SERS Substrates Effects on Electrochemical Reaction Dynamics
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SERS Technology Background and Objectives
Surface-Enhanced Raman Spectroscopy (SERS) emerged in the late 1970s when researchers observed anomalously intense Raman signals from molecules adsorbed on roughened silver electrodes. This discovery revolutionized analytical chemistry by enabling detection sensitivity at the single-molecule level. The evolution of SERS technology has been marked by significant advancements in substrate design, moving from simple roughened metal surfaces to sophisticated nanostructured materials engineered for maximum enhancement.
The fundamental principle behind SERS involves two primary enhancement mechanisms: electromagnetic enhancement from localized surface plasmon resonances and chemical enhancement from charge transfer between the substrate and analyte molecules. These mechanisms collectively amplify Raman signals by factors of 10^6 to 10^11, enabling unprecedented detection capabilities for trace analysis.
Recent technological trends have focused on developing reproducible, stable, and highly sensitive SERS substrates that can be integrated with electrochemical systems. This integration has opened new avenues for in-situ monitoring of electrochemical reactions, allowing researchers to observe reaction intermediates and dynamics that were previously undetectable using conventional analytical methods.
The intersection of SERS and electrochemistry represents a particularly promising frontier. Electrochemical SERS (EC-SERS) combines the molecular specificity of Raman spectroscopy with the ability to control and monitor electron transfer processes. This synergy enables real-time observation of redox reactions, catalytic processes, and interfacial phenomena at the electrode-electrolyte interface.
The primary objective of investigating SERS substrates effects on electrochemical reaction dynamics is to develop a comprehensive understanding of how substrate properties—including material composition, nanostructure morphology, surface chemistry, and plasmonic characteristics—influence both the spectroscopic enhancement and the electrochemical behavior of the system. This knowledge is crucial for designing next-generation sensors and catalysts with tailored properties.
Additional goals include establishing quantitative relationships between substrate parameters and reaction kinetics, identifying optimal substrate configurations for specific electrochemical applications, and developing standardized methodologies for EC-SERS measurements. These advances would address current challenges in reproducibility and comparability across different research platforms.
The ultimate technological objective is to translate fundamental insights into practical applications, including real-time monitoring of energy conversion devices, development of ultrasensitive biosensors, and creation of advanced analytical tools for environmental monitoring and pharmaceutical quality control. Success in this domain could significantly impact fields ranging from renewable energy to medical diagnostics.
The fundamental principle behind SERS involves two primary enhancement mechanisms: electromagnetic enhancement from localized surface plasmon resonances and chemical enhancement from charge transfer between the substrate and analyte molecules. These mechanisms collectively amplify Raman signals by factors of 10^6 to 10^11, enabling unprecedented detection capabilities for trace analysis.
Recent technological trends have focused on developing reproducible, stable, and highly sensitive SERS substrates that can be integrated with electrochemical systems. This integration has opened new avenues for in-situ monitoring of electrochemical reactions, allowing researchers to observe reaction intermediates and dynamics that were previously undetectable using conventional analytical methods.
The intersection of SERS and electrochemistry represents a particularly promising frontier. Electrochemical SERS (EC-SERS) combines the molecular specificity of Raman spectroscopy with the ability to control and monitor electron transfer processes. This synergy enables real-time observation of redox reactions, catalytic processes, and interfacial phenomena at the electrode-electrolyte interface.
The primary objective of investigating SERS substrates effects on electrochemical reaction dynamics is to develop a comprehensive understanding of how substrate properties—including material composition, nanostructure morphology, surface chemistry, and plasmonic characteristics—influence both the spectroscopic enhancement and the electrochemical behavior of the system. This knowledge is crucial for designing next-generation sensors and catalysts with tailored properties.
Additional goals include establishing quantitative relationships between substrate parameters and reaction kinetics, identifying optimal substrate configurations for specific electrochemical applications, and developing standardized methodologies for EC-SERS measurements. These advances would address current challenges in reproducibility and comparability across different research platforms.
The ultimate technological objective is to translate fundamental insights into practical applications, including real-time monitoring of energy conversion devices, development of ultrasensitive biosensors, and creation of advanced analytical tools for environmental monitoring and pharmaceutical quality control. Success in this domain could significantly impact fields ranging from renewable energy to medical diagnostics.
Market Applications and Demand Analysis for SERS
The Surface-Enhanced Raman Spectroscopy (SERS) market has experienced significant growth in recent years, driven by increasing demand for highly sensitive analytical techniques across multiple industries. The global SERS market was valued at approximately $103 million in 2020 and is projected to reach $257 million by 2025, representing a compound annual growth rate of 20.1% during this period.
Healthcare and pharmaceutical sectors constitute the largest application segments for SERS technology, accounting for nearly 40% of the total market share. Within these sectors, SERS is predominantly utilized for biomarker detection, drug discovery, and disease diagnosis. The ability of SERS substrates to enhance electrochemical reaction dynamics has proven particularly valuable for real-time monitoring of biological processes and drug interactions at the molecular level.
Food safety and environmental monitoring represent rapidly expanding application areas, collectively comprising about 25% of the market. Regulatory agencies worldwide have increasingly adopted SERS-based techniques for detecting contaminants, pesticides, and pathogens in food products and environmental samples. The sensitivity of SERS substrates in electrochemical applications allows for detection limits in the parts-per-billion range, meeting stringent regulatory requirements.
The academic research segment, while smaller in commercial value (approximately 15% of the market), drives significant innovation in SERS applications. Universities and research institutions continue to explore novel substrate designs that optimize electrochemical reaction dynamics, expanding the potential application landscape for this technology.
Industrial applications, including chemical process monitoring and quality control, represent about 20% of the current market. These sectors value SERS for its non-destructive nature and ability to provide real-time analysis of reaction kinetics and product formation.
Geographically, North America leads the SERS market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and South Korea, particularly in electrochemical SERS applications.
Customer demand increasingly focuses on substrate reproducibility, stability, and cost-effectiveness. End-users seek SERS substrates that maintain consistent enhancement factors under varying electrochemical conditions. Additionally, there is growing interest in portable and field-deployable SERS systems that can perform reliable electrochemical measurements outside laboratory environments, particularly in point-of-care diagnostics and on-site environmental monitoring applications.
Healthcare and pharmaceutical sectors constitute the largest application segments for SERS technology, accounting for nearly 40% of the total market share. Within these sectors, SERS is predominantly utilized for biomarker detection, drug discovery, and disease diagnosis. The ability of SERS substrates to enhance electrochemical reaction dynamics has proven particularly valuable for real-time monitoring of biological processes and drug interactions at the molecular level.
Food safety and environmental monitoring represent rapidly expanding application areas, collectively comprising about 25% of the market. Regulatory agencies worldwide have increasingly adopted SERS-based techniques for detecting contaminants, pesticides, and pathogens in food products and environmental samples. The sensitivity of SERS substrates in electrochemical applications allows for detection limits in the parts-per-billion range, meeting stringent regulatory requirements.
The academic research segment, while smaller in commercial value (approximately 15% of the market), drives significant innovation in SERS applications. Universities and research institutions continue to explore novel substrate designs that optimize electrochemical reaction dynamics, expanding the potential application landscape for this technology.
Industrial applications, including chemical process monitoring and quality control, represent about 20% of the current market. These sectors value SERS for its non-destructive nature and ability to provide real-time analysis of reaction kinetics and product formation.
Geographically, North America leads the SERS market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and South Korea, particularly in electrochemical SERS applications.
Customer demand increasingly focuses on substrate reproducibility, stability, and cost-effectiveness. End-users seek SERS substrates that maintain consistent enhancement factors under varying electrochemical conditions. Additionally, there is growing interest in portable and field-deployable SERS systems that can perform reliable electrochemical measurements outside laboratory environments, particularly in point-of-care diagnostics and on-site environmental monitoring applications.
Current SERS Substrate Challenges
Despite significant advancements in Surface-Enhanced Raman Spectroscopy (SERS) substrate development, several critical challenges persist that limit their widespread application in electrochemical reaction dynamics studies. The fundamental issue remains achieving consistent enhancement factors across the substrate surface. Current fabrication methods often produce "hot spots" with varying enhancement capabilities, resulting in signal inconsistency that complicates quantitative analysis of electrochemical processes.
Stability presents another major challenge, particularly in electrochemical environments. Many high-performance SERS substrates degrade rapidly under applied potentials or in electrolyte solutions, limiting their usefulness for in-situ monitoring of reaction dynamics. Noble metal substrates, while offering excellent enhancement, often undergo surface restructuring during electrochemical reactions, altering their plasmonic properties and consequently their enhancement capabilities.
Reproducibility in manufacturing remains problematic at scale. Laboratory-produced substrates often demonstrate excellent performance but translating these results to mass production while maintaining consistent quality presents significant engineering challenges. This manufacturing inconsistency creates barriers for systematic studies requiring multiple comparable measurements across different experimental conditions.
Biocompatibility and fouling resistance represent emerging concerns as SERS applications expand into biological systems. Current substrates frequently experience performance degradation due to non-specific adsorption of biomolecules, limiting their effectiveness in complex biological matrices where many electrochemical processes of interest occur.
Cost considerations further constrain widespread adoption. High-performance SERS substrates typically rely on expensive noble metals and sophisticated nanofabrication techniques, making routine application economically unfeasible for many research groups and industrial applications. This cost barrier significantly limits the technology's accessibility.
Signal specificity presents technical challenges in complex reaction environments. Distinguishing between target analyte signals and background contributions from electrolyte species or reaction intermediates remains difficult with current substrate designs. This signal-to-noise limitation restricts the detection sensitivity crucial for monitoring low-concentration intermediates in electrochemical reaction pathways.
Integration with existing electrochemical instrumentation poses practical challenges. Many high-performance SERS substrates are not readily compatible with standard electrochemical cells or require specialized equipment, limiting their practical utility in real-world applications. The development of standardized, easily integrated SERS-electrochemical platforms remains an unmet need in the field.
Stability presents another major challenge, particularly in electrochemical environments. Many high-performance SERS substrates degrade rapidly under applied potentials or in electrolyte solutions, limiting their usefulness for in-situ monitoring of reaction dynamics. Noble metal substrates, while offering excellent enhancement, often undergo surface restructuring during electrochemical reactions, altering their plasmonic properties and consequently their enhancement capabilities.
Reproducibility in manufacturing remains problematic at scale. Laboratory-produced substrates often demonstrate excellent performance but translating these results to mass production while maintaining consistent quality presents significant engineering challenges. This manufacturing inconsistency creates barriers for systematic studies requiring multiple comparable measurements across different experimental conditions.
Biocompatibility and fouling resistance represent emerging concerns as SERS applications expand into biological systems. Current substrates frequently experience performance degradation due to non-specific adsorption of biomolecules, limiting their effectiveness in complex biological matrices where many electrochemical processes of interest occur.
Cost considerations further constrain widespread adoption. High-performance SERS substrates typically rely on expensive noble metals and sophisticated nanofabrication techniques, making routine application economically unfeasible for many research groups and industrial applications. This cost barrier significantly limits the technology's accessibility.
Signal specificity presents technical challenges in complex reaction environments. Distinguishing between target analyte signals and background contributions from electrolyte species or reaction intermediates remains difficult with current substrate designs. This signal-to-noise limitation restricts the detection sensitivity crucial for monitoring low-concentration intermediates in electrochemical reaction pathways.
Integration with existing electrochemical instrumentation poses practical challenges. Many high-performance SERS substrates are not readily compatible with standard electrochemical cells or require specialized equipment, limiting their practical utility in real-world applications. The development of standardized, easily integrated SERS-electrochemical platforms remains an unmet need in the field.
Current SERS-Electrochemistry Interface Solutions
01 SERS substrate design and fabrication methods
Various methods for designing and fabricating SERS substrates to enhance Raman signals. These include nanostructured surfaces, metallic nanoparticles, and specialized coatings that create electromagnetic hotspots. The fabrication techniques focus on creating reproducible substrates with high enhancement factors and stability for consistent reaction monitoring.- SERS substrate design and fabrication: Various methods for designing and fabricating SERS substrates with enhanced sensitivity and reproducibility. These include nanostructured surfaces, metallic nanoparticles, and patterned arrays that provide consistent hot spots for signal enhancement. Advanced fabrication techniques enable precise control over substrate geometry and surface properties to optimize SERS performance for reaction dynamics studies.
- Real-time monitoring of reaction kinetics: SERS substrates designed specifically for monitoring chemical reactions in real-time, allowing researchers to observe reaction dynamics and kinetics at the molecular level. These systems incorporate microfluidic channels, temperature control mechanisms, and specialized detection setups to capture spectral changes during reactions with high temporal resolution, providing insights into reaction mechanisms and intermediate states.
- Plasmonic enhancement mechanisms: Investigation of plasmonic enhancement mechanisms that govern SERS substrate performance in reaction dynamics studies. This includes understanding how electromagnetic field enhancement occurs at metal-molecule interfaces, the role of hot electrons in catalytic processes, and how plasmon resonances can be tuned to specific reaction conditions. These mechanisms are crucial for designing substrates that can effectively monitor chemical transformations.
- Catalytic SERS substrates: Development of dual-function SERS substrates that simultaneously serve as catalysts and sensing platforms for reaction dynamics. These substrates incorporate catalytically active materials alongside plasmonic metals to enable in-situ monitoring of surface reactions. The design allows researchers to correlate spectroscopic signals with catalytic activity, providing fundamental insights into reaction mechanisms at catalytic interfaces.
- Advanced data analysis for reaction dynamics: Sophisticated data analysis methods for extracting meaningful reaction dynamics information from SERS measurements. These include machine learning algorithms, multivariate statistical approaches, and time-resolved spectral processing techniques that can identify reaction intermediates, determine rate constants, and elucidate reaction pathways from complex SERS datasets. These computational tools are essential for interpreting the wealth of information contained in dynamic SERS measurements.
02 Real-time monitoring of reaction dynamics using SERS
Systems and methods for real-time monitoring of chemical reactions and catalytic processes using SERS. These approaches allow for in-situ observation of reaction intermediates, kinetics, and mechanisms at the molecular level. The techniques provide insights into transient species and reaction pathways that are difficult to detect with conventional analytical methods.Expand Specific Solutions03 Plasmonic nanomaterials for enhanced SERS detection
Development of plasmonic nanomaterials specifically designed for SERS applications. These materials include gold and silver nanostructures with optimized shapes and sizes to maximize electromagnetic field enhancement. The plasmonic properties are tailored to specific wavelengths and reaction conditions to improve sensitivity and selectivity in detecting reaction dynamics.Expand Specific Solutions04 Integration of SERS with microfluidic systems
Integration of SERS substrates with microfluidic platforms for controlled reaction environments. These systems enable precise manipulation of reagents, temperature control, and continuous monitoring of reactions in confined spaces. The combination allows for high-throughput screening of reaction conditions while minimizing sample volumes and providing spatially resolved information about reaction dynamics.Expand Specific Solutions05 Data analysis and computational methods for SERS reaction dynamics
Advanced computational and data analysis methods for interpreting SERS spectra in reaction dynamics studies. These include machine learning algorithms, chemometric approaches, and theoretical modeling to extract meaningful information from complex spectral data. The methods help in identifying reaction intermediates, determining rate constants, and elucidating reaction mechanisms from time-resolved SERS measurements.Expand Specific Solutions
Leading Research Groups and Companies in SERS Field
The SERS substrates market for electrochemical reaction dynamics is currently in a growth phase, characterized by increasing research activity across academic and industrial sectors. The market size is expanding as SERS technology finds applications in chemical sensing, biomedical diagnostics, and environmental monitoring. From a technological maturity perspective, the field shows varying degrees of development among key players. Academic institutions like University of South Carolina, Jilin University, and Texas A&M University are driving fundamental research, while companies such as Intel Corp., SICPA Holding SA, and Becton, Dickinson & Co. are focusing on commercial applications. Government research entities including the Naval Research Laboratory and National Research Council of Canada are bridging the gap between theoretical advancements and practical implementations, creating a competitive landscape where collaboration between sectors is increasingly important for innovation in SERS substrate technology.
University of South Carolina
Technical Solution: The University of South Carolina has developed proprietary SERS substrate technologies specifically optimized for electrochemical reaction monitoring. Their approach utilizes nanopatterned gold and silver surfaces with controlled roughness to create reproducible hot spots for SERS enhancement. The university's research teams have pioneered dual-function substrates that serve both as working electrodes and SERS-active surfaces, enabling direct correlation between electrochemical signals and molecular vibrational information. Their technology incorporates unique surface functionalization strategies that maintain SERS activity while allowing selective binding of target analytes in complex electrochemical environments. Recent innovations include the development of flow-cell configurations that permit real-time monitoring of electrochemical reaction intermediates with sub-second temporal resolution, providing unprecedented insights into reaction pathways and kinetics. The university has also demonstrated the application of these substrates in energy conversion systems, particularly for understanding oxygen reduction reaction mechanisms.
Strengths: Excellent reproducibility and stability under varying electrochemical conditions, high sensitivity for detecting reaction intermediates, and versatile platform adaptable to different electrochemical systems. Weaknesses: Limited commercial availability of the specialized substrates and relatively high production costs compared to conventional electrodes.
Jilin University
Technical Solution: Jilin University has developed advanced SERS substrates utilizing hierarchical nanostructures with precisely controlled morphology for enhanced electrochemical reaction monitoring. Their technology combines gold-silver core-shell nanoparticles with graphene oxide supports to create high-performance SERS platforms that enable simultaneous electrochemical measurements and spectroscopic detection. The university's research teams have pioneered the development of in-situ SERS monitoring systems that can track electrochemical reaction intermediates with temporal resolution below 1 second, allowing for real-time observation of reaction dynamics. Their substrates demonstrate enhancement factors exceeding 10^7, significantly improving detection sensitivity for trace electroactive species. Recent innovations include the integration of machine learning algorithms to correlate SERS spectral changes with electrochemical parameters, enabling more accurate interpretation of complex reaction mechanisms.
Strengths: Exceptional signal enhancement capabilities, high temporal resolution for dynamic studies, and excellent reproducibility across batches. Weaknesses: Relatively complex fabrication process requiring specialized equipment, and potential challenges in mass production for commercial applications.
Key SERS Substrate Enhancement Mechanisms
Surface enhanced raman spectroscopy (SERS) substrates exhibiting uniform high enhancement and stability
PatentWO2006137885A8
Innovation
- The development of porous metal substrates, such as gold-silver alloy films that are acid-etched or electrochemically roughened, providing uniform and high enhancement factors for SERS, with methods like sputter deposition and electrochemical roughening to enhance substrate properties.
Surface enhanced Raman spectroscopy using shaped gold nanoparticles
PatentInactiveUS8241922B2
Innovation
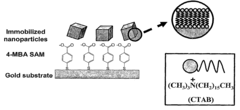
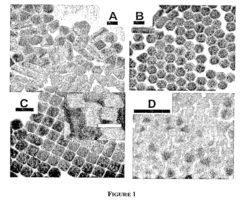
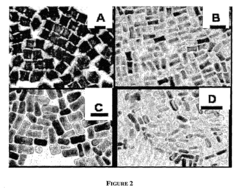
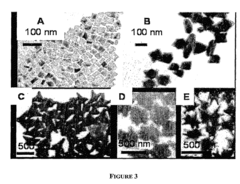
- A method for preparing cetyltrimethylammonium bromide-capped gold nanoparticles of specific shapes, such as cubes, rods, and tetrapods, is developed, which are immobilized on self-assembled monolayers to enhance Raman signals by overlapping excitation wavelengths with their plasmon resonance bands, achieving significant signal enhancement factors.
Materials Science Considerations for SERS Substrates
The selection of appropriate materials for Surface-Enhanced Raman Spectroscopy (SERS) substrates is critical for optimizing electrochemical reaction dynamics studies. Noble metals, particularly gold and silver, remain the predominant materials due to their exceptional plasmonic properties in the visible to near-infrared spectral range. These metals exhibit strong localized surface plasmon resonance (LSPR) effects, which significantly enhance the electromagnetic field near the substrate surface, resulting in Raman signal amplification factors of 10^6 to 10^10.
Material morphology plays a decisive role in SERS performance. Nanostructured surfaces with controlled roughness, including nanospheres, nanorods, nanostars, and hierarchical structures, create "hot spots" where electromagnetic fields are concentrated. Recent advances in nanofabrication techniques have enabled precise control over these morphological features, allowing researchers to tailor substrates for specific electrochemical applications.
The interface between the SERS substrate and the electrolyte solution represents a critical consideration for electrochemical studies. Surface chemistry modifications, including functionalization with self-assembled monolayers (SAMs), polymers, or biomolecules, can significantly alter the substrate's interaction with target analytes. These modifications influence not only signal enhancement but also the selectivity and stability of the substrate during electrochemical processes.
Stability under electrochemical conditions presents a significant materials science challenge. Substrate degradation through oxidation, dissolution, or restructuring during potential cycling can compromise measurement reliability. Protective coatings, such as ultrathin graphene or metal oxide layers, have emerged as promising solutions to enhance durability while maintaining enhancement capabilities.
Composite SERS substrates, incorporating multiple materials, have gained attention for electrochemical applications. Metal-semiconductor hybrids (Au-TiO2, Ag-ZnO) and metal-carbon nanomaterial composites (Au-graphene, Ag-carbon nanotubes) offer synergistic benefits, including enhanced stability, tunable plasmonic properties, and improved charge transfer characteristics during electrochemical reactions.
Manufacturing scalability and reproducibility remain significant considerations for practical applications. While techniques like electron-beam lithography offer precise control over nanostructure geometry, they face limitations in production scale. Alternative approaches, including chemical synthesis methods, nanoimprint lithography, and self-assembly techniques, present promising pathways for large-scale, cost-effective production of SERS substrates with consistent enhancement factors across the substrate surface.
Material morphology plays a decisive role in SERS performance. Nanostructured surfaces with controlled roughness, including nanospheres, nanorods, nanostars, and hierarchical structures, create "hot spots" where electromagnetic fields are concentrated. Recent advances in nanofabrication techniques have enabled precise control over these morphological features, allowing researchers to tailor substrates for specific electrochemical applications.
The interface between the SERS substrate and the electrolyte solution represents a critical consideration for electrochemical studies. Surface chemistry modifications, including functionalization with self-assembled monolayers (SAMs), polymers, or biomolecules, can significantly alter the substrate's interaction with target analytes. These modifications influence not only signal enhancement but also the selectivity and stability of the substrate during electrochemical processes.
Stability under electrochemical conditions presents a significant materials science challenge. Substrate degradation through oxidation, dissolution, or restructuring during potential cycling can compromise measurement reliability. Protective coatings, such as ultrathin graphene or metal oxide layers, have emerged as promising solutions to enhance durability while maintaining enhancement capabilities.
Composite SERS substrates, incorporating multiple materials, have gained attention for electrochemical applications. Metal-semiconductor hybrids (Au-TiO2, Ag-ZnO) and metal-carbon nanomaterial composites (Au-graphene, Ag-carbon nanotubes) offer synergistic benefits, including enhanced stability, tunable plasmonic properties, and improved charge transfer characteristics during electrochemical reactions.
Manufacturing scalability and reproducibility remain significant considerations for practical applications. While techniques like electron-beam lithography offer precise control over nanostructure geometry, they face limitations in production scale. Alternative approaches, including chemical synthesis methods, nanoimprint lithography, and self-assembly techniques, present promising pathways for large-scale, cost-effective production of SERS substrates with consistent enhancement factors across the substrate surface.
Reproducibility and Standardization Issues
One of the most significant challenges in SERS-based electrochemical studies is the lack of reproducibility across experiments and laboratories. The variability in SERS substrate preparation methods creates substantial inconsistencies in signal enhancement factors, which can range from 10^4 to 10^10 depending on the substrate characteristics. This wide variation undermines the reliability of quantitative analyses and hinders the broader adoption of SERS techniques in electrochemical reaction dynamics research.
Manufacturing processes for SERS substrates often suffer from batch-to-batch variations, even when produced by the same laboratory using identical protocols. These inconsistencies arise from subtle differences in nanostructure morphology, surface roughness, and metal deposition parameters that significantly impact the electromagnetic enhancement mechanisms. Commercial SERS substrates, while offering improved consistency compared to laboratory-fabricated alternatives, still exhibit performance variations that complicate cross-study comparisons.
The absence of universally accepted standardization protocols further exacerbates reproducibility issues. Currently, there is no consensus on reference materials or calibration procedures for SERS measurements in electrochemical environments. This lack of standardization makes it difficult to normalize results across different experimental setups and prevents meaningful meta-analyses of published data. Several international organizations, including ASTM International and IUPAC, have initiated efforts to develop standardized protocols, but these remain in preliminary stages.
Environmental factors introduce additional variability in SERS measurements during electrochemical reactions. Temperature fluctuations, solution pH, ionic strength, and dissolved oxygen levels can all affect both the SERS enhancement and the electrochemical processes being studied. These parameters are often inadequately controlled or reported in literature, further complicating reproducibility efforts.
Recent advances in machine learning and automated fabrication techniques offer promising approaches to address these challenges. AI-driven quality control systems can identify and compensate for substrate variations, while robotics-assisted manufacturing processes are improving consistency in substrate production. Additionally, the development of internal standard methodologies, where known reference molecules are co-adsorbed with the analytes of interest, provides a pathway to normalize enhancement factors across different experimental conditions.
Collaborative initiatives between academic institutions and industry partners are emerging to establish shared databases of SERS substrate characteristics and performance metrics. These efforts aim to create a standardized framework for reporting experimental conditions and results, potentially transforming the field by enabling more reliable comparisons between studies conducted across different laboratories and time periods.
Manufacturing processes for SERS substrates often suffer from batch-to-batch variations, even when produced by the same laboratory using identical protocols. These inconsistencies arise from subtle differences in nanostructure morphology, surface roughness, and metal deposition parameters that significantly impact the electromagnetic enhancement mechanisms. Commercial SERS substrates, while offering improved consistency compared to laboratory-fabricated alternatives, still exhibit performance variations that complicate cross-study comparisons.
The absence of universally accepted standardization protocols further exacerbates reproducibility issues. Currently, there is no consensus on reference materials or calibration procedures for SERS measurements in electrochemical environments. This lack of standardization makes it difficult to normalize results across different experimental setups and prevents meaningful meta-analyses of published data. Several international organizations, including ASTM International and IUPAC, have initiated efforts to develop standardized protocols, but these remain in preliminary stages.
Environmental factors introduce additional variability in SERS measurements during electrochemical reactions. Temperature fluctuations, solution pH, ionic strength, and dissolved oxygen levels can all affect both the SERS enhancement and the electrochemical processes being studied. These parameters are often inadequately controlled or reported in literature, further complicating reproducibility efforts.
Recent advances in machine learning and automated fabrication techniques offer promising approaches to address these challenges. AI-driven quality control systems can identify and compensate for substrate variations, while robotics-assisted manufacturing processes are improving consistency in substrate production. Additionally, the development of internal standard methodologies, where known reference molecules are co-adsorbed with the analytes of interest, provides a pathway to normalize enhancement factors across different experimental conditions.
Collaborative initiatives between academic institutions and industry partners are emerging to establish shared databases of SERS substrate characteristics and performance metrics. These efforts aim to create a standardized framework for reporting experimental conditions and results, potentially transforming the field by enabling more reliable comparisons between studies conducted across different laboratories and time periods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!