The Role of Ammonium Hydroxide in pH Control of Analytical Mixtures
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide pH Control Background
Ammonium hydroxide has played a crucial role in pH control of analytical mixtures for decades. This compound, also known as aqueous ammonia, is a versatile and widely used base in various analytical procedures. Its importance stems from its ability to effectively adjust and maintain pH levels in solution, which is critical for many chemical analyses and experimental setups.
The history of ammonium hydroxide in analytical chemistry dates back to the early 20th century when researchers began to recognize the significance of pH control in chemical reactions and analyses. As the field of analytical chemistry evolved, so did the understanding of the impact of pH on chemical equilibria, reaction rates, and the behavior of analytes in solution.
Ammonium hydroxide's unique properties make it particularly suitable for pH control in analytical mixtures. It is a weak base that dissociates partially in water, forming ammonium ions (NH4+) and hydroxide ions (OH-). This partial dissociation allows for a more gradual and controlled adjustment of pH compared to strong bases like sodium hydroxide. Additionally, the volatile nature of ammonia enables its easy removal from solutions when necessary, making it an ideal choice for applications where residual base contamination is undesirable.
The use of ammonium hydroxide in pH control extends across various analytical techniques. In spectrophotometry, it helps maintain optimal pH conditions for color development in colorimetric assays. In chromatography, it plays a crucial role in mobile phase preparation and pH adjustment of buffers, influencing the retention and separation of analytes. Electrochemical analyses often rely on ammonium hydroxide to create suitable pH environments for electrode reactions and potential measurements.
One of the key advantages of ammonium hydroxide in analytical mixtures is its buffer capacity. When used in conjunction with its conjugate acid (ammonium ion), it forms an effective buffer system that can resist pH changes upon the addition of small amounts of acids or bases. This buffering action is essential in maintaining stable pH conditions throughout analytical procedures, ensuring reproducibility and reliability of results.
The concentration range of ammonium hydroxide used in analytical applications typically varies from dilute solutions (around 0.1%) to more concentrated forms (up to 30%). The choice of concentration depends on the specific requirements of the analytical method, the desired pH range, and the nature of the analytes being studied. Proper handling and storage of ammonium hydroxide solutions are crucial to maintain their effectiveness and ensure laboratory safety.
The history of ammonium hydroxide in analytical chemistry dates back to the early 20th century when researchers began to recognize the significance of pH control in chemical reactions and analyses. As the field of analytical chemistry evolved, so did the understanding of the impact of pH on chemical equilibria, reaction rates, and the behavior of analytes in solution.
Ammonium hydroxide's unique properties make it particularly suitable for pH control in analytical mixtures. It is a weak base that dissociates partially in water, forming ammonium ions (NH4+) and hydroxide ions (OH-). This partial dissociation allows for a more gradual and controlled adjustment of pH compared to strong bases like sodium hydroxide. Additionally, the volatile nature of ammonia enables its easy removal from solutions when necessary, making it an ideal choice for applications where residual base contamination is undesirable.
The use of ammonium hydroxide in pH control extends across various analytical techniques. In spectrophotometry, it helps maintain optimal pH conditions for color development in colorimetric assays. In chromatography, it plays a crucial role in mobile phase preparation and pH adjustment of buffers, influencing the retention and separation of analytes. Electrochemical analyses often rely on ammonium hydroxide to create suitable pH environments for electrode reactions and potential measurements.
One of the key advantages of ammonium hydroxide in analytical mixtures is its buffer capacity. When used in conjunction with its conjugate acid (ammonium ion), it forms an effective buffer system that can resist pH changes upon the addition of small amounts of acids or bases. This buffering action is essential in maintaining stable pH conditions throughout analytical procedures, ensuring reproducibility and reliability of results.
The concentration range of ammonium hydroxide used in analytical applications typically varies from dilute solutions (around 0.1%) to more concentrated forms (up to 30%). The choice of concentration depends on the specific requirements of the analytical method, the desired pH range, and the nature of the analytes being studied. Proper handling and storage of ammonium hydroxide solutions are crucial to maintain their effectiveness and ensure laboratory safety.
Market Analysis for pH Control Solutions
The global market for pH control solutions in analytical applications is experiencing steady growth, driven by increasing demand for precise and reliable pH management across various industries. The pharmaceutical and biotechnology sectors are major contributors to this growth, as they require stringent pH control for drug development, quality assurance, and research processes. Additionally, the food and beverage industry relies heavily on pH control solutions for product quality and safety, further fueling market expansion.
Environmental monitoring and water treatment applications also represent significant market segments for pH control solutions. As regulations become more stringent and awareness of environmental issues grows, the need for accurate pH measurement and control in these areas continues to rise. The chemical industry, too, depends on pH control for numerous processes, contributing to the overall market demand.
Ammonium hydroxide, as a key component in pH control solutions, plays a crucial role in this market. Its ability to act as both a weak base and a buffer makes it particularly valuable in analytical mixtures where precise pH adjustment is required. The market for ammonium hydroxide in pH control applications is expected to grow in parallel with the broader pH control solutions market.
Geographically, North America and Europe currently dominate the market for pH control solutions, owing to their well-established pharmaceutical, biotechnology, and chemical industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, expanding research and development activities, and growing awareness of the importance of pH control in various applications.
The market is characterized by a mix of large multinational corporations and specialized niche players. Key market players are focusing on developing innovative pH control solutions that offer improved accuracy, reliability, and ease of use. There is also a growing trend towards the integration of digital technologies and automation in pH control systems, which is expected to drive further market growth and differentiation among competitors.
Challenges in the market include the need for solutions that can maintain stable pH levels in complex analytical mixtures and the development of more environmentally friendly pH control agents. These challenges present opportunities for innovation and product development, potentially reshaping the competitive landscape in the coming years.
Environmental monitoring and water treatment applications also represent significant market segments for pH control solutions. As regulations become more stringent and awareness of environmental issues grows, the need for accurate pH measurement and control in these areas continues to rise. The chemical industry, too, depends on pH control for numerous processes, contributing to the overall market demand.
Ammonium hydroxide, as a key component in pH control solutions, plays a crucial role in this market. Its ability to act as both a weak base and a buffer makes it particularly valuable in analytical mixtures where precise pH adjustment is required. The market for ammonium hydroxide in pH control applications is expected to grow in parallel with the broader pH control solutions market.
Geographically, North America and Europe currently dominate the market for pH control solutions, owing to their well-established pharmaceutical, biotechnology, and chemical industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization, expanding research and development activities, and growing awareness of the importance of pH control in various applications.
The market is characterized by a mix of large multinational corporations and specialized niche players. Key market players are focusing on developing innovative pH control solutions that offer improved accuracy, reliability, and ease of use. There is also a growing trend towards the integration of digital technologies and automation in pH control systems, which is expected to drive further market growth and differentiation among competitors.
Challenges in the market include the need for solutions that can maintain stable pH levels in complex analytical mixtures and the development of more environmentally friendly pH control agents. These challenges present opportunities for innovation and product development, potentially reshaping the competitive landscape in the coming years.
Current Challenges in Analytical pH Control
The field of analytical chemistry faces several significant challenges in pH control, particularly when using ammonium hydroxide in analytical mixtures. One of the primary issues is the volatility of ammonium hydroxide, which can lead to inconsistent pH levels over time. As the ammonia evaporates, the pH of the solution can drift, affecting the accuracy and reproducibility of analytical results. This is especially problematic in long-term experiments or when precise pH control is critical for the analysis.
Another challenge is the temperature dependence of ammonium hydroxide's dissociation. The pH of ammonium hydroxide solutions can vary significantly with temperature changes, making it difficult to maintain a stable pH in environments where temperature fluctuations occur. This can be particularly problematic in field applications or in laboratories without stringent temperature control.
The buffering capacity of ammonium hydroxide solutions presents an additional challenge. While ammonium hydroxide can act as a buffer in certain pH ranges, its buffering capacity is limited compared to other common buffer systems. This can result in rapid pH changes when acids or bases are added to the analytical mixture, potentially compromising the integrity of pH-sensitive analyses.
Furthermore, the presence of ammonium ions in the solution can interfere with certain analytical techniques. For instance, in mass spectrometry, ammonium adducts can form, complicating spectral interpretation. In electrochemical analyses, ammonium ions can affect electrode performance and alter redox potentials, leading to inaccurate measurements.
The purity and concentration of commercially available ammonium hydroxide solutions also pose challenges. Variations in concentration between batches or degradation during storage can lead to inconsistencies in pH adjustment. This necessitates frequent standardization and careful handling of ammonium hydroxide solutions to ensure reliable pH control.
Lastly, the environmental and safety concerns associated with ammonium hydroxide use cannot be overlooked. Its strong odor and potential for releasing ammonia gas require proper ventilation and handling procedures. This can limit its applicability in certain analytical settings and increase the complexity of experimental setups.
Addressing these challenges requires a multifaceted approach, including the development of more stable pH control methods, improved temperature compensation techniques, and alternative buffer systems that can provide more robust pH control in analytical mixtures. Research into novel pH-sensing technologies and automated pH adjustment systems may also offer solutions to enhance the precision and reliability of pH control in analytical chemistry.
Another challenge is the temperature dependence of ammonium hydroxide's dissociation. The pH of ammonium hydroxide solutions can vary significantly with temperature changes, making it difficult to maintain a stable pH in environments where temperature fluctuations occur. This can be particularly problematic in field applications or in laboratories without stringent temperature control.
The buffering capacity of ammonium hydroxide solutions presents an additional challenge. While ammonium hydroxide can act as a buffer in certain pH ranges, its buffering capacity is limited compared to other common buffer systems. This can result in rapid pH changes when acids or bases are added to the analytical mixture, potentially compromising the integrity of pH-sensitive analyses.
Furthermore, the presence of ammonium ions in the solution can interfere with certain analytical techniques. For instance, in mass spectrometry, ammonium adducts can form, complicating spectral interpretation. In electrochemical analyses, ammonium ions can affect electrode performance and alter redox potentials, leading to inaccurate measurements.
The purity and concentration of commercially available ammonium hydroxide solutions also pose challenges. Variations in concentration between batches or degradation during storage can lead to inconsistencies in pH adjustment. This necessitates frequent standardization and careful handling of ammonium hydroxide solutions to ensure reliable pH control.
Lastly, the environmental and safety concerns associated with ammonium hydroxide use cannot be overlooked. Its strong odor and potential for releasing ammonia gas require proper ventilation and handling procedures. This can limit its applicability in certain analytical settings and increase the complexity of experimental setups.
Addressing these challenges requires a multifaceted approach, including the development of more stable pH control methods, improved temperature compensation techniques, and alternative buffer systems that can provide more robust pH control in analytical mixtures. Research into novel pH-sensing technologies and automated pH adjustment systems may also offer solutions to enhance the precision and reliability of pH control in analytical chemistry.
Ammonium Hydroxide-based pH Control Techniques
01 pH adjustment in industrial processes
Ammonium hydroxide is commonly used for pH adjustment in various industrial processes. Its alkaline nature makes it suitable for neutralizing acidic solutions and maintaining desired pH levels in manufacturing, water treatment, and chemical production.- pH adjustment in industrial processes: Ammonium hydroxide is commonly used for pH adjustment in various industrial processes. Its alkaline nature makes it suitable for neutralizing acidic solutions and maintaining desired pH levels in manufacturing, water treatment, and chemical production.
- Cleaning and degreasing applications: The alkaline properties of ammonium hydroxide make it effective for cleaning and degreasing purposes. It is used in household and industrial cleaning products to remove dirt, grease, and stains from various surfaces.
- Use in agricultural fertilizers: Ammonium hydroxide is utilized in the production of agricultural fertilizers. Its high nitrogen content makes it valuable for enhancing soil fertility and promoting plant growth when applied in appropriate concentrations.
- pH control in food processing: In food processing, ammonium hydroxide is used for pH control and as a leavening agent. It helps regulate acidity levels in certain food products and contributes to the texture and appearance of baked goods.
- Environmental applications: Ammonium hydroxide plays a role in environmental applications, including air pollution control and wastewater treatment. It can be used to neutralize acidic emissions and adjust pH levels in water treatment processes to meet environmental regulations.
02 Cleaning and degreasing applications
The high pH of ammonium hydroxide makes it effective for cleaning and degreasing purposes. It is used in household and industrial cleaning products to remove stubborn stains, grease, and grime from various surfaces.Expand Specific Solutions03 Wastewater treatment and pH control
Ammonium hydroxide is utilized in wastewater treatment processes to adjust pH levels and remove contaminants. Its ability to neutralize acidic effluents helps in maintaining environmental standards and improving water quality.Expand Specific Solutions04 Agricultural applications
In agriculture, ammonium hydroxide is used as a nitrogen source for fertilizers and soil amendments. Its high pH can help neutralize acidic soils and improve nutrient uptake by plants.Expand Specific Solutions05 pH-dependent chemical reactions
The pH of ammonium hydroxide plays a crucial role in various chemical reactions and processes. It is used to control reaction conditions, catalyze certain reactions, and influence the solubility of compounds in different industrial and laboratory applications.Expand Specific Solutions
Key Players in Analytical Chemistry Industry
The field of pH control in analytical mixtures using ammonium hydroxide is in a mature stage of development, with established techniques and applications across various industries. The market size for this technology is significant, driven by its widespread use in laboratories, chemical processing, and environmental monitoring. Companies like Kurita Water Industries, DuPont de Nemours, and BASF Corp. have well-established positions in this sector, offering a range of products and solutions. The technology's maturity is evident in the involvement of academic institutions like The University of Queensland and Central South University, which contribute to ongoing research and development. While the core technology is well-understood, there is ongoing innovation in specialized applications and integration with advanced analytical systems.
Kurita Water Industries Ltd.
Technical Solution: Kurita Water Industries Ltd. has developed an advanced pH control system utilizing ammonium hydroxide for analytical mixtures. Their approach involves a precise dosing mechanism that adjusts the concentration of ammonium hydroxide based on real-time pH measurements[1]. The system employs a feedback loop with high-sensitivity pH sensors to maintain optimal pH levels in various analytical processes[3]. Kurita's technology also incorporates a predictive algorithm that anticipates pH changes based on the chemical composition of the mixture, allowing for proactive adjustments[5]. This method ensures stable pH control even in complex analytical environments with multiple interfering compounds.
Strengths: Precise pH control, real-time adjustment capability, and adaptability to complex mixtures. Weaknesses: May require frequent calibration and maintenance of sensors for optimal performance.
DuPont de Nemours, Inc.
Technical Solution: DuPont has engineered a novel pH control system for analytical mixtures using ammonium hydroxide as a key component. Their approach integrates a microfluidic delivery system that allows for precise control of ammonium hydroxide concentration at the nanoliter scale[2]. The technology utilizes advanced materials science to create pH-responsive surfaces within analytical devices, enhancing the efficiency of ammonium hydroxide in pH regulation[4]. DuPont's system also incorporates a proprietary buffering agent that works synergistically with ammonium hydroxide to provide extended pH stability in a wide range of analytical conditions[6]. This innovative approach enables rapid pH adjustments while minimizing sample dilution, crucial for maintaining the integrity of analytical results.
Strengths: High precision at microscale, minimal sample dilution, and extended pH stability. Weaknesses: May be more complex to implement in existing analytical systems and potentially higher cost.
Innovations in Ammonium Hydroxide Application
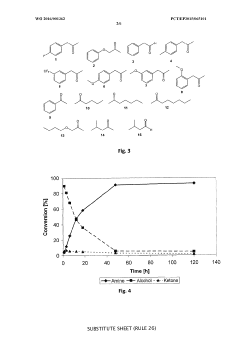
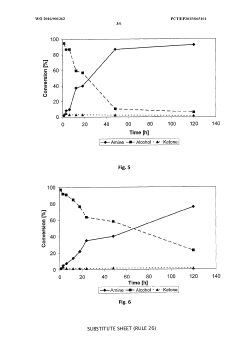
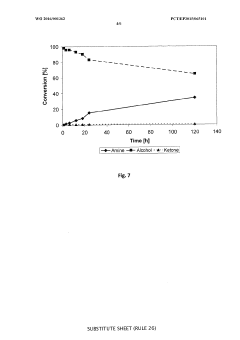
Redox self-sufficient biocatalytic amination of alcohols
PatentWO2016001362A1
Innovation
- A biocatalytic method using a combination of alcohol dehydrogenase and amine dehydrogenase enzymes in a redox self-sufficient process, where the enzymes operate simultaneously to regenerate cofactors, eliminating the need for external reducing equivalents and achieving high chemoselectivity and stereoselectivity.
High temperature gas desulfurization sorbents
PatentInactiveUS8308848B1
Innovation
- Development of sorbents with a nickel phase dispersed on high surface area metal oxide supports, such as γ-alumina, that are thermally stable up to 800°C, combined with rare earth doping and stabilizers like magnesium or manganese, to achieve efficient sulfur removal down to ppbv levels.
Safety and Handling of Ammonium Hydroxide
Ammonium hydroxide, while an essential component in pH control for analytical mixtures, requires careful handling due to its corrosive and toxic nature. Proper safety measures are crucial when working with this chemical. Personal protective equipment (PPE) is mandatory, including chemical-resistant gloves, safety goggles, and a lab coat. A well-ventilated workspace or fume hood is necessary to prevent inhalation of ammonia vapors.
Storage of ammonium hydroxide demands specific conditions. It should be kept in tightly sealed containers made of compatible materials such as glass or high-density polyethylene. The storage area must be cool, dry, and well-ventilated, away from direct sunlight and heat sources. Segregation from incompatible substances, particularly strong acids and oxidizing agents, is essential to prevent hazardous reactions.
Handling procedures for ammonium hydroxide require strict adherence to safety protocols. Always work with small quantities and use appropriate transfer techniques to minimize spills and splashes. Dilutions should be performed by adding the base to water, never the reverse, to avoid violent reactions. In case of accidental contact, immediate flushing with copious amounts of water is crucial, followed by seeking medical attention if necessary.
Spill management is a critical aspect of ammonium hydroxide handling. Small spills can be neutralized with dilute acids and absorbed using inert materials like vermiculite or sand. Larger spills necessitate professional cleanup and may require evacuation of the area. Proper disposal of ammonium hydroxide and its solutions must comply with local environmental regulations.
Training and education of personnel working with ammonium hydroxide are paramount. This includes understanding the chemical properties, potential hazards, and appropriate emergency response procedures. Regular safety drills and updates on handling protocols help maintain a safe working environment.
Environmental considerations are also significant when handling ammonium hydroxide. Its release into water systems can be harmful to aquatic life, and atmospheric emissions contribute to air pollution. Implementing closed systems and efficient scrubbing technologies can minimize environmental impact.
In analytical laboratories, where precise pH control is crucial, the use of automated dispensing systems can enhance safety and accuracy. These systems reduce direct handling and exposure risks while ensuring consistent and controlled addition of ammonium hydroxide to analytical mixtures.
Storage of ammonium hydroxide demands specific conditions. It should be kept in tightly sealed containers made of compatible materials such as glass or high-density polyethylene. The storage area must be cool, dry, and well-ventilated, away from direct sunlight and heat sources. Segregation from incompatible substances, particularly strong acids and oxidizing agents, is essential to prevent hazardous reactions.
Handling procedures for ammonium hydroxide require strict adherence to safety protocols. Always work with small quantities and use appropriate transfer techniques to minimize spills and splashes. Dilutions should be performed by adding the base to water, never the reverse, to avoid violent reactions. In case of accidental contact, immediate flushing with copious amounts of water is crucial, followed by seeking medical attention if necessary.
Spill management is a critical aspect of ammonium hydroxide handling. Small spills can be neutralized with dilute acids and absorbed using inert materials like vermiculite or sand. Larger spills necessitate professional cleanup and may require evacuation of the area. Proper disposal of ammonium hydroxide and its solutions must comply with local environmental regulations.
Training and education of personnel working with ammonium hydroxide are paramount. This includes understanding the chemical properties, potential hazards, and appropriate emergency response procedures. Regular safety drills and updates on handling protocols help maintain a safe working environment.
Environmental considerations are also significant when handling ammonium hydroxide. Its release into water systems can be harmful to aquatic life, and atmospheric emissions contribute to air pollution. Implementing closed systems and efficient scrubbing technologies can minimize environmental impact.
In analytical laboratories, where precise pH control is crucial, the use of automated dispensing systems can enhance safety and accuracy. These systems reduce direct handling and exposure risks while ensuring consistent and controlled addition of ammonium hydroxide to analytical mixtures.
Environmental Impact of pH Control Chemicals
The use of ammonium hydroxide for pH control in analytical mixtures has significant environmental implications that warrant careful consideration. As a widely used alkaline reagent, ammonium hydroxide's environmental impact stems primarily from its potential to contribute to eutrophication and ammonia pollution when released into aquatic ecosystems.
When ammonium hydroxide enters water bodies, it dissociates into ammonium ions and hydroxide ions. The ammonium ions can be converted to ammonia, which is highly toxic to aquatic life, particularly fish. Even at low concentrations, ammonia can cause gill damage, impair growth, and affect reproduction in various aquatic species. Furthermore, the excess nutrients introduced by ammonium compounds can lead to algal blooms, resulting in oxygen depletion and ecosystem disruption.
The production and transportation of ammonium hydroxide also contribute to its environmental footprint. Manufacturing processes often involve energy-intensive methods and may result in greenhouse gas emissions. Additionally, accidental spills during transportation or handling can lead to localized environmental damage, affecting soil quality and potentially contaminating groundwater.
However, it is important to note that when used properly in controlled laboratory settings, the environmental impact of ammonium hydroxide can be minimized. Proper disposal practices, such as neutralization before release or collection for specialized treatment, can significantly reduce its environmental burden. Many laboratories have implemented strict protocols for handling and disposing of ammonium hydroxide and other pH control chemicals to mitigate potential environmental risks.
Compared to other pH control chemicals, ammonium hydroxide's environmental impact is relatively moderate. For instance, sodium hydroxide, another common alkaline agent, poses less risk of nutrient pollution but can cause more severe localized damage due to its highly corrosive nature. Phosphate-based buffers, while effective for pH control, can contribute more significantly to eutrophication if released into the environment.
In recent years, there has been a growing trend towards developing and adopting more environmentally friendly alternatives for pH control in analytical applications. These include the use of organic buffers, which are less harmful to aquatic ecosystems, and the exploration of bio-based pH control agents derived from renewable resources. Such innovations aim to maintain analytical precision while reducing the environmental footprint of laboratory operations.
As environmental regulations become more stringent, the analytical chemistry community is increasingly focusing on green chemistry principles. This shift involves not only selecting less harmful pH control agents but also optimizing analytical methods to reduce the overall use of chemicals. Miniaturization of analytical techniques and the development of sensor-based pH monitoring systems are examples of approaches that can indirectly mitigate the environmental impact of pH control chemicals by reducing the volume of reagents required.
When ammonium hydroxide enters water bodies, it dissociates into ammonium ions and hydroxide ions. The ammonium ions can be converted to ammonia, which is highly toxic to aquatic life, particularly fish. Even at low concentrations, ammonia can cause gill damage, impair growth, and affect reproduction in various aquatic species. Furthermore, the excess nutrients introduced by ammonium compounds can lead to algal blooms, resulting in oxygen depletion and ecosystem disruption.
The production and transportation of ammonium hydroxide also contribute to its environmental footprint. Manufacturing processes often involve energy-intensive methods and may result in greenhouse gas emissions. Additionally, accidental spills during transportation or handling can lead to localized environmental damage, affecting soil quality and potentially contaminating groundwater.
However, it is important to note that when used properly in controlled laboratory settings, the environmental impact of ammonium hydroxide can be minimized. Proper disposal practices, such as neutralization before release or collection for specialized treatment, can significantly reduce its environmental burden. Many laboratories have implemented strict protocols for handling and disposing of ammonium hydroxide and other pH control chemicals to mitigate potential environmental risks.
Compared to other pH control chemicals, ammonium hydroxide's environmental impact is relatively moderate. For instance, sodium hydroxide, another common alkaline agent, poses less risk of nutrient pollution but can cause more severe localized damage due to its highly corrosive nature. Phosphate-based buffers, while effective for pH control, can contribute more significantly to eutrophication if released into the environment.
In recent years, there has been a growing trend towards developing and adopting more environmentally friendly alternatives for pH control in analytical applications. These include the use of organic buffers, which are less harmful to aquatic ecosystems, and the exploration of bio-based pH control agents derived from renewable resources. Such innovations aim to maintain analytical precision while reducing the environmental footprint of laboratory operations.
As environmental regulations become more stringent, the analytical chemistry community is increasingly focusing on green chemistry principles. This shift involves not only selecting less harmful pH control agents but also optimizing analytical methods to reduce the overall use of chemicals. Miniaturization of analytical techniques and the development of sensor-based pH monitoring systems are examples of approaches that can indirectly mitigate the environmental impact of pH control chemicals by reducing the volume of reagents required.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!