demand for chiral catalysts is also on the rise. Manufacturers of flavors, fragrances, and specialty chemicals are increasingly seeking ways to produce enantiopure compounds efficiently. The use of magnesium carbonate in chiral catalyst synthesis offers a promising avenue for meeting this demand, as it can lead to more selective and efficient production processes.
The market for chiral catalysts is not limited to these industries alone. Emerging applications in materials science, such as the development of advanced polymers and liquid crystals, are opening up new avenues for chiral catalyst technologies. This diversification of applications is expected to further drive the demand for innovative chiral catalysts, including those involving magnesium carbonate in their synthesis.
Geographically, North America and Europe currently dominate the chiral technology market, but rapid growth is expected in Asia-Pacific regions, particularly in China and India. This regional expansion is driven by the growing pharmaceutical and chemical industries in these countries, coupled with increasing research and development activities in chiral technologies.
MgCO3 in Catalysis
Magnesium carbonate (MgCO3) has emerged as a significant component in the synthesis of chiral catalysts, playing a crucial role in advancing asymmetric catalysis. The use of MgCO3 in catalysis has gained attention due to its unique properties and versatile applications in various chemical transformations.
In the context of chiral catalyst synthesis, MgCO3 serves as a valuable precursor and support material. Its basic nature and high surface area make it an ideal candidate for immobilizing catalytically active species. The incorporation of MgCO3 into catalyst systems has been shown to enhance stereoselectivity and reaction efficiency in numerous asymmetric transformations.
One of the key advantages of using MgCO3 in chiral catalyst synthesis is its ability to act as a heterogeneous support. This property allows for easy separation and recycling of the catalyst, addressing sustainability concerns in chemical processes. Furthermore, the porous structure of MgCO3 provides a large surface area for catalyst immobilization, leading to improved catalytic activity and selectivity.
Recent studies have demonstrated the effectiveness of MgCO3-supported chiral catalysts in various organic transformations, including asymmetric hydrogenation, aldol reactions, and Michael additions. These catalysts have shown remarkable enantioselectivity and high turnover numbers, often surpassing their homogeneous counterparts in terms of efficiency and recyclability.
The role of MgCO3 extends beyond its function as a support material. In some cases, it actively participates in the catalytic cycle, acting as a co-catalyst or modifying the electronic properties of the active site. This dual functionality of MgCO3 has opened up new possibilities for designing highly efficient and selective chiral catalysts.
Moreover, the use of MgCO3 in chiral catalyst synthesis aligns well with the principles of green chemistry. Its abundance, low toxicity, and biodegradability make it an environmentally friendly alternative to traditional catalyst supports. This aspect has garnered significant interest from both academia and industry, driving further research into MgCO3-based catalytic systems.
The integration of MgCO3 into chiral catalyst design has also led to the development of novel synthetic methodologies. Researchers have explored various approaches to modify the surface of MgCO3 to enhance its catalytic properties, including doping with other metal ions and functionalization with organic ligands. These strategies have resulted in the creation of highly specialized catalysts tailored for specific asymmetric transformations.
In the context of chiral catalyst synthesis, MgCO3 serves as a valuable precursor and support material. Its basic nature and high surface area make it an ideal candidate for immobilizing catalytically active species. The incorporation of MgCO3 into catalyst systems has been shown to enhance stereoselectivity and reaction efficiency in numerous asymmetric transformations.
One of the key advantages of using MgCO3 in chiral catalyst synthesis is its ability to act as a heterogeneous support. This property allows for easy separation and recycling of the catalyst, addressing sustainability concerns in chemical processes. Furthermore, the porous structure of MgCO3 provides a large surface area for catalyst immobilization, leading to improved catalytic activity and selectivity.
Recent studies have demonstrated the effectiveness of MgCO3-supported chiral catalysts in various organic transformations, including asymmetric hydrogenation, aldol reactions, and Michael additions. These catalysts have shown remarkable enantioselectivity and high turnover numbers, often surpassing their homogeneous counterparts in terms of efficiency and recyclability.
The role of MgCO3 extends beyond its function as a support material. In some cases, it actively participates in the catalytic cycle, acting as a co-catalyst or modifying the electronic properties of the active site. This dual functionality of MgCO3 has opened up new possibilities for designing highly efficient and selective chiral catalysts.
Moreover, the use of MgCO3 in chiral catalyst synthesis aligns well with the principles of green chemistry. Its abundance, low toxicity, and biodegradability make it an environmentally friendly alternative to traditional catalyst supports. This aspect has garnered significant interest from both academia and industry, driving further research into MgCO3-based catalytic systems.
The integration of MgCO3 into chiral catalyst design has also led to the development of novel synthetic methodologies. Researchers have explored various approaches to modify the surface of MgCO3 to enhance its catalytic properties, including doping with other metal ions and functionalization with organic ligands. These strategies have resulted in the creation of highly specialized catalysts tailored for specific asymmetric transformations.
Current MgCO3 Methods
01 Magnesium carbonate in pharmaceutical compositions
Magnesium carbonate is used in various pharmaceutical compositions as an excipient or active ingredient. It can be utilized in antacid formulations, oral care products, and as a filler or binder in tablets and capsules. Its properties make it suitable for improving drug stability, controlling release rates, and enhancing bioavailability of certain medications.- Magnesium carbonate in pharmaceutical compositions: Magnesium carbonate is used in various pharmaceutical compositions as an excipient or active ingredient. It can be utilized in antacid formulations, oral care products, and as a filler or binder in tablets and capsules. Its properties make it suitable for improving drug stability, controlling release rates, and enhancing bioavailability of certain medications.
- Industrial applications of magnesium carbonate: Magnesium carbonate finds use in various industrial applications due to its unique properties. It is employed as a filler in rubber and plastic products, a whitening agent in paper production, and a component in fireproofing materials. Additionally, it serves as a raw material in the production of magnesium oxide and other magnesium compounds used in refractory materials and ceramics.
- Magnesium carbonate in food and beverage industry: In the food and beverage industry, magnesium carbonate is utilized as an anticaking agent, acidity regulator, and color retention agent. It is commonly used in salt products, powdered drinks, and dairy products to prevent clumping and improve flow properties. Its ability to neutralize acids makes it valuable in certain food processing applications.
- Magnesium carbonate in personal care and cosmetics: Magnesium carbonate is incorporated into various personal care and cosmetic products. It serves as an absorbent in deodorants and antiperspirants, a bulking agent in powders, and a pH adjuster in skincare formulations. Its mild abrasive properties make it suitable for use in certain exfoliating products and toothpastes.
- Environmental and agricultural applications of magnesium carbonate: Magnesium carbonate has applications in environmental remediation and agriculture. It is used in soil treatment to adjust pH levels and improve soil structure. In wastewater treatment, it can help remove heavy metals and other contaminants. Additionally, it is utilized in the production of slow-release fertilizers and as a feed supplement for livestock to provide essential magnesium.
02 Magnesium carbonate in personal care products
Magnesium carbonate finds applications in personal care products such as deodorants, antiperspirants, and cosmetics. It can act as an absorbent, pH regulator, and anti-caking agent. In these formulations, it helps control moisture, improve texture, and enhance the overall performance of the products.Expand Specific Solutions03 Industrial applications of magnesium carbonate
Magnesium carbonate is widely used in various industrial processes and products. It serves as a raw material in the production of magnesium oxide, as a filler in rubber and plastics, and as a flame retardant in certain materials. Additionally, it is utilized in the manufacturing of ceramics, glass, and as a component in construction materials.Expand Specific Solutions04 Magnesium carbonate in food and beverage applications
In the food and beverage industry, magnesium carbonate is employed as an additive and processing aid. It functions as an anticaking agent, acidity regulator, and color retention agent. It can be found in various products such as salt, dairy alternatives, and certain beverages to improve their quality and shelf life.Expand Specific Solutions05 Environmental and agricultural uses of magnesium carbonate
Magnesium carbonate has applications in environmental remediation and agriculture. It can be used for soil pH adjustment, as a fertilizer component, and in water treatment processes. In environmental applications, it aids in neutralizing acidic conditions and removing contaminants from water and soil.Expand Specific Solutions
Key Industry Players
The role of magnesium carbonate in chiral catalyst synthesis is an emerging field within the broader context of catalysis and green chemistry. The industry is in its early growth stage, with increasing research focus and potential for commercial applications. The global chiral catalyst market is projected to expand significantly, driven by demand in pharmaceutical and fine chemical sectors. Technologically, the field is rapidly evolving, with companies like Chiyoda Corp., Zhejiang Jiuzhou Pharmaceutical, and Pujing Chemical Industry leading innovation. Academic institutions such as Caltech, Zhejiang University, and Tianjin University are contributing to fundamental research, while collaborations between industry and academia are accelerating progress in this specialized area of catalysis.
Zhejiang University
Technical Solution: Zhejiang University has developed a groundbreaking approach to utilizing magnesium carbonate in chiral catalyst synthesis. Their research focuses on the development of chiral magnesium carbonate-based organocatalysts for asymmetric transformations. The university's team has successfully synthesized chiral magnesium carbonate complexes by incorporating chiral organic ligands into the carbonate structure[7]. These complexes have shown exceptional catalytic activity in asymmetric Michael additions and Diels-Alder reactions. Furthermore, they have explored the use of magnesium carbonate as a chiral modifier for heterogeneous metal catalysts, enhancing their enantioselectivity in hydrogenation reactions[9]. The university has also pioneered the use of magnesium carbonate-supported chiral ionic liquids as recyclable catalysts for various asymmetric transformations, demonstrating high efficiency and easy separation from reaction mixtures[11].
Strengths: Development of novel chiral magnesium carbonate-based organocatalysts, enhanced enantioselectivity in metal-catalyzed reactions, creation of recyclable chiral ionic liquid catalysts. Weaknesses: Potential sensitivity of organocatalysts to moisture and air, possible limitations in substrate scope for some reactions.
Tianjin University
Technical Solution: Tianjin University has made significant contributions to the field of chiral catalyst synthesis involving magnesium carbonate. Their research team has developed a novel approach using magnesium carbonate as a base and structure-directing agent in the synthesis of chiral metal-organic frameworks (MOFs)[2]. These MOFs serve as heterogeneous catalysts for asymmetric reactions. The university's method involves the in-situ formation of magnesium carbonate during the MOF synthesis, which helps control the crystal growth and imparts chirality to the framework. This approach has shown remarkable success in catalyzing asymmetric aldol reactions and Michael additions with high enantioselectivity[4]. Additionally, they have explored the use of magnesium carbonate-derived chiral nanoparticles as supports for immobilizing homogeneous chiral catalysts, resulting in improved catalyst recovery and reusability[6].
Strengths: Innovative use of magnesium carbonate in MOF synthesis, high enantioselectivity in various asymmetric reactions, improved catalyst recovery. Weaknesses: Potential challenges in large-scale production of chiral MOFs, possible limitations in the scope of applicable reactions.
Innovative MgCO3 Uses
Chiral compound suitable as a catalyst for asymmetric transfer hydrogenation
PatentActiveEP1824868B1
Innovation
- A chiral compound with a transition metal M, comprising four, five, or six coordinating groups, where at least one pair forms a bidentate ligand linked to an aromatic ring or amino group, allowing for easy synthesis and high enantiomeric purity, particularly using cyclometallated ligands with metals like iron, ruthenium, or osmium.
Method for the preparation of chiral beta-fluoroalkylated carbonyl compounds using chiral catalyst
PatentActiveKR1020110039826A
Innovation
- A method involving the reaction of an alpha, beta-unsaturated ketone compound with 1-fluorobis(phenylsulfonyl)methane in the presence of a chiral catalyst, specifically a chiral bifunctional organic catalyst with a chiral primary amine and tertiary amine group, to produce beta-fluoroalkylated carbonyl compounds.
Green Chemistry Impact
The incorporation of magnesium carbonate in chiral catalyst synthesis has significant implications for green chemistry, aligning with the principles of sustainability and environmental responsibility. This approach contributes to the development of more eco-friendly catalytic processes, reducing the environmental impact of chemical manufacturing.
Magnesium carbonate, as a readily available and non-toxic compound, serves as an excellent base in various catalytic reactions. Its use in chiral catalyst synthesis promotes the principles of atom economy and waste reduction, key tenets of green chemistry. By utilizing this abundant and benign material, researchers can minimize the reliance on more hazardous or resource-intensive alternatives.
The role of magnesium carbonate extends beyond its function as a base. It can act as a template or scaffold in the synthesis of chiral catalysts, facilitating the formation of specific three-dimensional structures crucial for enantioselective reactions. This structural control enables the design of more efficient catalysts, potentially reducing reaction times and energy requirements in chemical processes.
Furthermore, the use of magnesium carbonate in chiral catalyst synthesis often allows for milder reaction conditions. This aspect aligns with green chemistry's emphasis on energy efficiency and the reduction of auxiliary substances. By operating under less harsh conditions, these catalytic systems can contribute to safer and more sustainable chemical manufacturing processes.
The recyclability of magnesium carbonate-based catalysts is another significant green chemistry benefit. Many of these catalysts can be recovered and reused multiple times without significant loss of activity or selectivity. This characteristic not only reduces waste but also enhances the overall efficiency and cost-effectiveness of catalytic processes.
In the context of life cycle assessment, the use of magnesium carbonate in chiral catalyst synthesis generally results in a lower environmental footprint compared to traditional methods. The reduced reliance on rare or toxic metals, coupled with the potential for catalyst recycling, contributes to a more sustainable approach in asymmetric synthesis.
The green chemistry impact of magnesium carbonate in chiral catalyst synthesis also extends to the realm of biocatalysis. By mimicking natural enzymatic processes, these catalysts can facilitate more environmentally benign routes to complex chiral molecules, particularly in the pharmaceutical and fine chemical industries.
Magnesium carbonate, as a readily available and non-toxic compound, serves as an excellent base in various catalytic reactions. Its use in chiral catalyst synthesis promotes the principles of atom economy and waste reduction, key tenets of green chemistry. By utilizing this abundant and benign material, researchers can minimize the reliance on more hazardous or resource-intensive alternatives.
The role of magnesium carbonate extends beyond its function as a base. It can act as a template or scaffold in the synthesis of chiral catalysts, facilitating the formation of specific three-dimensional structures crucial for enantioselective reactions. This structural control enables the design of more efficient catalysts, potentially reducing reaction times and energy requirements in chemical processes.
Furthermore, the use of magnesium carbonate in chiral catalyst synthesis often allows for milder reaction conditions. This aspect aligns with green chemistry's emphasis on energy efficiency and the reduction of auxiliary substances. By operating under less harsh conditions, these catalytic systems can contribute to safer and more sustainable chemical manufacturing processes.
The recyclability of magnesium carbonate-based catalysts is another significant green chemistry benefit. Many of these catalysts can be recovered and reused multiple times without significant loss of activity or selectivity. This characteristic not only reduces waste but also enhances the overall efficiency and cost-effectiveness of catalytic processes.
In the context of life cycle assessment, the use of magnesium carbonate in chiral catalyst synthesis generally results in a lower environmental footprint compared to traditional methods. The reduced reliance on rare or toxic metals, coupled with the potential for catalyst recycling, contributes to a more sustainable approach in asymmetric synthesis.
The green chemistry impact of magnesium carbonate in chiral catalyst synthesis also extends to the realm of biocatalysis. By mimicking natural enzymatic processes, these catalysts can facilitate more environmentally benign routes to complex chiral molecules, particularly in the pharmaceutical and fine chemical industries.
Scalability Challenges
The scalability of chiral catalyst synthesis involving magnesium carbonate presents several significant challenges that must be addressed for industrial-scale production. One of the primary issues is the heterogeneous nature of the reaction system, which can lead to inconsistent product quality and yield when scaling up from laboratory to industrial levels.
The particle size and distribution of magnesium carbonate play a crucial role in the synthesis process. As the scale increases, maintaining uniform particle characteristics becomes increasingly difficult, potentially affecting the catalytic activity and selectivity of the final product. This variability can result in batch-to-batch inconsistencies, a major concern for large-scale manufacturing.
Temperature control is another critical factor that becomes more complex at larger scales. The exothermic nature of some reactions involving magnesium carbonate can lead to localized hot spots in large reactors, potentially causing unwanted side reactions or degradation of the catalyst. Implementing effective heat transfer systems that can maintain precise temperature control throughout the entire reaction vessel is a significant engineering challenge.
Mass transfer limitations also become more pronounced in larger reactors. The efficient mixing of reagents and the uniform distribution of magnesium carbonate throughout the reaction medium are essential for optimal catalyst synthesis. As reactor size increases, achieving homogeneous mixing becomes more difficult, potentially leading to reduced reaction rates and lower overall yields.
The recovery and purification of the chiral catalyst from the reaction mixture present additional scalability challenges. Separation techniques that work well at laboratory scale may not be as effective or economically viable when applied to industrial-scale production. Developing efficient and cost-effective purification methods that can handle large volumes while maintaining high product purity is crucial for commercial viability.
Environmental and safety considerations also become more prominent at larger scales. The handling and disposal of large quantities of magnesium carbonate and other reagents require careful planning and implementation of appropriate safety measures. Additionally, the potential for dust formation during handling of dry magnesium carbonate necessitates the development of robust containment and filtration systems to protect workers and the environment.
Lastly, the economic aspects of scaling up chiral catalyst synthesis using magnesium carbonate must be carefully evaluated. The cost of raw materials, energy consumption, and capital investment in specialized equipment all contribute to the overall production costs. Optimizing these factors while maintaining product quality and meeting regulatory requirements is essential for the commercial success of large-scale chiral catalyst production.
The particle size and distribution of magnesium carbonate play a crucial role in the synthesis process. As the scale increases, maintaining uniform particle characteristics becomes increasingly difficult, potentially affecting the catalytic activity and selectivity of the final product. This variability can result in batch-to-batch inconsistencies, a major concern for large-scale manufacturing.
Temperature control is another critical factor that becomes more complex at larger scales. The exothermic nature of some reactions involving magnesium carbonate can lead to localized hot spots in large reactors, potentially causing unwanted side reactions or degradation of the catalyst. Implementing effective heat transfer systems that can maintain precise temperature control throughout the entire reaction vessel is a significant engineering challenge.
Mass transfer limitations also become more pronounced in larger reactors. The efficient mixing of reagents and the uniform distribution of magnesium carbonate throughout the reaction medium are essential for optimal catalyst synthesis. As reactor size increases, achieving homogeneous mixing becomes more difficult, potentially leading to reduced reaction rates and lower overall yields.
The recovery and purification of the chiral catalyst from the reaction mixture present additional scalability challenges. Separation techniques that work well at laboratory scale may not be as effective or economically viable when applied to industrial-scale production. Developing efficient and cost-effective purification methods that can handle large volumes while maintaining high product purity is crucial for commercial viability.
Environmental and safety considerations also become more prominent at larger scales. The handling and disposal of large quantities of magnesium carbonate and other reagents require careful planning and implementation of appropriate safety measures. Additionally, the potential for dust formation during handling of dry magnesium carbonate necessitates the development of robust containment and filtration systems to protect workers and the environment.
Lastly, the economic aspects of scaling up chiral catalyst synthesis using magnesium carbonate must be carefully evaluated. The cost of raw materials, energy consumption, and capital investment in specialized equipment all contribute to the overall production costs. Optimizing these factors while maintaining product quality and meeting regulatory requirements is essential for the commercial success of large-scale chiral catalyst production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!