Why Ammonium Hydroxide is Key in Polymer Gel Electrolytes Production
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide in PGE: Background and Objectives
Polymer gel electrolytes (PGEs) have emerged as a promising solution in the field of energy storage, particularly for advanced battery technologies. The development of PGEs has been driven by the need for safer, more efficient, and flexible energy storage systems. Ammonium hydroxide plays a crucial role in the production of these innovative materials, serving as a key component in the synthesis process.
The evolution of PGEs can be traced back to the early 1970s when the concept of polymer electrolytes was first introduced. Since then, researchers have been working tirelessly to improve their properties and performance. The incorporation of ammonium hydroxide into the PGE production process represents a significant milestone in this technological journey, addressing several challenges that had previously hindered widespread adoption.
One of the primary objectives in utilizing ammonium hydroxide in PGE production is to enhance the ionic conductivity of the resulting electrolyte. By introducing ammonium ions into the polymer matrix, researchers aim to create a more efficient pathway for ion transport, which is essential for improved battery performance. This approach has shown promising results in increasing the overall energy density and power output of batteries incorporating PGEs.
Another critical goal is to improve the mechanical stability of the gel electrolytes. Ammonium hydroxide contributes to the formation of a robust, cross-linked polymer network, which helps maintain the structural integrity of the electrolyte under various operating conditions. This enhanced stability is particularly important for applications in flexible and wearable electronics, where the electrolyte must withstand repeated bending and stretching.
Furthermore, the use of ammonium hydroxide in PGE production addresses safety concerns associated with traditional liquid electrolytes. By creating a gel-like structure, the risk of electrolyte leakage is significantly reduced, minimizing the potential for short circuits and thermal runaway events. This improvement in safety is a key driver for the adoption of PGEs in consumer electronics and electric vehicles.
The technological trend in PGE development is moving towards more sustainable and environmentally friendly production methods. Ammonium hydroxide, being a relatively benign and easily handled compound, aligns well with this trend. Researchers are exploring ways to optimize its use in PGE synthesis to reduce environmental impact and improve the overall sustainability of battery production processes.
As we look to the future, the role of ammonium hydroxide in PGE production is expected to expand further. Ongoing research is focused on fine-tuning the chemistry and processing techniques to achieve even higher performance metrics. The ultimate goal is to develop PGEs that can outperform traditional liquid electrolytes in all aspects, paving the way for the next generation of energy storage solutions.
The evolution of PGEs can be traced back to the early 1970s when the concept of polymer electrolytes was first introduced. Since then, researchers have been working tirelessly to improve their properties and performance. The incorporation of ammonium hydroxide into the PGE production process represents a significant milestone in this technological journey, addressing several challenges that had previously hindered widespread adoption.
One of the primary objectives in utilizing ammonium hydroxide in PGE production is to enhance the ionic conductivity of the resulting electrolyte. By introducing ammonium ions into the polymer matrix, researchers aim to create a more efficient pathway for ion transport, which is essential for improved battery performance. This approach has shown promising results in increasing the overall energy density and power output of batteries incorporating PGEs.
Another critical goal is to improve the mechanical stability of the gel electrolytes. Ammonium hydroxide contributes to the formation of a robust, cross-linked polymer network, which helps maintain the structural integrity of the electrolyte under various operating conditions. This enhanced stability is particularly important for applications in flexible and wearable electronics, where the electrolyte must withstand repeated bending and stretching.
Furthermore, the use of ammonium hydroxide in PGE production addresses safety concerns associated with traditional liquid electrolytes. By creating a gel-like structure, the risk of electrolyte leakage is significantly reduced, minimizing the potential for short circuits and thermal runaway events. This improvement in safety is a key driver for the adoption of PGEs in consumer electronics and electric vehicles.
The technological trend in PGE development is moving towards more sustainable and environmentally friendly production methods. Ammonium hydroxide, being a relatively benign and easily handled compound, aligns well with this trend. Researchers are exploring ways to optimize its use in PGE synthesis to reduce environmental impact and improve the overall sustainability of battery production processes.
As we look to the future, the role of ammonium hydroxide in PGE production is expected to expand further. Ongoing research is focused on fine-tuning the chemistry and processing techniques to achieve even higher performance metrics. The ultimate goal is to develop PGEs that can outperform traditional liquid electrolytes in all aspects, paving the way for the next generation of energy storage solutions.
Market Demand for Advanced Electrolytes
The demand for advanced electrolytes, particularly polymer gel electrolytes, has been steadily increasing in recent years due to the rapid growth of the energy storage industry. This surge in demand is primarily driven by the expanding electric vehicle (EV) market, the growing adoption of renewable energy systems, and the increasing need for high-performance portable electronic devices.
In the automotive sector, the global shift towards electrification has created a substantial market for advanced battery technologies. Polymer gel electrolytes offer several advantages over traditional liquid electrolytes, including improved safety, higher energy density, and better thermal stability. These characteristics make them particularly attractive for use in EVs, where battery performance and safety are paramount concerns.
The renewable energy sector is another key driver of demand for advanced electrolytes. As the world transitions towards cleaner energy sources, there is a growing need for efficient and reliable energy storage solutions. Polymer gel electrolytes can play a crucial role in improving the performance and longevity of grid-scale energy storage systems, which are essential for managing the intermittent nature of renewable energy sources such as solar and wind power.
In the consumer electronics market, the demand for longer-lasting and faster-charging devices continues to grow. Polymer gel electrolytes have the potential to significantly enhance the performance of lithium-ion batteries used in smartphones, laptops, and other portable devices. This improvement can lead to extended battery life, reduced charging times, and increased overall device efficiency.
The industrial sector is also showing increased interest in advanced electrolytes for applications in backup power systems, uninterruptible power supplies, and specialized equipment. The improved safety and stability of polymer gel electrolytes make them particularly suitable for use in harsh environments or critical applications where reliability is essential.
Market analysts project that the global advanced electrolyte market will experience substantial growth in the coming years. This growth is expected to be driven by ongoing research and development efforts, technological advancements, and increasing investments in energy storage technologies. The role of ammonium hydroxide in the production of polymer gel electrolytes is likely to become increasingly important as manufacturers seek to optimize their production processes and improve the performance of their products.
As environmental concerns continue to shape industry regulations and consumer preferences, there is also a growing demand for more sustainable and environmentally friendly electrolyte solutions. This trend is likely to further boost the market for advanced electrolytes, including those produced using ammonium hydroxide, as they offer potential improvements in both performance and environmental impact compared to traditional electrolyte technologies.
In the automotive sector, the global shift towards electrification has created a substantial market for advanced battery technologies. Polymer gel electrolytes offer several advantages over traditional liquid electrolytes, including improved safety, higher energy density, and better thermal stability. These characteristics make them particularly attractive for use in EVs, where battery performance and safety are paramount concerns.
The renewable energy sector is another key driver of demand for advanced electrolytes. As the world transitions towards cleaner energy sources, there is a growing need for efficient and reliable energy storage solutions. Polymer gel electrolytes can play a crucial role in improving the performance and longevity of grid-scale energy storage systems, which are essential for managing the intermittent nature of renewable energy sources such as solar and wind power.
In the consumer electronics market, the demand for longer-lasting and faster-charging devices continues to grow. Polymer gel electrolytes have the potential to significantly enhance the performance of lithium-ion batteries used in smartphones, laptops, and other portable devices. This improvement can lead to extended battery life, reduced charging times, and increased overall device efficiency.
The industrial sector is also showing increased interest in advanced electrolytes for applications in backup power systems, uninterruptible power supplies, and specialized equipment. The improved safety and stability of polymer gel electrolytes make them particularly suitable for use in harsh environments or critical applications where reliability is essential.
Market analysts project that the global advanced electrolyte market will experience substantial growth in the coming years. This growth is expected to be driven by ongoing research and development efforts, technological advancements, and increasing investments in energy storage technologies. The role of ammonium hydroxide in the production of polymer gel electrolytes is likely to become increasingly important as manufacturers seek to optimize their production processes and improve the performance of their products.
As environmental concerns continue to shape industry regulations and consumer preferences, there is also a growing demand for more sustainable and environmentally friendly electrolyte solutions. This trend is likely to further boost the market for advanced electrolytes, including those produced using ammonium hydroxide, as they offer potential improvements in both performance and environmental impact compared to traditional electrolyte technologies.
Current Challenges in PGE Production
The production of Polymer Gel Electrolytes (PGEs) faces several significant challenges that hinder their widespread adoption and optimal performance. One of the primary issues is achieving the right balance between mechanical strength and ionic conductivity. PGEs need to be robust enough to prevent short circuits while maintaining high ionic conductivity for efficient battery operation. This delicate balance is often difficult to achieve, as increasing mechanical strength typically results in decreased ionic conductivity, and vice versa.
Another major challenge is the long-term stability of PGEs. Over time, these electrolytes can degrade due to various factors such as temperature fluctuations, chemical reactions with electrode materials, and mechanical stress. This degradation can lead to reduced battery performance and shortened lifespan, which is a significant concern for commercial applications.
The scalability of PGE production also presents a considerable hurdle. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing while maintaining consistent quality and performance is challenging. This scaling issue often leads to increased production costs, making PGEs less economically viable compared to traditional liquid electrolytes.
Furthermore, the choice of polymer matrix and its interaction with the electrolyte salt and solvent is crucial. Finding the optimal combination that provides high ionic conductivity, good mechanical properties, and excellent electrochemical stability is an ongoing challenge. The polymer matrix must also be compatible with the electrode materials and stable over a wide range of operating conditions.
The presence of impurities and residual solvents in PGEs is another significant concern. These contaminants can negatively impact the electrochemical performance and safety of the battery. Developing efficient purification methods and ensuring complete solvent removal without compromising the gel structure are critical challenges that researchers continue to address.
Lastly, the environmental impact and safety of PGEs are important considerations. Many current PGE formulations use organic solvents that may be flammable or toxic. Developing more environmentally friendly and safer alternatives without sacrificing performance is a key challenge in advancing PGE technology.
In this context, the use of ammonium hydroxide in PGE production emerges as a potential solution to address some of these challenges. Its role in enhancing ionic conductivity, improving mechanical properties, and potentially offering a more environmentally friendly alternative to traditional solvents makes it a key area of focus in ongoing PGE research and development efforts.
Another major challenge is the long-term stability of PGEs. Over time, these electrolytes can degrade due to various factors such as temperature fluctuations, chemical reactions with electrode materials, and mechanical stress. This degradation can lead to reduced battery performance and shortened lifespan, which is a significant concern for commercial applications.
The scalability of PGE production also presents a considerable hurdle. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing while maintaining consistent quality and performance is challenging. This scaling issue often leads to increased production costs, making PGEs less economically viable compared to traditional liquid electrolytes.
Furthermore, the choice of polymer matrix and its interaction with the electrolyte salt and solvent is crucial. Finding the optimal combination that provides high ionic conductivity, good mechanical properties, and excellent electrochemical stability is an ongoing challenge. The polymer matrix must also be compatible with the electrode materials and stable over a wide range of operating conditions.
The presence of impurities and residual solvents in PGEs is another significant concern. These contaminants can negatively impact the electrochemical performance and safety of the battery. Developing efficient purification methods and ensuring complete solvent removal without compromising the gel structure are critical challenges that researchers continue to address.
Lastly, the environmental impact and safety of PGEs are important considerations. Many current PGE formulations use organic solvents that may be flammable or toxic. Developing more environmentally friendly and safer alternatives without sacrificing performance is a key challenge in advancing PGE technology.
In this context, the use of ammonium hydroxide in PGE production emerges as a potential solution to address some of these challenges. Its role in enhancing ionic conductivity, improving mechanical properties, and potentially offering a more environmentally friendly alternative to traditional solvents makes it a key area of focus in ongoing PGE research and development efforts.
Ammonium Hydroxide-based PGE Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it useful for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. It also finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.
- Role in environmental applications: Ammonium hydroxide is employed in environmental applications, particularly in air pollution control and water treatment. It is used to neutralize acidic gases in flue gas desulfurization processes and to remove nitrogen oxides from exhaust gases. In water treatment, it helps in adjusting pH levels and removing heavy metals through precipitation.
- Use in agricultural and horticultural products: Ammonium hydroxide is an important component in the production of agricultural and horticultural products. It is used in the manufacture of nitrogen-based fertilizers, providing a source of readily available nitrogen for plants. Additionally, it is utilized in the formulation of pesticides and herbicides, contributing to crop protection and yield improvement.
- Application in personal care and cosmetic products: Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in hair dyes, helping to open the hair cuticle for better color penetration. In some cosmetic formulations, it acts as a buffering agent to maintain product stability. It is also employed in certain hair straightening treatments to break and reform hair bonds.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental remediation
Ammonium hydroxide is employed in environmental remediation processes, particularly in air pollution control. It is used in flue gas treatment systems to neutralize acidic components and remove sulfur dioxide. In water treatment, it helps in adjusting pH levels and removing heavy metals through precipitation. Its ability to form complexes with certain pollutants makes it valuable in soil remediation techniques.Expand Specific Solutions04 Use in agricultural applications
In agriculture, ammonium hydroxide serves as a source of nitrogen for fertilizers. It can be directly applied to soil or used in the production of other nitrogen-based fertilizers. Its alkaline nature helps in adjusting soil pH, making it useful for treating acidic soils. Additionally, it is used in the preservation of silage and as a component in some pesticide formulations.Expand Specific Solutions05 Applications in personal care and cosmetics
Ammonium hydroxide finds applications in personal care products and cosmetics. It is used as a pH adjuster in hair dyes, helping to open the hair cuticle for better dye penetration. In some cosmetic formulations, it acts as a buffering agent to maintain product stability. Its ability to neutralize fatty acids makes it useful in the production of soaps and detergents.Expand Specific Solutions
Key Players in PGE Manufacturing
The competitive landscape for ammonium hydroxide in polymer gel electrolytes production is evolving rapidly, reflecting the growing importance of advanced energy storage technologies. The market is in an early growth stage, with significant potential for expansion as demand for high-performance batteries increases. Key players like Toshiba, BASF, and LG Chem are investing heavily in research and development, driving technological advancements. The market size is expected to grow substantially in the coming years, fueled by the electric vehicle revolution and renewable energy integration. While the technology is still maturing, companies such as SK On and Svolt Energy Technology are making significant strides in improving the performance and scalability of polymer gel electrolytes using ammonium hydroxide.
BASF Corp.
Technical Solution: BASF has developed advanced polymer gel electrolytes using ammonium hydroxide as a key component. Their approach involves incorporating ammonium hydroxide into the polymer matrix to enhance ionic conductivity and mechanical stability. The process includes mixing a polymer base with ammonium hydroxide and other additives, followed by a controlled gelation process. This results in a gel electrolyte with improved lithium-ion transport properties and reduced interfacial resistance[1]. BASF's method also incorporates nanoparticles to further enhance the electrolyte's performance, achieving conductivities up to 10^-3 S/cm at room temperature[3].
Strengths: High ionic conductivity, improved mechanical stability, and enhanced safety. Weaknesses: Potential for ammonia off-gassing, which may require additional containment measures.
SK On Co., Ltd.
Technical Solution: SK On has developed an innovative polymer gel electrolyte production method utilizing ammonium hydroxide. Their approach focuses on creating a highly porous polymer structure by incorporating ammonium hydroxide as a porogen. During the gelation process, the ammonium hydroxide creates micro and nano-scale pores, which are then filled with a liquid electrolyte. This unique structure allows for high ionic conductivity while maintaining mechanical integrity. SK On's research has demonstrated that this method can achieve conductivities of up to 8 × 10^-3 S/cm at room temperature, with excellent cycling stability in lithium-ion batteries[5]. The company has also developed a proprietary coating technique that further enhances the electrolyte's interface stability with electrode materials[6].
Strengths: High ionic conductivity, excellent mechanical properties, and improved electrode-electrolyte interface stability. Weaknesses: Potential for increased manufacturing complexity due to the multi-step production process.
Innovations in Ammonium Hydroxide Usage
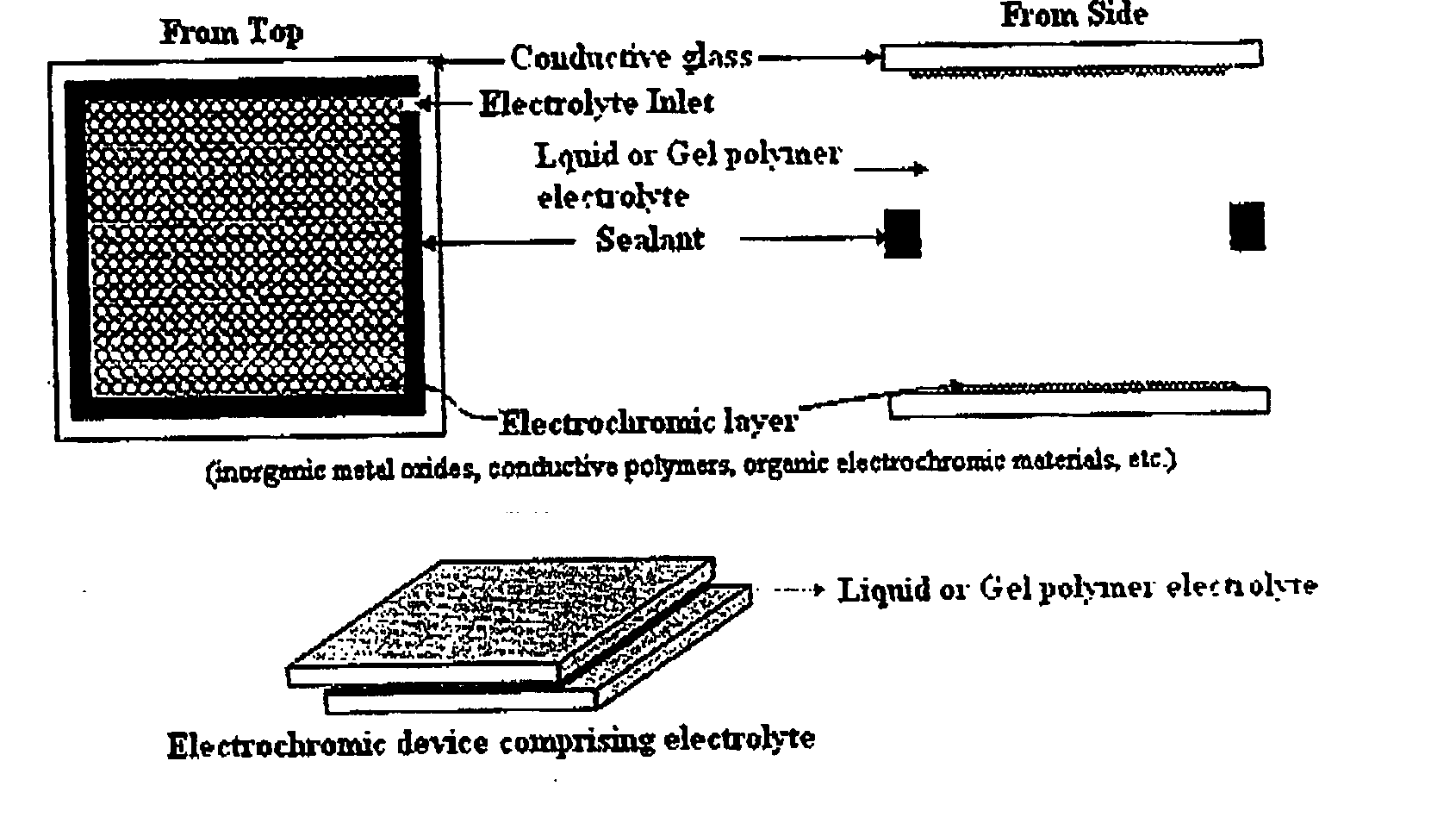
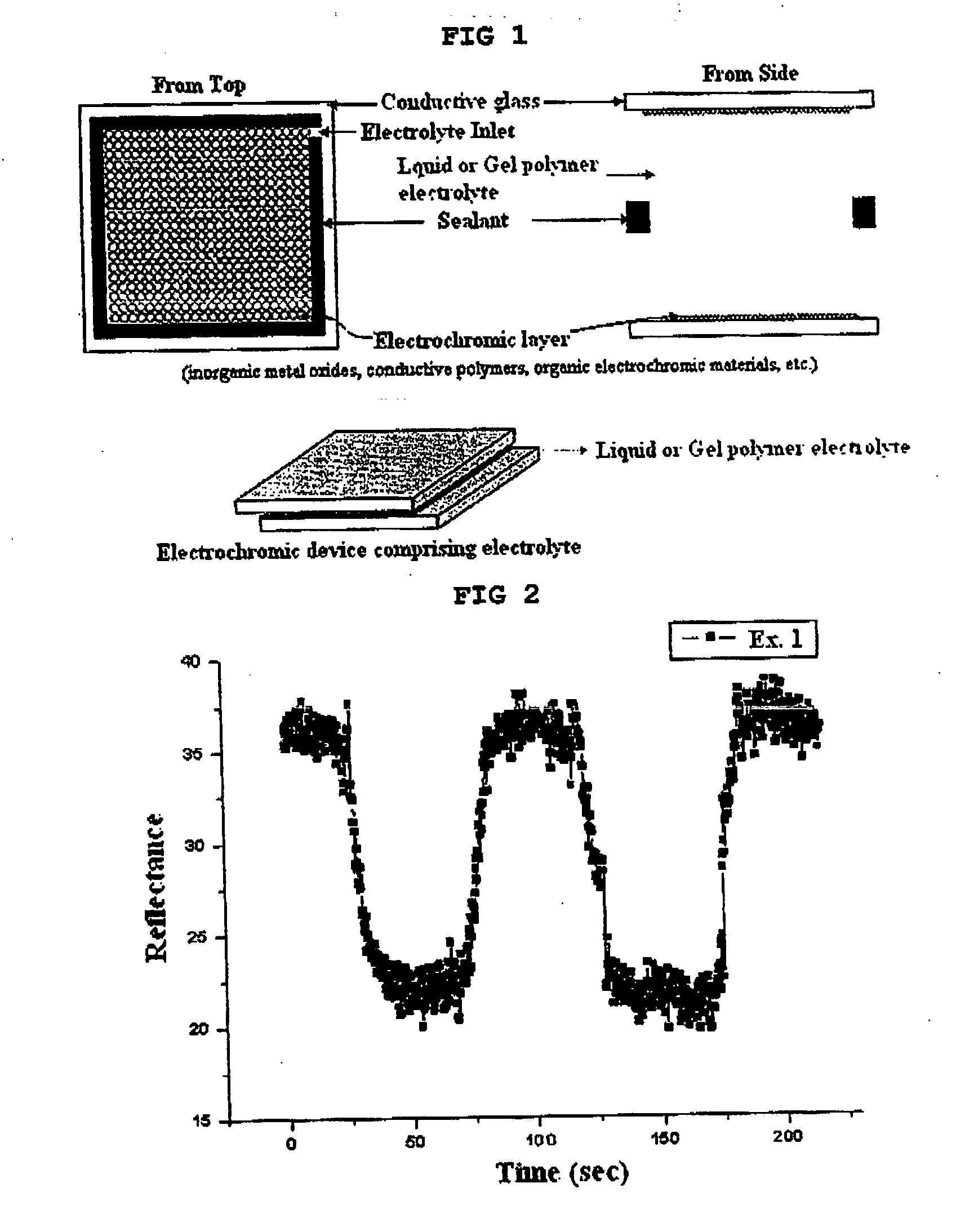
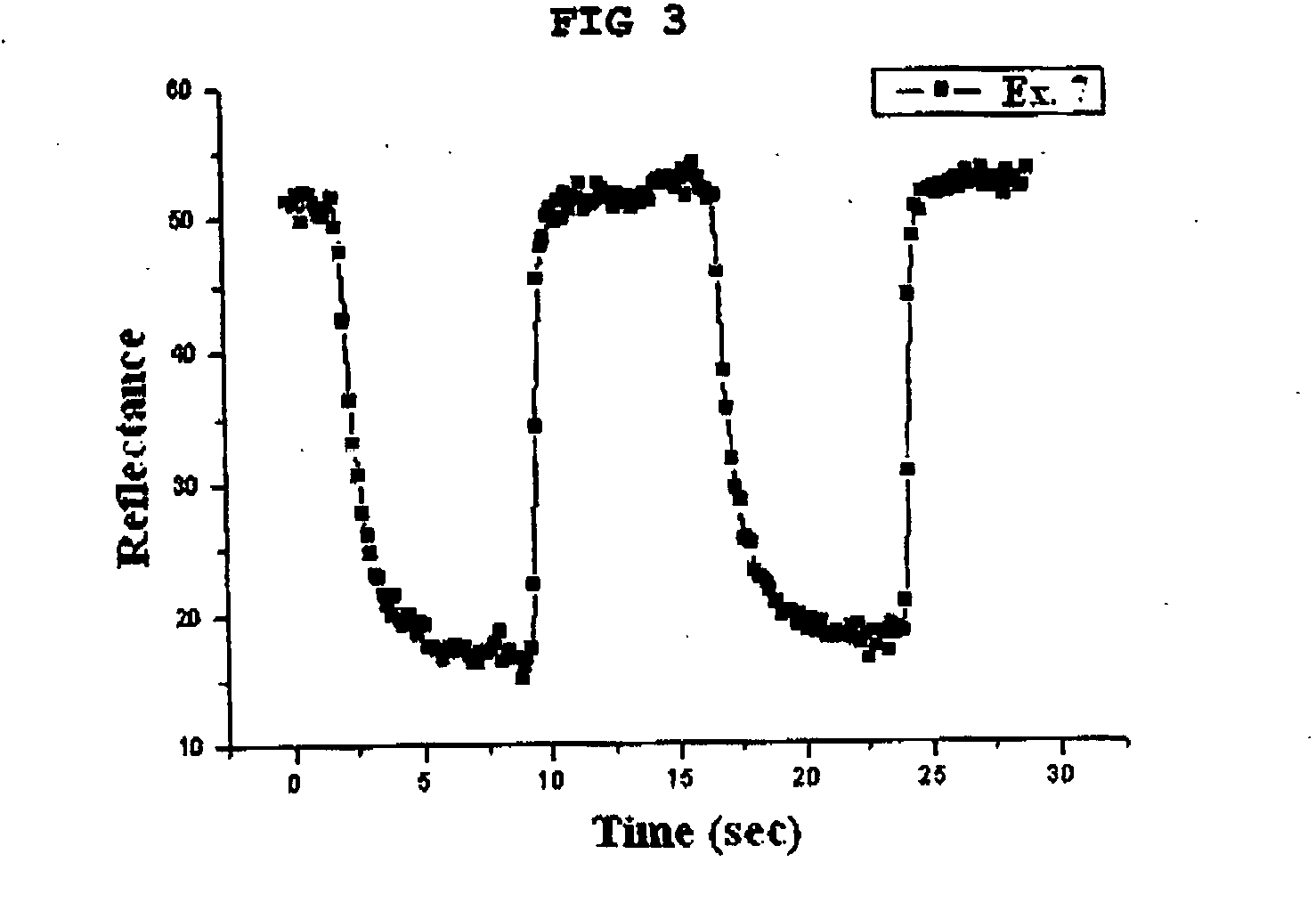
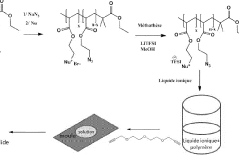
Gel polymer electrolyte containing ionic liquid and electrochromic device using the same
PatentActiveUS20050231785A1
Innovation
- The development of an electrochromic device utilizing a gel polymer electrolyte formed by polymerizing an ionic liquid with a monomer, eliminating the need for organic solvents and enabling in-situ polymerization, which enhances ion conductivity and prevents electrolyte leakage.
ELECTROLYTE gel COMPRISING A POLYMER AND AN IONIC LIQUID, METHOD FOR PREPARATION AND USE
PatentInactiveFR3036856A1
Innovation
- A gel composed of a crosslinked polymer network and an ionic liquid, where the polymer network ensures compatibility and stability with the ionic liquid, enhancing mechanical strength and ionic conductivity.
Environmental Impact of PGE Production
The production of Polymer Gel Electrolytes (PGEs) using ammonium hydroxide has significant environmental implications that warrant careful consideration. The process involves the use of various chemicals and energy-intensive steps, which can contribute to environmental pollution and resource depletion if not managed properly.
One of the primary environmental concerns is the potential release of ammonia gas during the production process. Ammonia is a toxic substance that can cause respiratory irritation and damage to aquatic ecosystems if released into the environment. Proper ventilation systems and emission control measures are essential to mitigate this risk and ensure compliance with environmental regulations.
Water consumption is another critical factor in PGE production. The process requires substantial amounts of water for mixing, washing, and cooling purposes. This can strain local water resources, particularly in water-scarce regions. Implementing water recycling and conservation techniques can help reduce the overall water footprint of PGE manufacturing.
The disposal of waste products generated during PGE production also poses environmental challenges. Residual chemicals, including unreacted ammonium hydroxide and other additives, must be treated and disposed of responsibly to prevent soil and groundwater contamination. Developing efficient waste management protocols and exploring opportunities for byproduct utilization can minimize the environmental impact of waste disposal.
Energy consumption is a significant contributor to the carbon footprint of PGE production. The process often requires high temperatures and prolonged reaction times, leading to substantial energy use. Adopting energy-efficient technologies and exploring renewable energy sources can help reduce greenhouse gas emissions associated with PGE manufacturing.
The sourcing of raw materials for PGE production, including ammonium hydroxide, also has upstream environmental implications. The production of these chemicals may involve energy-intensive processes and potential environmental risks at their respective manufacturing sites. Implementing sustainable procurement practices and considering the lifecycle impact of raw materials can help address these concerns.
Lastly, the end-of-life management of PGE-containing products is an important environmental consideration. Developing effective recycling and disposal methods for used PGEs can help recover valuable materials and prevent potential environmental contamination from improper disposal.
One of the primary environmental concerns is the potential release of ammonia gas during the production process. Ammonia is a toxic substance that can cause respiratory irritation and damage to aquatic ecosystems if released into the environment. Proper ventilation systems and emission control measures are essential to mitigate this risk and ensure compliance with environmental regulations.
Water consumption is another critical factor in PGE production. The process requires substantial amounts of water for mixing, washing, and cooling purposes. This can strain local water resources, particularly in water-scarce regions. Implementing water recycling and conservation techniques can help reduce the overall water footprint of PGE manufacturing.
The disposal of waste products generated during PGE production also poses environmental challenges. Residual chemicals, including unreacted ammonium hydroxide and other additives, must be treated and disposed of responsibly to prevent soil and groundwater contamination. Developing efficient waste management protocols and exploring opportunities for byproduct utilization can minimize the environmental impact of waste disposal.
Energy consumption is a significant contributor to the carbon footprint of PGE production. The process often requires high temperatures and prolonged reaction times, leading to substantial energy use. Adopting energy-efficient technologies and exploring renewable energy sources can help reduce greenhouse gas emissions associated with PGE manufacturing.
The sourcing of raw materials for PGE production, including ammonium hydroxide, also has upstream environmental implications. The production of these chemicals may involve energy-intensive processes and potential environmental risks at their respective manufacturing sites. Implementing sustainable procurement practices and considering the lifecycle impact of raw materials can help address these concerns.
Lastly, the end-of-life management of PGE-containing products is an important environmental consideration. Developing effective recycling and disposal methods for used PGEs can help recover valuable materials and prevent potential environmental contamination from improper disposal.
Safety Regulations in Electrolyte Manufacturing
Safety regulations in electrolyte manufacturing are crucial for ensuring the protection of workers, the environment, and the quality of the final product. In the context of polymer gel electrolytes production using ammonium hydroxide, several key safety considerations must be addressed.
Firstly, proper handling and storage of ammonium hydroxide is paramount. This compound is corrosive and can release harmful ammonia vapors. Manufacturers must implement strict protocols for storage in well-ventilated areas, use of appropriate containment systems, and regular inspections to prevent leaks or spills. Personal protective equipment (PPE) including chemical-resistant gloves, safety goggles, and respiratory protection is mandatory for personnel working with ammonium hydroxide.
Ventilation systems play a critical role in maintaining a safe working environment. Industrial-grade exhaust systems must be installed to remove ammonia vapors and other potentially harmful fumes generated during the production process. Regular air quality monitoring should be conducted to ensure that exposure levels remain within permissible limits set by occupational health and safety authorities.
Emergency response procedures are essential components of safety regulations. Facilities must have clearly defined protocols for handling spills, leaks, or accidental exposures. This includes the availability of eyewash stations, safety showers, and spill containment kits. Staff should be thoroughly trained in emergency procedures and regular drills conducted to ensure readiness.
Waste management is another crucial aspect of safety regulations in electrolyte manufacturing. Proper disposal of ammonium hydroxide and other chemical waste must comply with local and national environmental regulations. This may involve neutralization processes, specialized waste treatment facilities, or licensed disposal services.
Quality control measures are integral to safety regulations, as they ensure the consistency and purity of the electrolyte product. Rigorous testing protocols must be established to verify the composition and properties of the polymer gel electrolytes. This includes checks for contaminants that could compromise safety or performance.
Employee training and certification programs are fundamental to maintaining a safe working environment. All personnel involved in the manufacturing process should receive comprehensive training on the properties of ammonium hydroxide, safe handling procedures, and emergency response protocols. Regular refresher courses and competency assessments should be conducted to ensure ongoing compliance with safety standards.
Lastly, documentation and record-keeping are essential for demonstrating compliance with safety regulations. Detailed logs of safety inspections, incident reports, training records, and material safety data sheets must be maintained and readily accessible for review by regulatory authorities.
Firstly, proper handling and storage of ammonium hydroxide is paramount. This compound is corrosive and can release harmful ammonia vapors. Manufacturers must implement strict protocols for storage in well-ventilated areas, use of appropriate containment systems, and regular inspections to prevent leaks or spills. Personal protective equipment (PPE) including chemical-resistant gloves, safety goggles, and respiratory protection is mandatory for personnel working with ammonium hydroxide.
Ventilation systems play a critical role in maintaining a safe working environment. Industrial-grade exhaust systems must be installed to remove ammonia vapors and other potentially harmful fumes generated during the production process. Regular air quality monitoring should be conducted to ensure that exposure levels remain within permissible limits set by occupational health and safety authorities.
Emergency response procedures are essential components of safety regulations. Facilities must have clearly defined protocols for handling spills, leaks, or accidental exposures. This includes the availability of eyewash stations, safety showers, and spill containment kits. Staff should be thoroughly trained in emergency procedures and regular drills conducted to ensure readiness.
Waste management is another crucial aspect of safety regulations in electrolyte manufacturing. Proper disposal of ammonium hydroxide and other chemical waste must comply with local and national environmental regulations. This may involve neutralization processes, specialized waste treatment facilities, or licensed disposal services.
Quality control measures are integral to safety regulations, as they ensure the consistency and purity of the electrolyte product. Rigorous testing protocols must be established to verify the composition and properties of the polymer gel electrolytes. This includes checks for contaminants that could compromise safety or performance.
Employee training and certification programs are fundamental to maintaining a safe working environment. All personnel involved in the manufacturing process should receive comprehensive training on the properties of ammonium hydroxide, safe handling procedures, and emergency response protocols. Regular refresher courses and competency assessments should be conducted to ensure ongoing compliance with safety standards.
Lastly, documentation and record-keeping are essential for demonstrating compliance with safety regulations. Detailed logs of safety inspections, incident reports, training records, and material safety data sheets must be maintained and readily accessible for review by regulatory authorities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!