TMDs in Biosensing: Surface Functionalization and Sensitivity Control
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMDs Biosensing Background and Objectives
Transition metal dichalcogenides (TMDs) have emerged as a revolutionary class of two-dimensional materials that have garnered significant attention in the scientific community since the successful isolation of graphene in 2004. These atomically thin semiconductors, with their general formula MX2 (where M represents transition metals such as Mo, W, and X represents chalcogens like S, Se, Te), possess unique electronic, optical, and mechanical properties that make them exceptionally suitable for biosensing applications.
The evolution of TMDs in biosensing technology has witnessed remarkable progress over the past decade. Initially, research focused primarily on graphene-based sensors, but limitations in bandgap engineering prompted exploration of alternative 2D materials. TMDs filled this gap by offering tunable bandgaps, high surface-to-volume ratios, and excellent electrical conductivity, enabling more sensitive and selective biosensing platforms.
Current technological trends indicate a shift toward hybrid sensing systems that leverage the complementary properties of different TMDs. The integration of TMDs with other nanomaterials and biological recognition elements has opened new avenues for detecting biomarkers at unprecedented sensitivity levels. Furthermore, recent advances in surface functionalization techniques have significantly enhanced the biocompatibility and specificity of TMD-based biosensors.
The primary objective of this technical research is to comprehensively evaluate the current state and future potential of surface functionalization strategies for TMDs in biosensing applications. We aim to identify optimal approaches for controlling and enhancing the sensitivity of TMD-based biosensors through systematic surface modification techniques. This includes investigating covalent and non-covalent functionalization methods, exploring bioconjugation strategies, and assessing the impact of various surface treatments on sensor performance.
Additionally, this research seeks to establish correlations between specific surface functionalization techniques and resulting improvements in key biosensor parameters, including detection limit, response time, selectivity, and stability. By mapping these relationships, we intend to develop a comprehensive framework that guides the rational design of next-generation TMD biosensors tailored for specific biomedical applications.
The ultimate goal is to overcome existing limitations in TMD biosensor technology, particularly addressing challenges related to reproducibility, scalability, and long-term stability. Through systematic investigation of surface chemistry and interface engineering, we aim to establish standardized protocols for TMD functionalization that can accelerate the translation of these promising materials from laboratory demonstrations to practical clinical and point-of-care diagnostic platforms.
The evolution of TMDs in biosensing technology has witnessed remarkable progress over the past decade. Initially, research focused primarily on graphene-based sensors, but limitations in bandgap engineering prompted exploration of alternative 2D materials. TMDs filled this gap by offering tunable bandgaps, high surface-to-volume ratios, and excellent electrical conductivity, enabling more sensitive and selective biosensing platforms.
Current technological trends indicate a shift toward hybrid sensing systems that leverage the complementary properties of different TMDs. The integration of TMDs with other nanomaterials and biological recognition elements has opened new avenues for detecting biomarkers at unprecedented sensitivity levels. Furthermore, recent advances in surface functionalization techniques have significantly enhanced the biocompatibility and specificity of TMD-based biosensors.
The primary objective of this technical research is to comprehensively evaluate the current state and future potential of surface functionalization strategies for TMDs in biosensing applications. We aim to identify optimal approaches for controlling and enhancing the sensitivity of TMD-based biosensors through systematic surface modification techniques. This includes investigating covalent and non-covalent functionalization methods, exploring bioconjugation strategies, and assessing the impact of various surface treatments on sensor performance.
Additionally, this research seeks to establish correlations between specific surface functionalization techniques and resulting improvements in key biosensor parameters, including detection limit, response time, selectivity, and stability. By mapping these relationships, we intend to develop a comprehensive framework that guides the rational design of next-generation TMD biosensors tailored for specific biomedical applications.
The ultimate goal is to overcome existing limitations in TMD biosensor technology, particularly addressing challenges related to reproducibility, scalability, and long-term stability. Through systematic investigation of surface chemistry and interface engineering, we aim to establish standardized protocols for TMD functionalization that can accelerate the translation of these promising materials from laboratory demonstrations to practical clinical and point-of-care diagnostic platforms.
Market Analysis for TMD-based Biosensors
The global market for biosensors is experiencing robust growth, with the TMD-based biosensor segment emerging as a particularly promising niche. Current market valuations place the overall biosensor market at approximately $25 billion, with projections indicating a compound annual growth rate of 8-10% through 2028. Within this broader landscape, TMD-based biosensing technologies are gaining significant traction due to their exceptional sensitivity, selectivity, and potential for miniaturization.
Healthcare applications represent the largest market segment for TMD-based biosensors, driven by increasing demand for point-of-care diagnostics, continuous health monitoring, and personalized medicine solutions. The ability of TMD-based sensors to detect biomarkers at ultra-low concentrations positions them as ideal candidates for early disease detection, particularly for cancer, cardiovascular conditions, and infectious diseases.
Environmental monitoring constitutes another substantial market opportunity, with growing regulatory pressure for real-time detection of pollutants, pathogens, and toxins in water and air. TMD-based sensors offer advantages in terms of portability, low power consumption, and multi-analyte detection capabilities that traditional sensing technologies cannot match.
Food safety applications represent a rapidly expanding market segment, particularly in developing economies where supply chain monitoring infrastructure is being established. The ability of TMD-based sensors to detect contaminants, adulterants, and pathogens with high specificity addresses critical needs in this sector.
Regionally, North America currently dominates the TMD biosensor market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate, driven by increasing healthcare expenditure, expanding biotechnology sectors in China, Japan, and South Korea, and government initiatives supporting advanced sensor technologies.
Key market restraints include high initial development costs, challenges in mass production of consistent TMD materials, and regulatory hurdles for clinical applications. The cost factor is particularly significant, as current TMD sensor fabrication processes have not yet achieved economies of scale comparable to established sensing technologies.
Customer adoption patterns indicate that medical device manufacturers and pharmaceutical companies are the early adopters, primarily integrating TMD-based sensing elements into existing diagnostic platforms. Consumer electronics manufacturers are also showing increasing interest, particularly for wearable health monitoring applications.
Market penetration strategies will likely follow a premium-to-mass market trajectory, with initial applications in high-value medical diagnostics gradually expanding to more cost-sensitive applications as manufacturing processes mature and costs decrease.
Healthcare applications represent the largest market segment for TMD-based biosensors, driven by increasing demand for point-of-care diagnostics, continuous health monitoring, and personalized medicine solutions. The ability of TMD-based sensors to detect biomarkers at ultra-low concentrations positions them as ideal candidates for early disease detection, particularly for cancer, cardiovascular conditions, and infectious diseases.
Environmental monitoring constitutes another substantial market opportunity, with growing regulatory pressure for real-time detection of pollutants, pathogens, and toxins in water and air. TMD-based sensors offer advantages in terms of portability, low power consumption, and multi-analyte detection capabilities that traditional sensing technologies cannot match.
Food safety applications represent a rapidly expanding market segment, particularly in developing economies where supply chain monitoring infrastructure is being established. The ability of TMD-based sensors to detect contaminants, adulterants, and pathogens with high specificity addresses critical needs in this sector.
Regionally, North America currently dominates the TMD biosensor market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate, driven by increasing healthcare expenditure, expanding biotechnology sectors in China, Japan, and South Korea, and government initiatives supporting advanced sensor technologies.
Key market restraints include high initial development costs, challenges in mass production of consistent TMD materials, and regulatory hurdles for clinical applications. The cost factor is particularly significant, as current TMD sensor fabrication processes have not yet achieved economies of scale comparable to established sensing technologies.
Customer adoption patterns indicate that medical device manufacturers and pharmaceutical companies are the early adopters, primarily integrating TMD-based sensing elements into existing diagnostic platforms. Consumer electronics manufacturers are also showing increasing interest, particularly for wearable health monitoring applications.
Market penetration strategies will likely follow a premium-to-mass market trajectory, with initial applications in high-value medical diagnostics gradually expanding to more cost-sensitive applications as manufacturing processes mature and costs decrease.
Current TMD Surface Functionalization Challenges
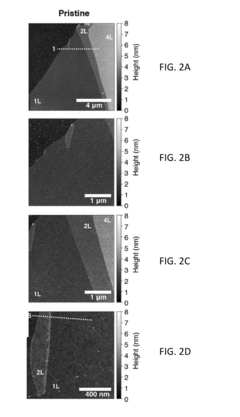
Despite significant advancements in TMD-based biosensing platforms, surface functionalization remains a critical bottleneck that limits widespread adoption. The inherent chemical inertness of pristine TMD surfaces presents a fundamental challenge, as it restricts the direct attachment of biomolecules necessary for specific target recognition. This intrinsic property, while beneficial for certain electronic applications, significantly hampers biosensor development by limiting available binding sites for biorecognition elements.
Current covalent functionalization approaches often disrupt the electronic structure of TMDs, leading to degraded sensing performance. The introduction of defects during functionalization processes can alter carrier mobility and concentration, resulting in inconsistent sensor responses. Moreover, these modifications frequently compromise the exceptional electronic properties that make TMDs attractive sensing materials in the first place, creating a challenging trade-off between functionalization density and device performance.
Non-covalent functionalization strategies, while preserving electronic properties, suffer from stability issues in complex biological environments. The weak interactions underpinning these approaches are susceptible to displacement by competing biomolecules in real samples, leading to poor sensor durability and reliability. Additionally, achieving uniform surface coverage remains problematic, resulting in batch-to-batch variability that complicates manufacturing scale-up and clinical translation.
The lack of standardized functionalization protocols represents another significant hurdle. Current literature reveals a fragmented landscape of methodologies, with limited systematic studies comparing different approaches under identical conditions. This absence of benchmarking makes it difficult to identify optimal functionalization strategies for specific sensing applications and impedes knowledge transfer between research groups.
Controlling the orientation of immobilized biomolecules presents yet another challenge. Random orientation of recognition elements (e.g., antibodies, aptamers) reduces binding efficiency and sensor sensitivity. Current functionalization methods provide limited control over biomolecule orientation, resulting in suboptimal target capture efficiency and diminished sensor performance in low-concentration detection scenarios.
Scalability concerns further complicate TMD surface functionalization. Many laboratory-scale functionalization techniques employ complex, multi-step processes that are difficult to implement in mass production environments. The lack of industrially viable functionalization methods represents a significant barrier to commercial translation of TMD biosensors, despite their promising performance in research settings.
Current covalent functionalization approaches often disrupt the electronic structure of TMDs, leading to degraded sensing performance. The introduction of defects during functionalization processes can alter carrier mobility and concentration, resulting in inconsistent sensor responses. Moreover, these modifications frequently compromise the exceptional electronic properties that make TMDs attractive sensing materials in the first place, creating a challenging trade-off between functionalization density and device performance.
Non-covalent functionalization strategies, while preserving electronic properties, suffer from stability issues in complex biological environments. The weak interactions underpinning these approaches are susceptible to displacement by competing biomolecules in real samples, leading to poor sensor durability and reliability. Additionally, achieving uniform surface coverage remains problematic, resulting in batch-to-batch variability that complicates manufacturing scale-up and clinical translation.
The lack of standardized functionalization protocols represents another significant hurdle. Current literature reveals a fragmented landscape of methodologies, with limited systematic studies comparing different approaches under identical conditions. This absence of benchmarking makes it difficult to identify optimal functionalization strategies for specific sensing applications and impedes knowledge transfer between research groups.
Controlling the orientation of immobilized biomolecules presents yet another challenge. Random orientation of recognition elements (e.g., antibodies, aptamers) reduces binding efficiency and sensor sensitivity. Current functionalization methods provide limited control over biomolecule orientation, resulting in suboptimal target capture efficiency and diminished sensor performance in low-concentration detection scenarios.
Scalability concerns further complicate TMD surface functionalization. Many laboratory-scale functionalization techniques employ complex, multi-step processes that are difficult to implement in mass production environments. The lack of industrially viable functionalization methods represents a significant barrier to commercial translation of TMD biosensors, despite their promising performance in research settings.
State-of-the-Art Surface Functionalization Methods
01 Surface functionalization methods for TMDs
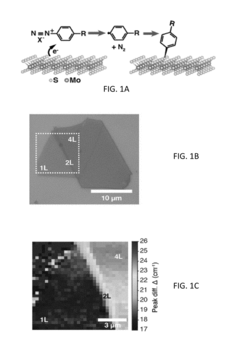
Various methods can be employed to functionalize the surface of transition metal dichalcogenides (TMDs) to enhance their properties. These methods include chemical vapor deposition, plasma treatment, and solution-based approaches. Surface functionalization can modify the electronic, optical, and chemical properties of TMDs, making them suitable for various applications. The functionalization process typically involves attaching specific functional groups or molecules to the TMD surface to achieve desired characteristics.- Surface functionalization methods for TMDs: Various methods can be employed to functionalize the surface of transition metal dichalcogenides (TMDs) to enhance their properties. These methods include chemical vapor deposition, plasma treatment, and solution-based approaches. Surface functionalization can modify the electronic, optical, and chemical properties of TMDs, making them suitable for various applications including sensing, electronics, and energy storage. The functionalization process typically involves attaching specific functional groups or molecules to the TMD surface to achieve desired characteristics.
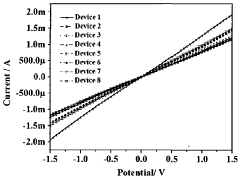
- TMD-based sensor devices with enhanced sensitivity: Transition metal dichalcogenides can be incorporated into sensor devices with high sensitivity for detecting various analytes. By functionalizing TMD surfaces with specific receptor molecules, these sensors can detect gases, biomolecules, and environmental pollutants with high specificity and low detection limits. The two-dimensional structure of TMDs provides a large surface area for interaction with target molecules, while their unique electronic properties allow for efficient signal transduction. These sensors often exhibit fast response times and good recovery characteristics, making them suitable for real-time monitoring applications.
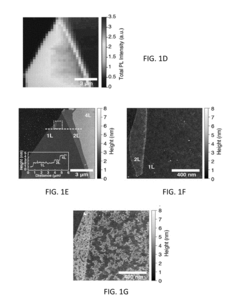
- Defect engineering and doping of TMDs: Controlled introduction of defects and dopants into the crystal structure of transition metal dichalcogenides can significantly alter their sensitivity and functionality. Techniques such as ion bombardment, thermal annealing, and chemical treatment can create vacancies, substitutional defects, or adatoms in the TMD lattice. These defects can serve as active sites for molecular adsorption and chemical reactions, enhancing the material's sensitivity to specific analytes. Additionally, doping with heteroatoms can modify the electronic band structure of TMDs, tuning their conductivity and optical properties for improved sensor performance.
- Hybrid TMD nanostructures for enhanced functionality: Combining transition metal dichalcogenides with other nanomaterials creates hybrid structures with enhanced functionality and sensitivity. These hybrids may incorporate noble metal nanoparticles, carbon nanomaterials, metal oxides, or polymers. The synergistic effects between TMDs and these materials can improve charge transfer, increase active surface area, and provide additional binding sites for target molecules. Such hybrid structures often demonstrate superior sensing performance compared to pristine TMDs, including lower detection limits, higher selectivity, and improved stability under various environmental conditions.
- Environmental and operational stability of functionalized TMDs: Improving the environmental and operational stability of functionalized transition metal dichalcogenides is crucial for their practical applications. TMDs can be susceptible to oxidation, humidity, and degradation under certain conditions, which may compromise their performance over time. Protective coatings, encapsulation techniques, and specific surface modifications can enhance the stability of TMDs in various environments. Additionally, optimizing the density and distribution of functional groups on the TMD surface can prevent agglomeration and maintain the material's active surface area, ensuring consistent sensitivity and reliable performance in sensing applications.
02 TMD-based sensing applications and sensitivity enhancement
Functionalized TMDs exhibit excellent sensing capabilities for various analytes including gases, biomolecules, and environmental pollutants. The sensitivity of TMD-based sensors can be significantly enhanced through strategic surface modifications that increase active sites for analyte interaction. These sensors demonstrate high selectivity, fast response times, and low detection limits. The sensing mechanism typically relies on changes in electrical conductivity, optical properties, or electrochemical behavior upon analyte adsorption on the functionalized TMD surface.Expand Specific Solutions03 Defect engineering and doping of TMDs
Controlled introduction of defects and dopants into the TMD lattice can significantly alter their electronic and optical properties. Techniques such as ion bombardment, thermal annealing, and chemical treatment can create vacancies, substitutional defects, or adatoms in TMD structures. These engineered defects can serve as active sites for molecular adsorption, enhancing sensitivity in sensing applications. Additionally, doping with heteroatoms can tune the band structure and charge carrier concentration, leading to improved electrical conductivity and sensing performance.Expand Specific Solutions04 TMD-based heterostructures and composites
Combining TMDs with other materials to form heterostructures or composites can enhance their functionality and sensitivity. These hybrid structures may include TMDs integrated with other 2D materials, metal nanoparticles, polymers, or carbon-based materials. The resulting composites often exhibit synergistic effects, including improved charge transfer, increased surface area, and enhanced stability. These properties make TMD-based heterostructures particularly valuable for high-performance sensing applications with improved sensitivity and selectivity.Expand Specific Solutions05 Novel synthesis and processing techniques for TMDs
Advanced synthesis and processing techniques have been developed to produce high-quality TMDs with controlled thickness, crystallinity, and surface properties. These methods include exfoliation techniques, hydrothermal synthesis, chemical vapor deposition with precise control over growth parameters, and solution-phase methods. Post-synthesis treatments such as annealing, plasma exposure, or chemical treatments can further modify the surface properties of TMDs. These techniques enable the production of TMDs with tailored characteristics for specific sensing applications, resulting in improved sensitivity and performance.Expand Specific Solutions
Leading Companies and Research Institutions in TMD Biosensing
The field of TMDs in biosensing is currently in a growth phase, with increasing market potential driven by advancements in surface functionalization and sensitivity control technologies. The global biosensor market is expanding rapidly, with TMD-based sensors representing an emerging segment poised for significant growth. Technologically, the field shows varying maturity levels across different applications, with companies like Koninklijke Philips NV and GLOBALFOUNDRIES leading commercial development, while academic institutions such as Zhejiang University and Arizona State University drive fundamental research. Research organizations like Southwest Research Institute and Wisconsin Alumni Research Foundation are bridging the gap between academic discoveries and commercial applications. The competitive landscape features both established electronics manufacturers and specialized biosensor companies, with increasing collaboration between industry and academia to overcome sensitivity and functionalization challenges.
Zhejiang University
Technical Solution: Zhejiang University has pioneered a defect engineering approach for TMD biosensors that strategically introduces and controls defects in the TMD lattice to enhance sensitivity while maintaining selectivity. Their technique involves creating sulfur vacancies in MoS2 through controlled thermal annealing in hydrogen atmosphere, followed by passivation of specific defect sites with organic linkers carrying functional groups for biomolecule attachment. This method has demonstrated remarkable improvements in sensor response, with up to 15-fold enhancement in sensitivity compared to pristine TMD sensors. The research team has further developed a gradient functionalization technique that creates spatially varying receptor densities across the TMD surface, enabling simultaneous multi-analyte detection with different sensitivity ranges on a single device. Recent work has focused on integrating these functionalized TMD sensors with flexible substrates for wearable biosensing applications, demonstrating stable performance under mechanical deformation and in complex biological fluids such as sweat and interstitial fluid.
Strengths: The defect engineering approach provides precise control over sensor sensitivity through tunable defect density, and the gradient functionalization enables versatile multi-analyte detection capabilities. Weaknesses: The thermal annealing process requires precise control to avoid excessive defect formation that could compromise device performance, and the approach may face challenges in large-scale manufacturing with consistent defect profiles.
President & Fellows of Harvard College
Technical Solution: Harvard has developed advanced surface functionalization techniques for TMD-based biosensors using thiol chemistry to covalently attach biomolecular receptors to MoS2 surfaces. Their approach involves creating defect sites on the TMD surface that serve as anchoring points for thiol-terminated molecules, enabling specific detection of biomarkers. The research team has demonstrated a novel method using mild oxygen plasma treatment to create controlled sulfur vacancies that enhance binding sites without compromising the electronic properties of the TMD material. This technique has shown remarkable sensitivity improvements, with detection limits in the picomolar range for various protein biomarkers and nucleic acids. Harvard researchers have also pioneered the integration of these functionalized TMD sensors with microfluidic platforms for sample handling and signal amplification, creating complete lab-on-chip diagnostic systems with potential applications in point-of-care testing.
Strengths: Harvard's approach offers exceptional sensitivity and selectivity through precisely controlled surface chemistry, maintaining the intrinsic electronic properties of TMDs. Their integrated microfluidic systems provide practical sample handling solutions. Weaknesses: The plasma treatment process requires careful optimization to avoid excessive damage to the TMD structure, and the long-term stability of the functionalized surfaces in biological environments remains a challenge.
Key Patents and Literature on TMD Sensitivity Control
Metallic transition metal dichalcogenide based chemiresistive biosensor
PatentActiveIN202241026382A
Innovation
- A chemiresistive biosensor using two-dimensional vanadium disulfide nanosheets is developed, where the nanosheets are biofunctionalized with monoclonal anti-MMP9 antibodies and exfoliated to create a conducting channel between interdigitated gold microelectrodes, enabling label-free detection with a wider linear range and lower detection limits.
Method for functionalizing transition metal dichalcogenides
PatentActiveUS10155782B2
Innovation
- A lithium-free method involving direct reaction of predominantly semiconducting 2H phase TMDCs with aryl diazonium salts, such as 4-nitrobenzenediazonium tetrafluoroborate, without converting to the metallic 1T phase, allowing for stable covalent functionalization and retention of semiconducting properties.
Biocompatibility and Toxicity Considerations
The integration of Transition Metal Dichalcogenides (TMDs) into biosensing applications necessitates thorough evaluation of their biocompatibility and potential toxicity. Recent studies have demonstrated varying degrees of cytotoxicity among different TMD materials, with factors such as layer thickness, lateral dimensions, and surface chemistry significantly influencing their biological interactions. MoS2, one of the most widely studied TMDs, has shown relatively low toxicity compared to other 2D materials, particularly when properly functionalized with biocompatible molecules.
Surface oxidation states of TMDs represent a critical consideration, as oxidized edges can generate reactive oxygen species (ROS) when in contact with biological systems. These ROS may induce cellular stress and potential damage to biomolecules. Research indicates that controlling the oxidation state through appropriate surface passivation techniques can substantially mitigate these effects, enhancing the biocompatibility profile of TMD-based biosensors.
The biodegradability of TMDs presents both advantages and challenges in biosensing applications. While controlled degradation can be beneficial for transient biosensing devices, unintended degradation may release potentially harmful metal ions into biological environments. Studies have shown that MoS2 and WS2 exhibit different degradation kinetics in physiological conditions, with degradation products requiring careful characterization to assess long-term safety profiles.
Protein corona formation—the adsorption of biomolecules onto TMD surfaces when exposed to biological fluids—significantly alters their biological identity and subsequent cellular interactions. This phenomenon can mask functionalized sensing elements, potentially compromising device performance. However, strategic surface engineering approaches can leverage this effect to enhance biocompatibility while maintaining sensing capabilities.
Immune system responses to TMD-based materials vary based on their physicochemical properties. Larger TMD flakes tend to trigger stronger inflammatory responses compared to smaller, well-exfoliated nanosheets. Surface functionalization with polyethylene glycol (PEG) or other biocompatible polymers has proven effective in reducing immunogenicity, enabling longer circulation times for in vivo applications.
Standardized toxicity assessment protocols specifically designed for TMD materials remain underdeveloped, creating challenges in comparing results across different studies. The establishment of comprehensive testing frameworks that address acute and chronic exposure scenarios, dose-dependent responses, and organ-specific accumulation patterns is essential for advancing TMD biosensors toward clinical applications.
Recent advances in green synthesis methods for TMDs have shown promise in reducing the environmental and biological impact of these materials. Approaches utilizing biocompatible reducing agents and environmentally friendly exfoliation techniques produce TMDs with inherently lower toxicity profiles while maintaining their exceptional sensing capabilities.
Surface oxidation states of TMDs represent a critical consideration, as oxidized edges can generate reactive oxygen species (ROS) when in contact with biological systems. These ROS may induce cellular stress and potential damage to biomolecules. Research indicates that controlling the oxidation state through appropriate surface passivation techniques can substantially mitigate these effects, enhancing the biocompatibility profile of TMD-based biosensors.
The biodegradability of TMDs presents both advantages and challenges in biosensing applications. While controlled degradation can be beneficial for transient biosensing devices, unintended degradation may release potentially harmful metal ions into biological environments. Studies have shown that MoS2 and WS2 exhibit different degradation kinetics in physiological conditions, with degradation products requiring careful characterization to assess long-term safety profiles.
Protein corona formation—the adsorption of biomolecules onto TMD surfaces when exposed to biological fluids—significantly alters their biological identity and subsequent cellular interactions. This phenomenon can mask functionalized sensing elements, potentially compromising device performance. However, strategic surface engineering approaches can leverage this effect to enhance biocompatibility while maintaining sensing capabilities.
Immune system responses to TMD-based materials vary based on their physicochemical properties. Larger TMD flakes tend to trigger stronger inflammatory responses compared to smaller, well-exfoliated nanosheets. Surface functionalization with polyethylene glycol (PEG) or other biocompatible polymers has proven effective in reducing immunogenicity, enabling longer circulation times for in vivo applications.
Standardized toxicity assessment protocols specifically designed for TMD materials remain underdeveloped, creating challenges in comparing results across different studies. The establishment of comprehensive testing frameworks that address acute and chronic exposure scenarios, dose-dependent responses, and organ-specific accumulation patterns is essential for advancing TMD biosensors toward clinical applications.
Recent advances in green synthesis methods for TMDs have shown promise in reducing the environmental and biological impact of these materials. Approaches utilizing biocompatible reducing agents and environmentally friendly exfoliation techniques produce TMDs with inherently lower toxicity profiles while maintaining their exceptional sensing capabilities.
Scalable Manufacturing Processes for TMD Biosensors
The scalable manufacturing of TMD biosensors represents a critical challenge for transitioning these promising devices from laboratory prototypes to commercial products. Current manufacturing approaches primarily rely on small-scale production methods such as mechanical exfoliation and chemical vapor deposition (CVD), which face significant limitations in throughput, consistency, and cost-effectiveness when considered for industrial-scale implementation.
Solution-phase synthesis methods have emerged as promising alternatives for large-scale TMD production. These techniques, including hydrothermal synthesis and liquid exfoliation, offer advantages in terms of scalability and cost-efficiency. Recent advancements in liquid exfoliation have demonstrated the ability to produce TMD nanosheets with controlled thickness and lateral dimensions, which are crucial parameters for biosensing applications.
Roll-to-roll processing represents another significant advancement toward scalable TMD biosensor manufacturing. This continuous production method enables the deposition of TMD materials onto flexible substrates at high throughput rates. Several research groups have successfully demonstrated roll-to-roll production of MoS2 and WS2 films with consistent quality across large areas, suggesting potential for industrial-scale manufacturing.
Integration with existing semiconductor fabrication infrastructure presents a viable pathway for scaling TMD biosensor production. Adaptation of conventional photolithography, etching, and thin-film deposition techniques for TMD materials has shown promising results. Companies like Applied Materials and Lam Research have begun developing specialized equipment for TMD processing that maintains compatibility with standard semiconductor manufacturing lines.
Quality control and standardization remain significant challenges in scaled manufacturing. The development of in-line characterization techniques, including optical spectroscopy and electrical testing methods, has improved production consistency. Organizations such as the International Electrotechnical Commission (IEC) have initiated efforts to establish standardized testing protocols for 2D materials, which will facilitate quality assurance in mass production scenarios.
Cost considerations significantly impact manufacturing strategy selection. Economic analyses indicate that solution-phase methods currently offer the lowest cost per unit area for TMD production ($0.1-1 per cm²) compared to CVD methods ($5-20 per cm²). However, the higher defect density in solution-processed materials may necessitate additional purification steps, potentially offsetting some cost advantages.
Environmental sustainability of manufacturing processes has gained increasing attention. Green chemistry approaches for TMD synthesis, utilizing non-toxic precursors and reducing hazardous waste, are being developed. Life cycle assessments of various manufacturing routes suggest that solution-phase methods generally have lower environmental impacts than vapor-phase techniques, though comprehensive sustainability metrics are still being established.
Solution-phase synthesis methods have emerged as promising alternatives for large-scale TMD production. These techniques, including hydrothermal synthesis and liquid exfoliation, offer advantages in terms of scalability and cost-efficiency. Recent advancements in liquid exfoliation have demonstrated the ability to produce TMD nanosheets with controlled thickness and lateral dimensions, which are crucial parameters for biosensing applications.
Roll-to-roll processing represents another significant advancement toward scalable TMD biosensor manufacturing. This continuous production method enables the deposition of TMD materials onto flexible substrates at high throughput rates. Several research groups have successfully demonstrated roll-to-roll production of MoS2 and WS2 films with consistent quality across large areas, suggesting potential for industrial-scale manufacturing.
Integration with existing semiconductor fabrication infrastructure presents a viable pathway for scaling TMD biosensor production. Adaptation of conventional photolithography, etching, and thin-film deposition techniques for TMD materials has shown promising results. Companies like Applied Materials and Lam Research have begun developing specialized equipment for TMD processing that maintains compatibility with standard semiconductor manufacturing lines.
Quality control and standardization remain significant challenges in scaled manufacturing. The development of in-line characterization techniques, including optical spectroscopy and electrical testing methods, has improved production consistency. Organizations such as the International Electrotechnical Commission (IEC) have initiated efforts to establish standardized testing protocols for 2D materials, which will facilitate quality assurance in mass production scenarios.
Cost considerations significantly impact manufacturing strategy selection. Economic analyses indicate that solution-phase methods currently offer the lowest cost per unit area for TMD production ($0.1-1 per cm²) compared to CVD methods ($5-20 per cm²). However, the higher defect density in solution-processed materials may necessitate additional purification steps, potentially offsetting some cost advantages.
Environmental sustainability of manufacturing processes has gained increasing attention. Green chemistry approaches for TMD synthesis, utilizing non-toxic precursors and reducing hazardous waste, are being developed. Life cycle assessments of various manufacturing routes suggest that solution-phase methods generally have lower environmental impacts than vapor-phase techniques, though comprehensive sustainability metrics are still being established.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!