The potential of neuromorphic computing in personalized medicine.
SEP 3, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neuromorphic Computing Evolution and Medical Applications
Neuromorphic computing represents a revolutionary approach to computational architecture, drawing inspiration from the structure and function of biological neural systems. This paradigm shift from traditional von Neumann architectures has evolved significantly since its conceptual inception in the late 1980s by Carver Mead. The evolution trajectory has moved from basic analog circuit implementations to sophisticated neuromorphic chips capable of mimicking various aspects of neural processing, including spike-timing-dependent plasticity and parallel information processing.
In the medical domain, particularly personalized medicine, neuromorphic computing offers unprecedented capabilities for processing complex, heterogeneous patient data. The integration of neuromorphic systems in healthcare has progressed from experimental applications to practical implementations in diagnostic tools and treatment optimization platforms. This evolution has been accelerated by advances in materials science, nanotechnology, and our deepening understanding of neural computation principles.
The application landscape in personalized medicine has expanded dramatically, with neuromorphic systems now being employed for genomic data analysis, medical imaging interpretation, drug response prediction, and real-time patient monitoring. These systems excel at identifying subtle patterns in patient data that might escape traditional analytical methods, potentially revealing new biomarkers and treatment targets.
Recent developments have seen neuromorphic computing platforms integrated with other emerging technologies such as quantum computing and advanced AI frameworks, creating hybrid systems with enhanced capabilities for medical applications. These systems demonstrate superior energy efficiency compared to conventional computing approaches, making them suitable for deployment in resource-constrained healthcare settings.
The evolution of neuromorphic hardware has been particularly significant, with specialized chips like IBM's TrueNorth, Intel's Loihi, and BrainChip's Akida demonstrating increasing sophistication in neural processing capabilities. These hardware advances have been complemented by the development of specialized algorithms and programming frameworks designed to leverage the unique characteristics of neuromorphic architectures.
In personalized medicine specifically, neuromorphic systems are evolving toward closed-loop applications that can continuously monitor patient parameters, analyze complex physiological signals, and adjust treatment protocols in real-time. This represents a fundamental shift from reactive to proactive healthcare delivery models, potentially transforming chronic disease management and preventive medicine approaches.
The convergence of neuromorphic computing with advances in biosensors, wearable technology, and telemedicine platforms is creating new possibilities for distributed healthcare delivery systems that can provide personalized interventions based on individual patient profiles and real-time physiological data.
In the medical domain, particularly personalized medicine, neuromorphic computing offers unprecedented capabilities for processing complex, heterogeneous patient data. The integration of neuromorphic systems in healthcare has progressed from experimental applications to practical implementations in diagnostic tools and treatment optimization platforms. This evolution has been accelerated by advances in materials science, nanotechnology, and our deepening understanding of neural computation principles.
The application landscape in personalized medicine has expanded dramatically, with neuromorphic systems now being employed for genomic data analysis, medical imaging interpretation, drug response prediction, and real-time patient monitoring. These systems excel at identifying subtle patterns in patient data that might escape traditional analytical methods, potentially revealing new biomarkers and treatment targets.
Recent developments have seen neuromorphic computing platforms integrated with other emerging technologies such as quantum computing and advanced AI frameworks, creating hybrid systems with enhanced capabilities for medical applications. These systems demonstrate superior energy efficiency compared to conventional computing approaches, making them suitable for deployment in resource-constrained healthcare settings.
The evolution of neuromorphic hardware has been particularly significant, with specialized chips like IBM's TrueNorth, Intel's Loihi, and BrainChip's Akida demonstrating increasing sophistication in neural processing capabilities. These hardware advances have been complemented by the development of specialized algorithms and programming frameworks designed to leverage the unique characteristics of neuromorphic architectures.
In personalized medicine specifically, neuromorphic systems are evolving toward closed-loop applications that can continuously monitor patient parameters, analyze complex physiological signals, and adjust treatment protocols in real-time. This represents a fundamental shift from reactive to proactive healthcare delivery models, potentially transforming chronic disease management and preventive medicine approaches.
The convergence of neuromorphic computing with advances in biosensors, wearable technology, and telemedicine platforms is creating new possibilities for distributed healthcare delivery systems that can provide personalized interventions based on individual patient profiles and real-time physiological data.
Market Analysis for Personalized Medicine Computing Solutions
The personalized medicine market is experiencing unprecedented growth, driven by advancements in genomics, biomarker discovery, and precision diagnostics. Current market valuations place the global personalized medicine sector at approximately $2.45 trillion, with projections indicating annual growth rates of 11.5% through 2030. Computing solutions specifically designed for personalized medicine applications represent a rapidly expanding subsegment, currently valued at $38.6 billion and expected to reach $89.2 billion by 2028.
Neuromorphic computing presents a disruptive opportunity within this landscape. Traditional computing architectures struggle with the massive datasets and complex pattern recognition requirements inherent to personalized medicine. Healthcare providers report that existing systems require an average of 4-6 hours to process comprehensive patient genomic profiles, creating bottlenecks in clinical workflows and limiting real-time treatment adjustments.
Market demand for neuromorphic computing solutions in personalized medicine stems from several critical needs. First, the exponential growth in patient-specific data—including multi-omics, longitudinal health records, and real-time monitoring—requires computational systems capable of efficient processing and integration. Second, clinicians increasingly demand real-time analysis capabilities to support point-of-care decision-making, particularly in oncology and critical care settings where treatment windows are narrow.
Regional analysis reveals significant market variations. North America currently dominates with 42% market share, driven by established healthcare infrastructure and substantial R&D investments. However, Asia-Pacific markets demonstrate the highest growth potential at 14.8% annually, fueled by expanding healthcare access and government initiatives in precision medicine, particularly in China, Singapore, and South Korea.
Customer segmentation identifies three primary market segments: academic medical centers (currently 38% of market share), pharmaceutical R&D (33%), and clinical healthcare providers (29%). The pharmaceutical segment shows the highest willingness to invest in advanced computing solutions, with average implementation budgets of $15-20 million for enterprise-scale platforms.
Key market drivers include the decreasing cost of genomic sequencing (now below $500 per genome), increasing prevalence of chronic diseases requiring personalized interventions, and regulatory shifts favoring precision medicine approaches. The FDA has approved 25% more personalized medicine products annually since 2020, creating expanded market opportunities for supporting computational technologies.
Market barriers include concerns regarding data security and privacy, high initial implementation costs, and integration challenges with existing healthcare IT infrastructure. Additionally, reimbursement uncertainties for personalized medicine approaches create hesitancy among some potential adopters, particularly smaller healthcare organizations with limited capital expenditure capabilities.
Neuromorphic computing presents a disruptive opportunity within this landscape. Traditional computing architectures struggle with the massive datasets and complex pattern recognition requirements inherent to personalized medicine. Healthcare providers report that existing systems require an average of 4-6 hours to process comprehensive patient genomic profiles, creating bottlenecks in clinical workflows and limiting real-time treatment adjustments.
Market demand for neuromorphic computing solutions in personalized medicine stems from several critical needs. First, the exponential growth in patient-specific data—including multi-omics, longitudinal health records, and real-time monitoring—requires computational systems capable of efficient processing and integration. Second, clinicians increasingly demand real-time analysis capabilities to support point-of-care decision-making, particularly in oncology and critical care settings where treatment windows are narrow.
Regional analysis reveals significant market variations. North America currently dominates with 42% market share, driven by established healthcare infrastructure and substantial R&D investments. However, Asia-Pacific markets demonstrate the highest growth potential at 14.8% annually, fueled by expanding healthcare access and government initiatives in precision medicine, particularly in China, Singapore, and South Korea.
Customer segmentation identifies three primary market segments: academic medical centers (currently 38% of market share), pharmaceutical R&D (33%), and clinical healthcare providers (29%). The pharmaceutical segment shows the highest willingness to invest in advanced computing solutions, with average implementation budgets of $15-20 million for enterprise-scale platforms.
Key market drivers include the decreasing cost of genomic sequencing (now below $500 per genome), increasing prevalence of chronic diseases requiring personalized interventions, and regulatory shifts favoring precision medicine approaches. The FDA has approved 25% more personalized medicine products annually since 2020, creating expanded market opportunities for supporting computational technologies.
Market barriers include concerns regarding data security and privacy, high initial implementation costs, and integration challenges with existing healthcare IT infrastructure. Additionally, reimbursement uncertainties for personalized medicine approaches create hesitancy among some potential adopters, particularly smaller healthcare organizations with limited capital expenditure capabilities.
Current Neuromorphic Technologies and Healthcare Integration Challenges
The neuromorphic computing landscape is currently dominated by several key technologies that attempt to mimic the brain's neural architecture. Spiking Neural Networks (SNNs) represent the most prominent approach, utilizing discrete spikes for information transmission similar to biological neurons. Major implementations include IBM's TrueNorth chip, Intel's Loihi, and BrainChip's Akida, each offering different trade-offs between power efficiency, processing capability, and integration potential. These systems demonstrate significant advantages in pattern recognition and time-series data analysis—capabilities particularly valuable for medical diagnostics and personalized treatment planning.
Memristive technologies have emerged as another critical component in neuromorphic systems, offering non-volatile memory capabilities that more closely resemble synaptic behavior. Recent advancements in phase-change memory (PCM) and resistive RAM (ReRAM) have enabled more efficient on-chip learning capabilities, though challenges in manufacturing consistency and long-term stability persist.
Despite these technological advances, integration with healthcare systems presents substantial challenges. Medical data heterogeneity represents a primary obstacle, as neuromorphic systems must process diverse data types including genomic sequences, medical imaging, continuous monitoring signals, and electronic health records. Current neuromorphic architectures excel at specific data modalities but struggle with the multi-modal integration necessary for comprehensive personalized medicine applications.
Regulatory compliance presents another significant barrier. Healthcare technologies must adhere to stringent regulatory frameworks such as HIPAA, GDPR, and FDA requirements. The novel nature of neuromorphic computing creates uncertainty in validation protocols and compliance verification, particularly regarding the "black box" nature of some neuromorphic learning systems that may not provide the transparency required in medical decision-making contexts.
Technical integration with existing healthcare IT infrastructure remains problematic. Legacy systems dominate healthcare environments, and neuromorphic computing solutions must demonstrate interoperability with established electronic health record systems, laboratory information management systems, and clinical decision support tools. Current neuromorphic platforms typically lack standardized interfaces for such integration.
Energy and computational constraints in clinical settings also limit deployment options. While neuromorphic systems offer theoretical energy efficiency advantages, practical implementations often require specialized hardware that may be impractical for widespread clinical adoption. Edge computing applications show promise but face limitations in processing capacity for complex medical analytics.
Lastly, the clinical validation pathway remains undefined for many neuromorphic applications in medicine. The technology requires extensive validation studies demonstrating improved patient outcomes, cost-effectiveness, and reliability compared to conventional computing approaches—evidence that is still emerging in this rapidly evolving field.
Memristive technologies have emerged as another critical component in neuromorphic systems, offering non-volatile memory capabilities that more closely resemble synaptic behavior. Recent advancements in phase-change memory (PCM) and resistive RAM (ReRAM) have enabled more efficient on-chip learning capabilities, though challenges in manufacturing consistency and long-term stability persist.
Despite these technological advances, integration with healthcare systems presents substantial challenges. Medical data heterogeneity represents a primary obstacle, as neuromorphic systems must process diverse data types including genomic sequences, medical imaging, continuous monitoring signals, and electronic health records. Current neuromorphic architectures excel at specific data modalities but struggle with the multi-modal integration necessary for comprehensive personalized medicine applications.
Regulatory compliance presents another significant barrier. Healthcare technologies must adhere to stringent regulatory frameworks such as HIPAA, GDPR, and FDA requirements. The novel nature of neuromorphic computing creates uncertainty in validation protocols and compliance verification, particularly regarding the "black box" nature of some neuromorphic learning systems that may not provide the transparency required in medical decision-making contexts.
Technical integration with existing healthcare IT infrastructure remains problematic. Legacy systems dominate healthcare environments, and neuromorphic computing solutions must demonstrate interoperability with established electronic health record systems, laboratory information management systems, and clinical decision support tools. Current neuromorphic platforms typically lack standardized interfaces for such integration.
Energy and computational constraints in clinical settings also limit deployment options. While neuromorphic systems offer theoretical energy efficiency advantages, practical implementations often require specialized hardware that may be impractical for widespread clinical adoption. Edge computing applications show promise but face limitations in processing capacity for complex medical analytics.
Lastly, the clinical validation pathway remains undefined for many neuromorphic applications in medicine. The technology requires extensive validation studies demonstrating improved patient outcomes, cost-effectiveness, and reliability compared to conventional computing approaches—evidence that is still emerging in this rapidly evolving field.
Existing Neuromorphic Architectures for Medical Data Processing
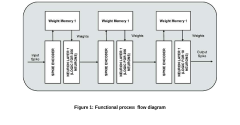
01 Neuromorphic hardware architectures
Neuromorphic computing systems implement hardware architectures that mimic the structure and function of the human brain. These architectures typically include specialized neural processing units, synaptic memory elements, and interconnection networks that enable parallel processing and efficient computation. By designing hardware specifically for neural network operations, these systems achieve higher energy efficiency and performance for AI workloads compared to traditional computing architectures.- Neuromorphic hardware architectures: Neuromorphic computing systems implement hardware architectures that mimic the structure and function of the human brain. These architectures typically include specialized neural processing units, synaptic elements, and memory structures designed to perform brain-inspired computations efficiently. By closely emulating biological neural networks, these systems can achieve higher energy efficiency and performance for certain AI tasks compared to traditional computing architectures.
- Memristive devices for neuromorphic computing: Memristive devices are key components in many neuromorphic computing implementations, serving as artificial synapses that can store and process information simultaneously. These devices can change their resistance based on the history of applied voltage or current, enabling them to mimic the plasticity of biological synapses. Memristive-based neuromorphic systems offer advantages in terms of power efficiency, density, and the ability to implement learning algorithms directly in hardware.
- Spiking neural networks implementation: Spiking neural networks (SNNs) represent a biologically plausible approach to neuromorphic computing where information is encoded in the timing and frequency of discrete spikes rather than continuous values. These networks process information asynchronously and event-driven, similar to biological neurons. Implementations of SNNs in hardware can achieve significant power efficiency advantages while maintaining computational capabilities for pattern recognition, classification, and other AI tasks.
- Learning algorithms for neuromorphic systems: Specialized learning algorithms have been developed for neuromorphic computing systems that enable on-chip learning and adaptation. These algorithms include spike-timing-dependent plasticity (STDP), reinforcement learning, and modified versions of backpropagation designed to work with spiking neurons. These approaches allow neuromorphic systems to learn from their environment, adapt to new data, and improve performance over time without requiring extensive retraining on external systems.
- Applications of neuromorphic computing: Neuromorphic computing systems are being applied to various domains including edge computing, robotics, autonomous vehicles, and IoT devices. These applications leverage the energy efficiency and real-time processing capabilities of neuromorphic hardware to perform tasks such as object recognition, natural language processing, and sensory data analysis. The low power consumption and ability to process sensory information in ways similar to biological systems make neuromorphic computing particularly suitable for embedded and mobile applications.
02 Memristive devices for neuromorphic computing
Memristive devices are key components in neuromorphic computing systems, functioning as artificial synapses that can store and process information simultaneously. These devices exhibit variable resistance states that can be modulated based on the history of applied voltage or current, enabling them to mimic biological synaptic plasticity. Memristive technologies enable high-density, low-power neural networks that can perform complex cognitive tasks with significantly reduced energy consumption.Expand Specific Solutions03 Spiking neural networks implementation
Spiking neural networks (SNNs) represent a biologically inspired approach to neuromorphic computing where information is encoded in the timing and frequency of discrete spikes rather than continuous values. These networks process information asynchronously and event-driven, similar to biological neurons, resulting in more energy-efficient computation. Implementation techniques include specialized encoding schemes, learning algorithms adapted for spike-based processing, and hardware optimizations that leverage the sparse and temporal nature of spike-based computation.Expand Specific Solutions04 Learning algorithms for neuromorphic systems
Specialized learning algorithms are developed for neuromorphic computing systems that account for the unique characteristics of brain-inspired hardware. These include spike-timing-dependent plasticity (STDP), reinforcement learning adapted for spiking networks, and hardware-aware training methods that optimize for the constraints of neuromorphic architectures. These algorithms enable on-chip learning capabilities, allowing neuromorphic systems to adapt to new data and tasks without requiring extensive retraining on conventional computing systems.Expand Specific Solutions05 Applications of neuromorphic computing
Neuromorphic computing systems are being applied to various domains that benefit from their energy efficiency and real-time processing capabilities. Key applications include edge AI for IoT devices, autonomous systems requiring low-latency decision making, pattern recognition in unstructured environments, and sensory processing tasks such as computer vision and audio processing. These systems excel in scenarios requiring continuous learning, adaptation to changing environments, and operation under power constraints.Expand Specific Solutions
Leading Organizations in Neuromorphic Computing for Medicine
Neuromorphic computing in personalized medicine is emerging as a transformative technology, currently in its early growth phase. The market is expanding rapidly, projected to reach significant scale as healthcare systems increasingly adopt AI-driven personalized treatments. Leading the technological development are established tech giants like IBM and Intel, who have made substantial investments in neuromorphic architectures specifically designed for healthcare applications. Academic institutions including Tsinghua University, National University of Singapore, and CNRS are advancing fundamental research, while healthcare-focused companies such as Siemens Healthineers, Novartis, and Roche are exploring clinical applications. The ecosystem demonstrates a collaborative approach between technology providers, research institutions, and healthcare organizations, with varying levels of technological maturity across different application domains.
International Business Machines Corp.
Technical Solution: IBM's neuromorphic computing approach for personalized medicine centers around their TrueNorth and subsequent neuromorphic chips. Their technology mimics the brain's neural architecture to process complex medical data with significantly lower power consumption. IBM has developed specialized neural networks that can analyze patient-specific genomic data, medical imaging, and electronic health records simultaneously to identify personalized treatment options. Their systems can detect subtle patterns in patient data that traditional computing might miss, enabling more precise diagnosis and treatment recommendations. IBM's neuromorphic platforms integrate with their Watson Health ecosystem, allowing for real-time processing of multimodal medical data while maintaining patient privacy through edge computing capabilities[1][3]. The company has demonstrated applications in cancer treatment optimization, where their neuromorphic systems analyze tumor heterogeneity and drug response data to suggest personalized therapy combinations with higher efficacy and lower side effects[7].
Strengths: Superior energy efficiency compared to traditional computing architectures; ability to process heterogeneous medical data types simultaneously; enhanced pattern recognition in complex biological systems. Weaknesses: Still requires significant specialized programming expertise; integration challenges with existing healthcare IT infrastructure; limited commercial deployment in clinical settings.
Intel Corp.
Technical Solution: Intel's neuromorphic computing solution for personalized medicine is built around their Loihi chip architecture. This self-learning neuromorphic system mimics the brain's basic mechanics to efficiently process complex, unstructured healthcare data. Intel has developed specialized neural networks that can analyze patient-specific genomic sequences, proteomics data, and clinical information with unprecedented energy efficiency. Their neuromorphic systems excel at identifying subtle patterns in patient data that might indicate disease susceptibility or medication response variations. Intel's Loihi-based platforms can process continuous streams of patient monitoring data at the edge, enabling real-time health insights while preserving privacy. The company has demonstrated applications in drug discovery optimization, where their neuromorphic systems can rapidly simulate molecular interactions and predict patient-specific drug responses based on genetic profiles[2][5]. Intel has also partnered with leading research hospitals to develop neuromorphic systems that can analyze complex medical imaging data to detect early disease markers that might be missed by conventional analysis.
Strengths: Extremely low power consumption enabling edge deployment in clinical settings; asynchronous processing well-suited to irregular medical data patterns; scalable architecture for handling population-level genomic analysis. Weaknesses: Requires new programming paradigms different from traditional computing approaches; limited commercial healthcare applications currently deployed; challenges in regulatory approval for clinical decision support.
Key Patents and Research in Brain-Inspired Medical Computing
Neuromorphic computing: brain-inspired hardware for efficient ai processing
PatentPendingIN202411005149A
Innovation
- Neuromorphic computing systems mimic the brain's neural networks and synapses to enable parallel and adaptive processing, leveraging advances in neuroscience and hardware to create energy-efficient AI systems that can learn and adapt in real-time.
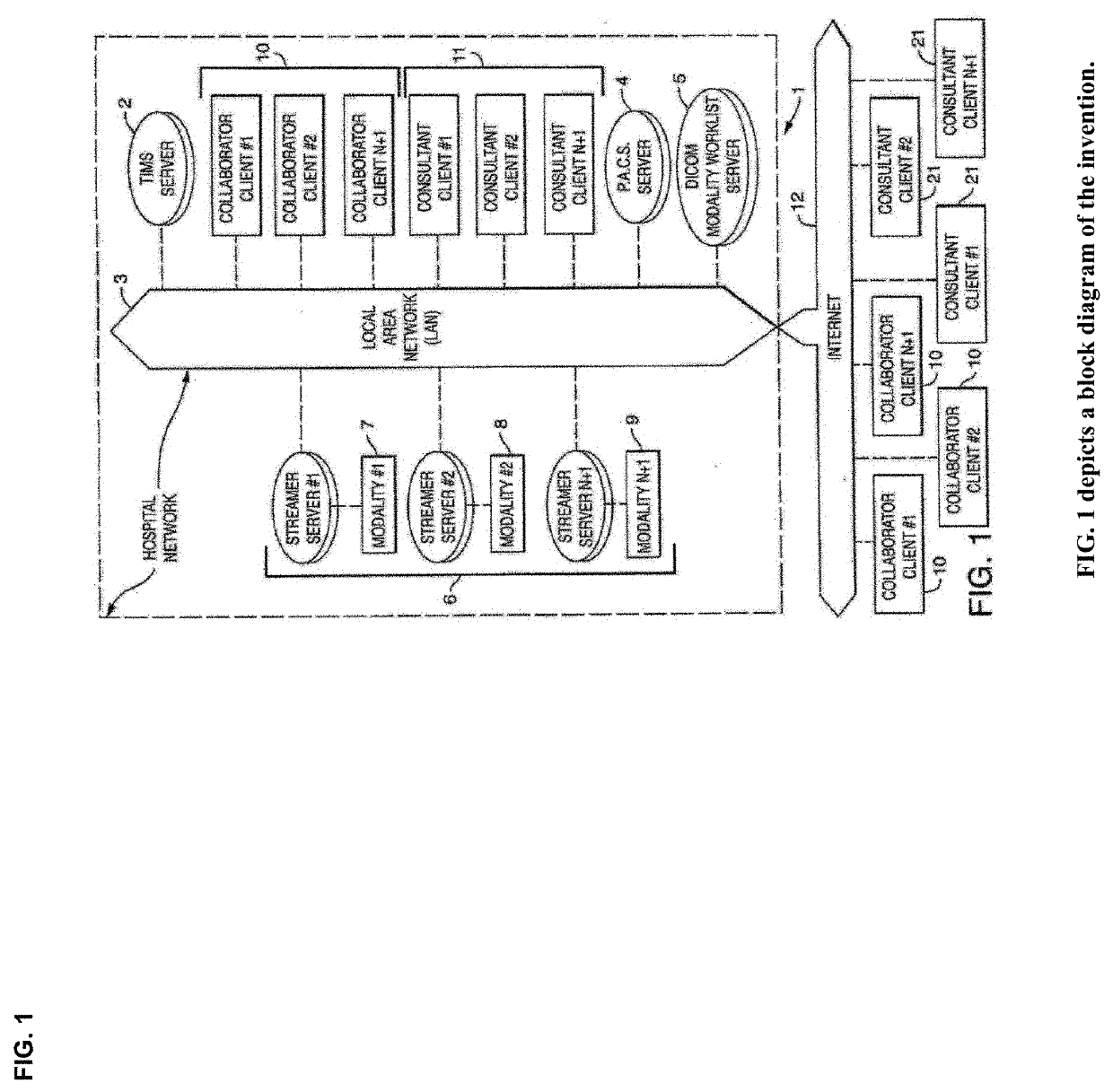
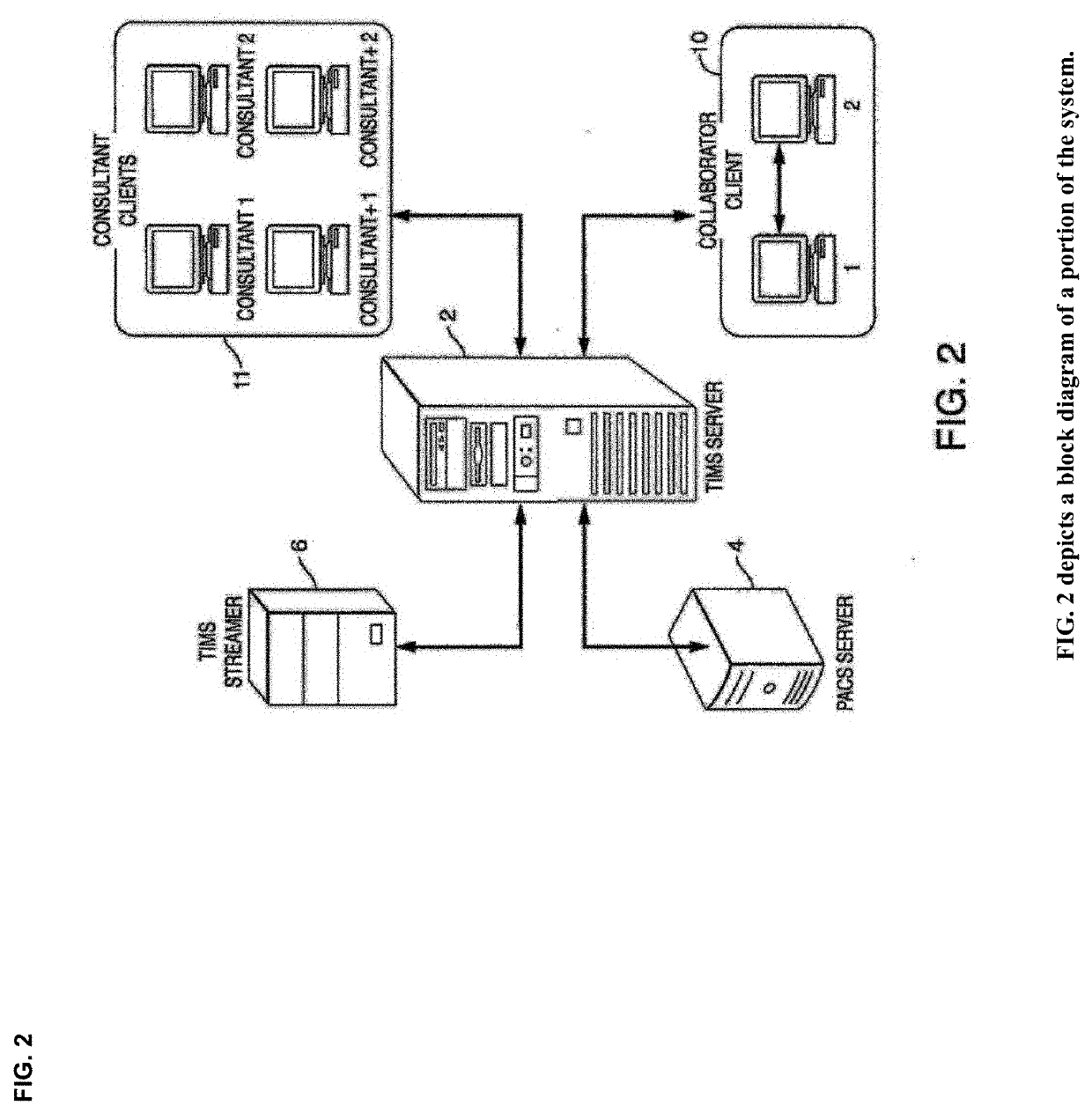
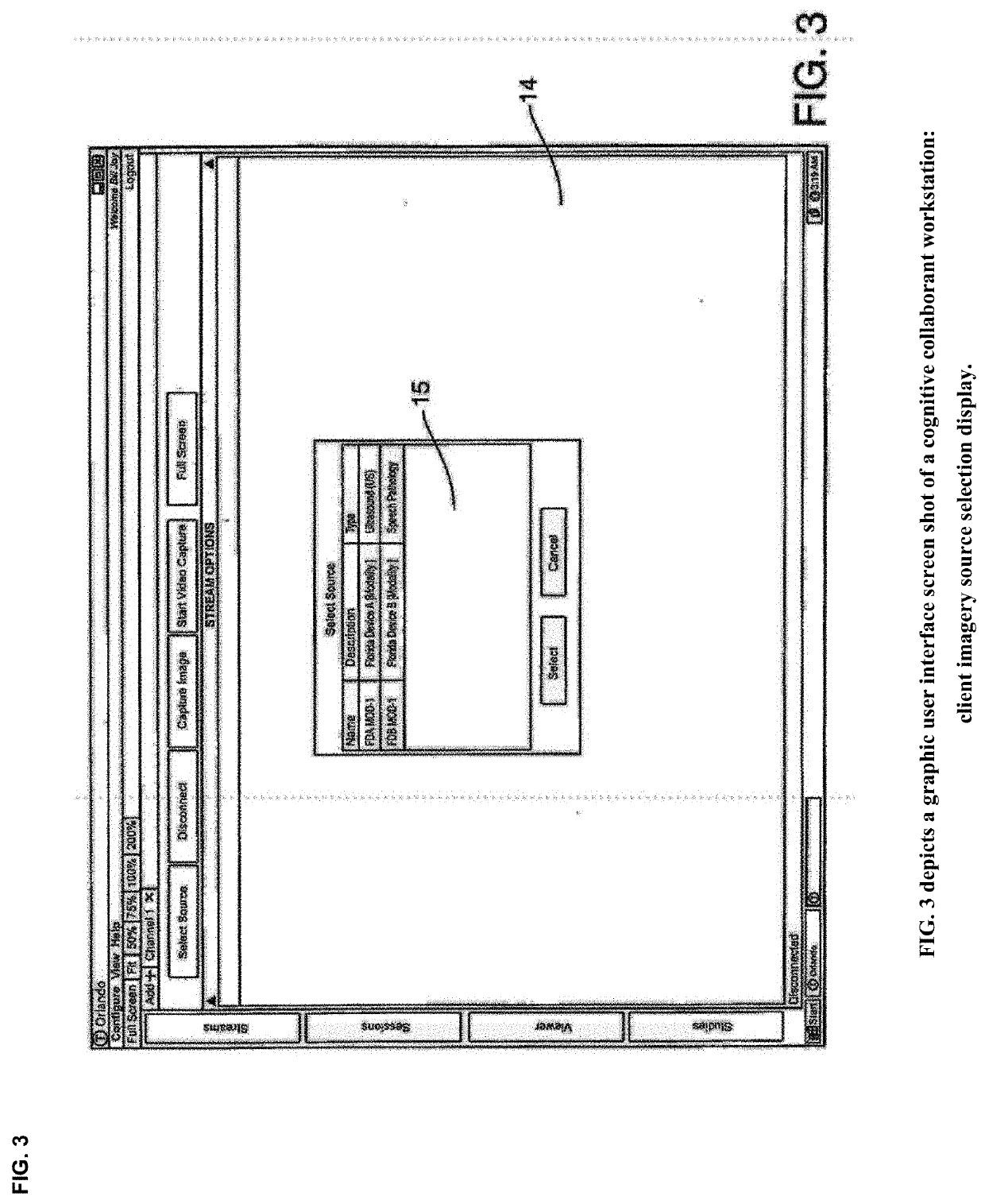
Cognitive Collaboration with Neurosynaptic Imaging Networks, Augmented Medical Intelligence and Cybernetic Workflow Streams
PatentActiveUS20190355483A1
Innovation
- A network system that enables concurrent collaboration by allowing multiple users to view and annotate streaming medical imagery data in real-time, using TIMS Clini-Pod Network Servers and Clini-Docks, which manage and synchronize data across various medical modalities, and encapsulate annotations and metadata into single file formats compliant with DICOM standards.
Data Privacy and Security Considerations in Medical Neuromorphic Systems
As neuromorphic computing systems increasingly integrate with personalized medicine applications, data privacy and security emerge as critical concerns. These systems process vast amounts of sensitive patient information, including genetic data, medical histories, and real-time physiological measurements, creating significant privacy vulnerabilities if not properly secured.
The unique architecture of neuromorphic systems presents distinct security challenges compared to traditional computing platforms. Their brain-inspired design, while efficient for medical pattern recognition, introduces novel attack vectors that conventional cybersecurity approaches may not adequately address. The distributed memory-processing architecture that makes these systems powerful also creates multiple potential points of compromise.
Healthcare regulations such as HIPAA in the United States, GDPR in Europe, and similar frameworks worldwide impose strict requirements on medical data handling. Neuromorphic systems must demonstrate compliance with these regulations while maintaining their computational advantages. This necessitates the development of privacy-preserving neuromorphic architectures that can process sensitive medical data without exposing patient identities or confidential information.
Encryption methodologies for neuromorphic systems require special consideration, as traditional encryption approaches may impede the performance benefits these systems offer. Researchers are exploring homomorphic encryption techniques that allow computations on encrypted data without decryption, potentially enabling neuromorphic systems to process sensitive medical information while maintaining privacy guarantees.
Edge computing implementations of neuromorphic systems offer promising security advantages by processing sensitive patient data locally rather than transmitting it to centralized servers. This approach minimizes data exposure during transmission and storage, though it requires careful implementation of device-level security measures to prevent physical tampering or unauthorized access.
The dynamic nature of neuromorphic learning presents additional security considerations. As these systems continuously adapt based on new medical data, they must be protected against adversarial attacks that could manipulate the learning process or extract sensitive information through model inversion techniques. Differential privacy approaches are being explored to ensure that individual patient data cannot be reverse-engineered from the trained models.
Establishing trust in neuromorphic medical systems requires transparent security auditing and certification processes. Healthcare providers and patients must have confidence that these systems protect sensitive information while delivering accurate medical insights. This necessitates the development of specialized security standards and testing methodologies specifically designed for neuromorphic computing in healthcare applications.
The unique architecture of neuromorphic systems presents distinct security challenges compared to traditional computing platforms. Their brain-inspired design, while efficient for medical pattern recognition, introduces novel attack vectors that conventional cybersecurity approaches may not adequately address. The distributed memory-processing architecture that makes these systems powerful also creates multiple potential points of compromise.
Healthcare regulations such as HIPAA in the United States, GDPR in Europe, and similar frameworks worldwide impose strict requirements on medical data handling. Neuromorphic systems must demonstrate compliance with these regulations while maintaining their computational advantages. This necessitates the development of privacy-preserving neuromorphic architectures that can process sensitive medical data without exposing patient identities or confidential information.
Encryption methodologies for neuromorphic systems require special consideration, as traditional encryption approaches may impede the performance benefits these systems offer. Researchers are exploring homomorphic encryption techniques that allow computations on encrypted data without decryption, potentially enabling neuromorphic systems to process sensitive medical information while maintaining privacy guarantees.
Edge computing implementations of neuromorphic systems offer promising security advantages by processing sensitive patient data locally rather than transmitting it to centralized servers. This approach minimizes data exposure during transmission and storage, though it requires careful implementation of device-level security measures to prevent physical tampering or unauthorized access.
The dynamic nature of neuromorphic learning presents additional security considerations. As these systems continuously adapt based on new medical data, they must be protected against adversarial attacks that could manipulate the learning process or extract sensitive information through model inversion techniques. Differential privacy approaches are being explored to ensure that individual patient data cannot be reverse-engineered from the trained models.
Establishing trust in neuromorphic medical systems requires transparent security auditing and certification processes. Healthcare providers and patients must have confidence that these systems protect sensitive information while delivering accurate medical insights. This necessitates the development of specialized security standards and testing methodologies specifically designed for neuromorphic computing in healthcare applications.
Clinical Validation Requirements for Neuromorphic Medical Solutions
The validation of neuromorphic computing solutions in personalized medicine requires rigorous clinical testing protocols that significantly differ from traditional computing system validations. These specialized requirements stem from the unique intersection of neuromorphic architecture capabilities and the high-stakes nature of medical applications.
Regulatory bodies such as the FDA in the United States and the EMA in Europe mandate comprehensive validation frameworks for any AI-based medical technologies. For neuromorphic systems specifically, these frameworks must address both the novel computing architecture and its application in patient care. Clinical validation typically follows a three-phase approach: preliminary validation using retrospective data, controlled prospective studies, and finally real-world implementation studies.
The validation process must demonstrate not only accuracy but also reliability under varying clinical conditions. Neuromorphic systems must maintain consistent performance across diverse patient populations, accounting for demographic variations, comorbidities, and genetic differences. This requires testing with stratified patient cohorts that represent the full spectrum of potential users.
Explainability presents a unique challenge for neuromorphic computing validation. Unlike traditional computing systems, neuromorphic architectures mimic biological neural networks, potentially creating a "black box" effect. Clinical validation protocols must therefore include methods to interpret system decisions, particularly for high-risk applications like treatment recommendation or disease progression prediction.
Safety validation requirements are especially stringent, focusing on failure mode analysis and graceful degradation capabilities. Neuromorphic systems must demonstrate predictable behavior even when processing incomplete or noisy medical data. This includes validation of built-in safeguards that prevent harmful recommendations when the system operates outside its validated parameters.
Longitudinal performance assessment forms another critical component of validation requirements. As neuromorphic systems often incorporate learning capabilities, validation protocols must verify consistent performance over time and across software updates. This includes testing for potential drift in diagnostic accuracy or treatment recommendations as the system processes more patient data.
Integration validation with existing clinical workflows represents the final major requirement. Neuromorphic solutions must demonstrate seamless operation within established healthcare IT infrastructures, including compatibility with electronic health record systems, imaging platforms, and clinical decision support frameworks. This validation ensures that theoretical benefits translate to practical improvements in clinical settings.
Regulatory bodies such as the FDA in the United States and the EMA in Europe mandate comprehensive validation frameworks for any AI-based medical technologies. For neuromorphic systems specifically, these frameworks must address both the novel computing architecture and its application in patient care. Clinical validation typically follows a three-phase approach: preliminary validation using retrospective data, controlled prospective studies, and finally real-world implementation studies.
The validation process must demonstrate not only accuracy but also reliability under varying clinical conditions. Neuromorphic systems must maintain consistent performance across diverse patient populations, accounting for demographic variations, comorbidities, and genetic differences. This requires testing with stratified patient cohorts that represent the full spectrum of potential users.
Explainability presents a unique challenge for neuromorphic computing validation. Unlike traditional computing systems, neuromorphic architectures mimic biological neural networks, potentially creating a "black box" effect. Clinical validation protocols must therefore include methods to interpret system decisions, particularly for high-risk applications like treatment recommendation or disease progression prediction.
Safety validation requirements are especially stringent, focusing on failure mode analysis and graceful degradation capabilities. Neuromorphic systems must demonstrate predictable behavior even when processing incomplete or noisy medical data. This includes validation of built-in safeguards that prevent harmful recommendations when the system operates outside its validated parameters.
Longitudinal performance assessment forms another critical component of validation requirements. As neuromorphic systems often incorporate learning capabilities, validation protocols must verify consistent performance over time and across software updates. This includes testing for potential drift in diagnostic accuracy or treatment recommendations as the system processes more patient data.
Integration validation with existing clinical workflows represents the final major requirement. Neuromorphic solutions must demonstrate seamless operation within established healthcare IT infrastructures, including compatibility with electronic health record systems, imaging platforms, and clinical decision support frameworks. This validation ensures that theoretical benefits translate to practical improvements in clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!