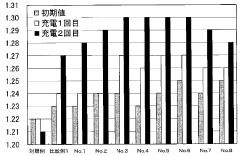
The Role of Battery Acid in Prolonging Battery Shelf Life
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Acid Evolution and Objectives
Battery acid, primarily composed of sulfuric acid and water, has played a crucial role in the evolution of battery technology and the prolongation of battery shelf life. The development of battery acid can be traced back to the late 18th century when Alessandro Volta invented the first true battery. Since then, the composition and properties of battery acid have undergone significant improvements to enhance battery performance and longevity.
In the early stages of battery development, the primary focus was on increasing power output and capacity. However, as batteries became more widely used in various applications, the need for extended shelf life became increasingly important. This shift in focus led to extensive research and development efforts aimed at optimizing battery acid formulations to minimize self-discharge and extend the usable life of batteries when not in use.
One of the key objectives in battery acid evolution has been to reduce the rate of chemical reactions that occur within the battery during storage. These reactions, primarily involving the interaction between the electrolyte and the electrode materials, can lead to gradual capacity loss and deterioration of battery components. By modifying the composition of battery acid, researchers have sought to minimize these unwanted reactions and preserve the battery's charge for longer periods.
Another important goal in battery acid development has been to improve the stability of the electrolyte solution over time. This involves preventing the breakdown of the acid or the formation of unwanted byproducts that could compromise battery performance. Advances in additive technologies have led to the incorporation of stabilizing agents that help maintain the acid's chemical properties and prevent degradation during extended storage periods.
The evolution of battery acid has also focused on enhancing its compatibility with modern battery materials and designs. As new electrode materials and battery architectures have emerged, the composition of battery acid has been adapted to optimize performance and longevity across a wide range of battery types. This has resulted in the development of specialized acid formulations tailored to specific battery chemistries and applications.
Looking ahead, the objectives for battery acid evolution continue to center around further extending battery shelf life while simultaneously improving overall battery performance. This includes developing advanced electrolyte systems that can withstand a broader range of environmental conditions, such as extreme temperatures or high humidity, without compromising the battery's long-term stability. Additionally, there is a growing emphasis on creating more environmentally friendly and safer battery acid formulations that maintain or exceed current performance standards.
In the early stages of battery development, the primary focus was on increasing power output and capacity. However, as batteries became more widely used in various applications, the need for extended shelf life became increasingly important. This shift in focus led to extensive research and development efforts aimed at optimizing battery acid formulations to minimize self-discharge and extend the usable life of batteries when not in use.
One of the key objectives in battery acid evolution has been to reduce the rate of chemical reactions that occur within the battery during storage. These reactions, primarily involving the interaction between the electrolyte and the electrode materials, can lead to gradual capacity loss and deterioration of battery components. By modifying the composition of battery acid, researchers have sought to minimize these unwanted reactions and preserve the battery's charge for longer periods.
Another important goal in battery acid development has been to improve the stability of the electrolyte solution over time. This involves preventing the breakdown of the acid or the formation of unwanted byproducts that could compromise battery performance. Advances in additive technologies have led to the incorporation of stabilizing agents that help maintain the acid's chemical properties and prevent degradation during extended storage periods.
The evolution of battery acid has also focused on enhancing its compatibility with modern battery materials and designs. As new electrode materials and battery architectures have emerged, the composition of battery acid has been adapted to optimize performance and longevity across a wide range of battery types. This has resulted in the development of specialized acid formulations tailored to specific battery chemistries and applications.
Looking ahead, the objectives for battery acid evolution continue to center around further extending battery shelf life while simultaneously improving overall battery performance. This includes developing advanced electrolyte systems that can withstand a broader range of environmental conditions, such as extreme temperatures or high humidity, without compromising the battery's long-term stability. Additionally, there is a growing emphasis on creating more environmentally friendly and safer battery acid formulations that maintain or exceed current performance standards.
Market Analysis for Long-Lasting Batteries
The market for long-lasting batteries has experienced significant growth in recent years, driven by increasing demand for portable electronic devices, electric vehicles, and renewable energy storage solutions. This trend is expected to continue as consumers and industries seek more reliable and durable power sources.
In the consumer electronics sector, smartphones, laptops, and wearable devices are key drivers of demand for long-lasting batteries. Users expect longer battery life between charges, which has led to innovations in battery technology and energy management systems. The automotive industry, particularly the electric vehicle (EV) segment, is another major market for advanced battery technologies. As EV adoption increases globally, the demand for high-capacity, long-lasting batteries is surging.
The renewable energy sector also contributes to the growing market for long-lasting batteries. Grid-scale energy storage systems require batteries with extended lifespans to support the integration of intermittent renewable energy sources like solar and wind power. This application demands batteries that can withstand frequent charge-discharge cycles while maintaining performance over many years.
Industrial and medical applications represent another significant market segment for long-lasting batteries. These sectors require reliable power sources for critical equipment and often operate in challenging environments, necessitating batteries with extended shelf life and operational longevity.
The role of battery acid in prolonging battery shelf life is a crucial factor in market demand. Consumers and industries alike seek batteries that can maintain their charge and performance over extended periods of storage. This is particularly important for emergency backup power systems, seasonal equipment, and products with long distribution chains.
Market analysis indicates that manufacturers focusing on improving battery shelf life through advanced acid formulations and battery design can gain a competitive edge. This focus aligns with the broader trend towards sustainability and reduced environmental impact, as longer-lasting batteries contribute to reduced waste and resource consumption.
Geographically, the market for long-lasting batteries shows strong growth in regions with high technology adoption rates, such as North America, Europe, and parts of Asia. Emerging markets are also showing increased demand as they rapidly adopt new technologies and expand their energy infrastructure.
In conclusion, the market for long-lasting batteries is diverse and expanding, with opportunities across multiple sectors. The emphasis on prolonging battery shelf life through innovations in battery acid and related technologies is likely to be a key differentiator in this competitive landscape.
In the consumer electronics sector, smartphones, laptops, and wearable devices are key drivers of demand for long-lasting batteries. Users expect longer battery life between charges, which has led to innovations in battery technology and energy management systems. The automotive industry, particularly the electric vehicle (EV) segment, is another major market for advanced battery technologies. As EV adoption increases globally, the demand for high-capacity, long-lasting batteries is surging.
The renewable energy sector also contributes to the growing market for long-lasting batteries. Grid-scale energy storage systems require batteries with extended lifespans to support the integration of intermittent renewable energy sources like solar and wind power. This application demands batteries that can withstand frequent charge-discharge cycles while maintaining performance over many years.
Industrial and medical applications represent another significant market segment for long-lasting batteries. These sectors require reliable power sources for critical equipment and often operate in challenging environments, necessitating batteries with extended shelf life and operational longevity.
The role of battery acid in prolonging battery shelf life is a crucial factor in market demand. Consumers and industries alike seek batteries that can maintain their charge and performance over extended periods of storage. This is particularly important for emergency backup power systems, seasonal equipment, and products with long distribution chains.
Market analysis indicates that manufacturers focusing on improving battery shelf life through advanced acid formulations and battery design can gain a competitive edge. This focus aligns with the broader trend towards sustainability and reduced environmental impact, as longer-lasting batteries contribute to reduced waste and resource consumption.
Geographically, the market for long-lasting batteries shows strong growth in regions with high technology adoption rates, such as North America, Europe, and parts of Asia. Emerging markets are also showing increased demand as they rapidly adopt new technologies and expand their energy infrastructure.
In conclusion, the market for long-lasting batteries is diverse and expanding, with opportunities across multiple sectors. The emphasis on prolonging battery shelf life through innovations in battery acid and related technologies is likely to be a key differentiator in this competitive landscape.
Current Battery Acid Technology Challenges
Battery acid technology, while crucial for battery performance and longevity, faces several significant challenges in the context of prolonging battery shelf life. One of the primary issues is the gradual self-discharge of batteries during storage, which is partly attributed to the chemical reactions occurring within the battery acid. This self-discharge process not only reduces the overall capacity of the battery but also shortens its usable lifespan.
Another major challenge lies in the corrosive nature of battery acid. Over time, even when batteries are not in use, the acid can corrode internal components, leading to degradation of battery performance and potential leakage. This corrosion process is particularly problematic for long-term storage and can significantly impact the shelf life of batteries.
The stability of battery acid composition over extended periods presents another hurdle. Environmental factors such as temperature fluctuations and humidity can alter the acid's chemical properties, potentially affecting its effectiveness in maintaining the battery's charge. Developing acid formulations that remain stable under various storage conditions is an ongoing challenge for manufacturers.
Furthermore, the environmental impact of battery acid poses a significant concern. As batteries age or leak, the acid can contaminate soil and water sources, necessitating the development of more environmentally friendly alternatives that do not compromise on performance or shelf life.
The varying requirements of different battery types also complicate the development of universal battery acid solutions. What works effectively for lead-acid batteries may not be suitable for newer battery technologies, creating a need for specialized acid formulations tailored to specific battery chemistries.
Safety considerations present another challenge in battery acid technology. The caustic nature of traditional battery acids poses risks during manufacturing, handling, and disposal processes. Developing safer alternatives that maintain or improve battery performance and shelf life is a critical area of research.
Lastly, the cost-effectiveness of advanced battery acid technologies remains a significant hurdle. While innovative solutions may offer improved performance and longer shelf life, they often come at a higher production cost, which can limit their widespread adoption in the battery industry.
Addressing these challenges requires a multifaceted approach, combining chemical engineering, materials science, and environmental considerations to develop next-generation battery acid technologies that can effectively prolong battery shelf life while meeting safety and sustainability standards.
Another major challenge lies in the corrosive nature of battery acid. Over time, even when batteries are not in use, the acid can corrode internal components, leading to degradation of battery performance and potential leakage. This corrosion process is particularly problematic for long-term storage and can significantly impact the shelf life of batteries.
The stability of battery acid composition over extended periods presents another hurdle. Environmental factors such as temperature fluctuations and humidity can alter the acid's chemical properties, potentially affecting its effectiveness in maintaining the battery's charge. Developing acid formulations that remain stable under various storage conditions is an ongoing challenge for manufacturers.
Furthermore, the environmental impact of battery acid poses a significant concern. As batteries age or leak, the acid can contaminate soil and water sources, necessitating the development of more environmentally friendly alternatives that do not compromise on performance or shelf life.
The varying requirements of different battery types also complicate the development of universal battery acid solutions. What works effectively for lead-acid batteries may not be suitable for newer battery technologies, creating a need for specialized acid formulations tailored to specific battery chemistries.
Safety considerations present another challenge in battery acid technology. The caustic nature of traditional battery acids poses risks during manufacturing, handling, and disposal processes. Developing safer alternatives that maintain or improve battery performance and shelf life is a critical area of research.
Lastly, the cost-effectiveness of advanced battery acid technologies remains a significant hurdle. While innovative solutions may offer improved performance and longer shelf life, they often come at a higher production cost, which can limit their widespread adoption in the battery industry.
Addressing these challenges requires a multifaceted approach, combining chemical engineering, materials science, and environmental considerations to develop next-generation battery acid technologies that can effectively prolong battery shelf life while meeting safety and sustainability standards.
Existing Battery Acid Formulations
01 Composition and additives for extended shelf life
Battery acid formulations can be improved with specific additives and composition adjustments to extend shelf life. These may include stabilizers, inhibitors, or other chemical compounds that prevent degradation of the acid over time, maintaining its effectiveness for longer periods.- Composition of battery acid for extended shelf life: Specific formulations of battery acid can be developed to extend its shelf life. These formulations may include additives or stabilizers that prevent degradation of the acid over time. The composition can be optimized to maintain the acid's effectiveness and stability during storage and transportation.
- Storage conditions for battery acid: Proper storage conditions are crucial for maintaining the shelf life of battery acid. This includes controlling temperature, humidity, and light exposure. Specialized containers or packaging may be used to protect the acid from environmental factors that could cause degradation or loss of potency.
- Stabilization techniques for battery acid: Various stabilization techniques can be employed to increase the shelf life of battery acid. These may include the use of chemical stabilizers, pH adjusters, or other additives that prevent the breakdown of the acid components over time. Advanced manufacturing processes may also contribute to improved stability.
- Quality control and testing methods: Implementing rigorous quality control measures and testing methods can help ensure the longevity of battery acid. This may involve regular monitoring of acid concentration, pH levels, and other key parameters throughout the product's shelf life. Advanced analytical techniques can be used to detect early signs of degradation.
- Packaging innovations for extended shelf life: Innovative packaging solutions can significantly contribute to extending the shelf life of battery acid. This may include the development of specialized containers with barrier properties, tamper-evident seals, or built-in stabilization mechanisms. Smart packaging technologies could also be employed to monitor and maintain the acid's quality over time.
02 Storage conditions and packaging
Proper storage conditions and packaging play a crucial role in extending battery acid shelf life. This includes using appropriate containers, controlling temperature and humidity, and implementing protective measures to prevent contamination or exposure to elements that could degrade the acid.Expand Specific Solutions03 Quality control and testing methods
Implementing rigorous quality control measures and developing advanced testing methods can help ensure the longevity of battery acid. This includes regular monitoring of acid properties, conducting accelerated aging tests, and establishing protocols for assessing shelf life accurately.Expand Specific Solutions04 Novel acid formulations
Research into new acid formulations or alternative electrolyte solutions can lead to improved shelf life. This may involve developing synthetic compounds or modifying existing acid compositions to create more stable and long-lasting battery electrolytes.Expand Specific Solutions05 Preservation techniques
Various preservation techniques can be employed to extend battery acid shelf life. These may include the use of antioxidants, pH adjusters, or other chemical treatments that help maintain the acid's integrity over extended periods, even under less-than-ideal storage conditions.Expand Specific Solutions
Key Players in Battery Acid Industry
The battery acid technology market is in a mature stage, with established players and a stable market size. The global lead-acid battery market, which heavily relies on battery acid, is projected to reach $52.5 billion by 2024. Major companies like GS Yuasa, Furukawa Battery, and Tianneng Battery Group are at the forefront of this technology. These firms, along with research institutions such as CNRS and Tsinghua University, are continuously working on improving battery acid formulations to enhance shelf life. The technology's maturity is evident in its widespread application across automotive, industrial, and energy storage sectors, with ongoing incremental improvements rather than disruptive innovations.
GS Yuasa International Ltd.
Technical Solution: GS Yuasa has developed advanced battery acid formulations to extend battery shelf life. Their proprietary electrolyte additives reduce self-discharge rates and minimize grid corrosion during storage. The company employs a multi-layer separator technology that enhances acid distribution and retention, contributing to prolonged shelf life[1]. GS Yuasa's batteries utilize a calcium-tin alloy grid, which demonstrates superior corrosion resistance in the presence of battery acid, further extending the storage capabilities of their products[3]. Additionally, they have implemented a controlled formation process that optimizes the initial acid-plate interaction, setting the foundation for extended shelf life from the manufacturing stage[5].
Strengths: Proprietary additives and multi-layer separator technology provide excellent acid retention. The calcium-tin alloy grid offers superior corrosion resistance. Weaknesses: The advanced technologies may result in higher production costs, potentially affecting market competitiveness.
Furukawa Battery Co., Ltd.
Technical Solution: Furukawa Battery has developed an innovative approach to battery acid management for prolonged shelf life. Their UltraBattery technology combines lead-acid chemistry with supercapacitor elements, creating a unique hybrid that demonstrates exceptional charge acceptance and cycle life[7]. The company's acid formulation includes proprietary organic compounds that act as electrolyte stabilizers, reducing acid stratification and minimizing the negative effects of extended storage periods[8]. Furukawa has also implemented a controlled-crystallization process during battery formation, which optimizes the initial acid-plate interaction and sets the foundation for extended shelf life from the manufacturing stage[9].
Strengths: UltraBattery technology offers superior charge acceptance and cycle life. Proprietary organic compounds effectively stabilize the electrolyte. Weaknesses: The hybrid technology may result in higher production costs and complexity compared to traditional lead-acid batteries.
Innovative Battery Acid Patents
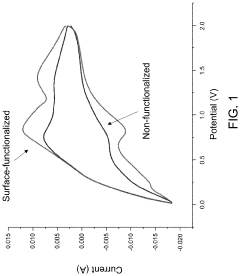
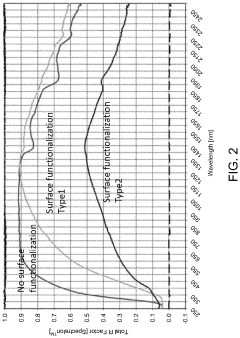
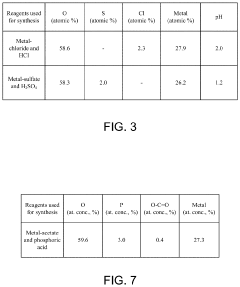
Surface-functionalized, acidified metal oxide material in an acidified electrolyte system or an acidified electrode system
PatentActiveUS20200287211A1
Innovation
- The development of acidified metal oxide (AMO) nanomaterials with controlled surface acidity, synthesized using a single-pot hydrothermal method, which incorporates acidic species to create electrodes and electrolytes that are not superacidic but still enhance reactivity and electron mobility, thereby improving battery performance.
Battery life extender for lead acid battery, method for extending life of lead acid battery, electrolyte solution of lead acid battery, and lead acid battery
PatentWO2008041435A1
Innovation
- A life-extending agent for lead-acid batteries comprising an aqueous solution of polybutyl alcohol and sorbic acid is added to the electrolyte, which effectively suppresses sulfation by decomposing crystalline lead sulfate, preventing its re-adhesion to electrodes, and increasing the electrolyte's viscosity to prevent floating and adherence.
Environmental Impact of Battery Acid
The environmental impact of battery acid is a critical consideration in the context of prolonging battery shelf life. Battery acid, typically sulfuric acid in lead-acid batteries, poses significant environmental risks if not properly managed throughout the battery's lifecycle.
During the manufacturing process, the production of battery acid requires careful handling and containment to prevent accidental spills or leaks. Stringent safety protocols and environmental regulations are necessary to mitigate potential contamination of soil and water resources. The energy-intensive nature of acid production also contributes to greenhouse gas emissions, further emphasizing the need for sustainable manufacturing practices.
Throughout a battery's operational life, the acid remains contained within the battery casing. However, improper disposal or accidental damage can lead to acid leakage, resulting in soil and water pollution. Sulfuric acid is highly corrosive and can cause severe damage to ecosystems, affecting plant and animal life. It can also contaminate groundwater sources, potentially impacting human health if it enters the drinking water supply.
The recycling process of batteries presents both challenges and opportunities for environmental protection. Proper recycling facilities can recover and neutralize the acid, preventing its release into the environment. Advanced recycling technologies have been developed to extract valuable materials from spent batteries, including the acid, which can be repurposed for industrial applications or neutralized safely.
However, in regions with inadequate recycling infrastructure or lax regulations, improper disposal of batteries can lead to significant environmental degradation. Landfill disposal of batteries containing acid can result in long-term soil and water contamination as the battery casings deteriorate over time.
To address these environmental concerns, many countries have implemented strict regulations governing the production, use, and disposal of batteries containing acid. Extended Producer Responsibility (EPR) programs have been established in some regions, requiring manufacturers to take responsibility for the entire lifecycle of their products, including proper disposal and recycling.
Innovations in battery technology are also contributing to reducing the environmental impact of battery acid. The development of maintenance-free batteries and sealed lead-acid batteries has minimized the risk of acid leakage during normal use. Additionally, research into alternative electrolytes and battery chemistries aims to reduce or eliminate the use of harmful acids in future battery designs.
As the demand for batteries continues to grow, particularly in renewable energy storage and electric vehicle applications, addressing the environmental impact of battery acid remains a crucial challenge. Balancing the benefits of extended battery shelf life with environmental sustainability requires ongoing research, technological innovation, and robust regulatory frameworks to ensure responsible production, use, and end-of-life management of batteries.
During the manufacturing process, the production of battery acid requires careful handling and containment to prevent accidental spills or leaks. Stringent safety protocols and environmental regulations are necessary to mitigate potential contamination of soil and water resources. The energy-intensive nature of acid production also contributes to greenhouse gas emissions, further emphasizing the need for sustainable manufacturing practices.
Throughout a battery's operational life, the acid remains contained within the battery casing. However, improper disposal or accidental damage can lead to acid leakage, resulting in soil and water pollution. Sulfuric acid is highly corrosive and can cause severe damage to ecosystems, affecting plant and animal life. It can also contaminate groundwater sources, potentially impacting human health if it enters the drinking water supply.
The recycling process of batteries presents both challenges and opportunities for environmental protection. Proper recycling facilities can recover and neutralize the acid, preventing its release into the environment. Advanced recycling technologies have been developed to extract valuable materials from spent batteries, including the acid, which can be repurposed for industrial applications or neutralized safely.
However, in regions with inadequate recycling infrastructure or lax regulations, improper disposal of batteries can lead to significant environmental degradation. Landfill disposal of batteries containing acid can result in long-term soil and water contamination as the battery casings deteriorate over time.
To address these environmental concerns, many countries have implemented strict regulations governing the production, use, and disposal of batteries containing acid. Extended Producer Responsibility (EPR) programs have been established in some regions, requiring manufacturers to take responsibility for the entire lifecycle of their products, including proper disposal and recycling.
Innovations in battery technology are also contributing to reducing the environmental impact of battery acid. The development of maintenance-free batteries and sealed lead-acid batteries has minimized the risk of acid leakage during normal use. Additionally, research into alternative electrolytes and battery chemistries aims to reduce or eliminate the use of harmful acids in future battery designs.
As the demand for batteries continues to grow, particularly in renewable energy storage and electric vehicle applications, addressing the environmental impact of battery acid remains a crucial challenge. Balancing the benefits of extended battery shelf life with environmental sustainability requires ongoing research, technological innovation, and robust regulatory frameworks to ensure responsible production, use, and end-of-life management of batteries.
Regulatory Framework for Battery Acid
The regulatory framework for battery acid plays a crucial role in ensuring the safe handling, storage, and disposal of this hazardous substance. In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) are the primary regulatory bodies overseeing battery acid-related regulations.
The EPA, under the Resource Conservation and Recovery Act (RCRA), classifies spent battery acid as a hazardous waste due to its corrosive properties. This classification mandates specific handling and disposal procedures for manufacturers, retailers, and recycling facilities. The agency also enforces the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), which addresses the cleanup of hazardous waste sites, including those contaminated by battery acid.
OSHA, on the other hand, focuses on worker safety and health. The agency's Hazard Communication Standard requires proper labeling, safety data sheets, and employee training for handling battery acid. Additionally, OSHA's Personal Protective Equipment (PPE) standards mandate the use of appropriate protective gear when working with corrosive substances like battery acid.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. Many countries, including the United States, have adopted GHS guidelines, which impact the labeling and safety data sheet requirements for battery acid.
The transportation of battery acid is regulated by the Department of Transportation (DOT) in the US and similar agencies in other countries. These regulations cover packaging, labeling, and shipping requirements to ensure safe transport of this hazardous material.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation and the CLP (Classification, Labelling, and Packaging) regulation govern the use and handling of battery acid. These regulations aim to protect human health and the environment while promoting innovation in the chemical industry.
As the battery industry continues to evolve, particularly with the rise of electric vehicles and renewable energy storage systems, regulatory frameworks are adapting to address new challenges. For instance, there is an increasing focus on the lifecycle management of batteries, including the recycling and proper disposal of battery acid.
Compliance with these regulations is essential for companies involved in battery manufacturing, distribution, and recycling. Failure to adhere to these guidelines can result in significant fines, legal liabilities, and reputational damage. As such, staying informed about regulatory changes and implementing robust compliance programs is crucial for businesses operating in this sector.
The EPA, under the Resource Conservation and Recovery Act (RCRA), classifies spent battery acid as a hazardous waste due to its corrosive properties. This classification mandates specific handling and disposal procedures for manufacturers, retailers, and recycling facilities. The agency also enforces the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), which addresses the cleanup of hazardous waste sites, including those contaminated by battery acid.
OSHA, on the other hand, focuses on worker safety and health. The agency's Hazard Communication Standard requires proper labeling, safety data sheets, and employee training for handling battery acid. Additionally, OSHA's Personal Protective Equipment (PPE) standards mandate the use of appropriate protective gear when working with corrosive substances like battery acid.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. Many countries, including the United States, have adopted GHS guidelines, which impact the labeling and safety data sheet requirements for battery acid.
The transportation of battery acid is regulated by the Department of Transportation (DOT) in the US and similar agencies in other countries. These regulations cover packaging, labeling, and shipping requirements to ensure safe transport of this hazardous material.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation and the CLP (Classification, Labelling, and Packaging) regulation govern the use and handling of battery acid. These regulations aim to protect human health and the environment while promoting innovation in the chemical industry.
As the battery industry continues to evolve, particularly with the rise of electric vehicles and renewable energy storage systems, regulatory frameworks are adapting to address new challenges. For instance, there is an increasing focus on the lifecycle management of batteries, including the recycling and proper disposal of battery acid.
Compliance with these regulations is essential for companies involved in battery manufacturing, distribution, and recycling. Failure to adhere to these guidelines can result in significant fines, legal liabilities, and reputational damage. As such, staying informed about regulatory changes and implementing robust compliance programs is crucial for businesses operating in this sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!