A Survey Of Bio-Inks For Printing Living Materials.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioprinting Evolution and Research Objectives
Bioprinting technology has evolved significantly over the past three decades, transforming from a conceptual idea to a practical application with immense potential in tissue engineering and regenerative medicine. The journey began in the early 1990s with the adaptation of conventional printing technologies for biological applications, primarily focusing on cell patterning. By the early 2000s, researchers had developed the first dedicated bioprinters capable of depositing living cells in predetermined patterns.
The evolution accelerated around 2010 with the emergence of more sophisticated bioprinting approaches, including extrusion-based, inkjet-based, laser-assisted, and stereolithography-based techniques. Each method offered distinct advantages in terms of resolution, cell viability, and structural complexity. This diversification of bioprinting methodologies coincided with significant advancements in bio-ink formulations, moving from simple cell suspensions to complex composite materials that better mimic the extracellular matrix.
Recent years have witnessed remarkable progress in multi-material and multi-cellular bioprinting capabilities, enabling the creation of heterogeneous tissue constructs with increasingly complex architectures and functionalities. The integration of bioprinting with complementary technologies such as microfluidics, artificial intelligence, and real-time monitoring systems has further expanded the possibilities for creating biomimetic tissues and organs.
The primary research objectives in the field of bio-inks for printing living materials are multifaceted and ambitious. First, there is a pressing need to develop bio-inks that maintain high cell viability throughout the printing process while providing adequate mechanical support for the resulting constructs. This balance between biocompatibility and structural integrity remains a significant challenge.
Second, researchers aim to create bio-inks that more accurately replicate the complex biochemical and mechanical properties of native tissues. This includes the incorporation of growth factors, cell-adhesion molecules, and other bioactive components that can guide cellular behavior and tissue development post-printing.
Third, there is growing interest in developing stimuli-responsive bio-inks that can undergo controlled changes in their properties in response to external triggers, enabling the creation of dynamic tissue constructs that can adapt to changing physiological conditions.
Finally, the field is moving toward the development of standardized bio-ink formulations and characterization methods to enhance reproducibility and facilitate regulatory approval of bioprinted products for clinical applications. This standardization is crucial for the translation of bioprinting technologies from laboratory research to practical medical applications.
The evolution accelerated around 2010 with the emergence of more sophisticated bioprinting approaches, including extrusion-based, inkjet-based, laser-assisted, and stereolithography-based techniques. Each method offered distinct advantages in terms of resolution, cell viability, and structural complexity. This diversification of bioprinting methodologies coincided with significant advancements in bio-ink formulations, moving from simple cell suspensions to complex composite materials that better mimic the extracellular matrix.
Recent years have witnessed remarkable progress in multi-material and multi-cellular bioprinting capabilities, enabling the creation of heterogeneous tissue constructs with increasingly complex architectures and functionalities. The integration of bioprinting with complementary technologies such as microfluidics, artificial intelligence, and real-time monitoring systems has further expanded the possibilities for creating biomimetic tissues and organs.
The primary research objectives in the field of bio-inks for printing living materials are multifaceted and ambitious. First, there is a pressing need to develop bio-inks that maintain high cell viability throughout the printing process while providing adequate mechanical support for the resulting constructs. This balance between biocompatibility and structural integrity remains a significant challenge.
Second, researchers aim to create bio-inks that more accurately replicate the complex biochemical and mechanical properties of native tissues. This includes the incorporation of growth factors, cell-adhesion molecules, and other bioactive components that can guide cellular behavior and tissue development post-printing.
Third, there is growing interest in developing stimuli-responsive bio-inks that can undergo controlled changes in their properties in response to external triggers, enabling the creation of dynamic tissue constructs that can adapt to changing physiological conditions.
Finally, the field is moving toward the development of standardized bio-ink formulations and characterization methods to enhance reproducibility and facilitate regulatory approval of bioprinted products for clinical applications. This standardization is crucial for the translation of bioprinting technologies from laboratory research to practical medical applications.
Market Analysis for Bioprinted Tissue Applications
The bioprinting market has experienced significant growth in recent years, driven by increasing demand for tissue engineering applications across various sectors. The global bioprinting market was valued at approximately $1.3 billion in 2020 and is projected to reach $4.2 billion by 2027, representing a compound annual growth rate (CAGR) of 18.2% during this period. This robust growth trajectory underscores the expanding commercial potential of bioprinted tissue applications.
Healthcare applications currently dominate the market landscape, with regenerative medicine and tissue engineering representing the largest segments. The demand for organ transplants continues to outpace supply significantly, creating a substantial market opportunity for bioprinted tissues and organs. In 2021, over 100,000 patients were on organ transplant waiting lists in the United States alone, highlighting the critical need for alternative solutions.
Skin tissue applications have gained considerable commercial traction, with products like bioprinted skin grafts for wound healing and burn treatment already entering clinical trials. The global wound care market, valued at $20.4 billion in 2021, presents a substantial opportunity for bioprinted skin substitutes. Cosmetic testing represents another significant market segment, driven by increasing regulatory restrictions on animal testing and growing consumer demand for cruelty-free products.
Pharmaceutical companies have begun integrating bioprinted tissue models into their drug discovery and development pipelines. These 3D tissue constructs offer more physiologically relevant testing platforms compared to traditional 2D cell cultures, potentially reducing drug development costs and accelerating time-to-market. The drug discovery market utilizing 3D bioprinted tissues is expected to grow at a CAGR of 20.4% through 2026.
Regionally, North America leads the bioprinting market, accounting for approximately 45% of global revenue, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to witness the fastest growth rate due to increasing healthcare expenditure, growing research activities, and supportive government initiatives in countries like China, Japan, and South Korea.
Key market challenges include high production costs, regulatory uncertainties, and technical limitations in creating complex vascularized tissues. Despite these challenges, the convergence of advances in bio-ink formulations, printing technologies, and increasing clinical validation is expected to drive continued market expansion and diversification of bioprinted tissue applications across multiple industries.
Healthcare applications currently dominate the market landscape, with regenerative medicine and tissue engineering representing the largest segments. The demand for organ transplants continues to outpace supply significantly, creating a substantial market opportunity for bioprinted tissues and organs. In 2021, over 100,000 patients were on organ transplant waiting lists in the United States alone, highlighting the critical need for alternative solutions.
Skin tissue applications have gained considerable commercial traction, with products like bioprinted skin grafts for wound healing and burn treatment already entering clinical trials. The global wound care market, valued at $20.4 billion in 2021, presents a substantial opportunity for bioprinted skin substitutes. Cosmetic testing represents another significant market segment, driven by increasing regulatory restrictions on animal testing and growing consumer demand for cruelty-free products.
Pharmaceutical companies have begun integrating bioprinted tissue models into their drug discovery and development pipelines. These 3D tissue constructs offer more physiologically relevant testing platforms compared to traditional 2D cell cultures, potentially reducing drug development costs and accelerating time-to-market. The drug discovery market utilizing 3D bioprinted tissues is expected to grow at a CAGR of 20.4% through 2026.
Regionally, North America leads the bioprinting market, accounting for approximately 45% of global revenue, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to witness the fastest growth rate due to increasing healthcare expenditure, growing research activities, and supportive government initiatives in countries like China, Japan, and South Korea.
Key market challenges include high production costs, regulatory uncertainties, and technical limitations in creating complex vascularized tissues. Despite these challenges, the convergence of advances in bio-ink formulations, printing technologies, and increasing clinical validation is expected to drive continued market expansion and diversification of bioprinted tissue applications across multiple industries.
Current Bio-Ink Technologies and Limitations
Bio-ink technology has evolved significantly over the past decade, with several distinct categories now established in the field. Hydrogel-based bio-inks remain the most widely utilized, offering excellent biocompatibility and structural support for cellular growth. These typically incorporate natural polymers such as alginate, gelatin, collagen, and hyaluronic acid, or synthetic alternatives like polyethylene glycol (PEG) and pluronic. Each material presents unique advantages in terms of printability, mechanical properties, and cell viability.
Decellularized extracellular matrix (dECM) bio-inks represent a significant advancement, preserving the native tissue microenvironment and biochemical cues essential for cell differentiation and function. These bio-inks are derived from specific tissues through decellularization processes that remove cellular components while maintaining the structural integrity of the extracellular matrix.
Composite bio-inks combine multiple materials to overcome limitations of single-component systems. For example, integrating nanoparticles or fibers into hydrogels can enhance mechanical properties without compromising biocompatibility. Similarly, hybrid bio-inks incorporating both synthetic and natural polymers aim to balance printability with biological functionality.
Despite these advances, current bio-ink technologies face several critical limitations. Printability versus biocompatibility remains a fundamental challenge - materials with optimal printing properties often compromise cell viability, while highly biocompatible materials frequently lack the mechanical stability required for precise printing. This trade-off necessitates careful formulation adjustments that often result in suboptimal performance in either category.
Mechanical properties present another significant hurdle. Many bio-inks lack sufficient strength to maintain structural integrity during and after printing, particularly for load-bearing applications. Additionally, achieving tissue-specific mechanical properties that mimic native tissue elasticity and stiffness remains difficult with current formulations.
Vascularization represents perhaps the most critical limitation in bio-printed constructs. Current bio-ink technologies struggle to create functional vascular networks necessary for nutrient delivery and waste removal in tissues exceeding 200 micrometers in thickness. This constraint severely limits the size and complexity of viable printed tissues.
Scalability and standardization issues also persist across the field. Batch-to-batch variability in natural materials, complex processing requirements, and limited shelf-life impede widespread adoption and commercialization. Furthermore, regulatory pathways for bio-printed constructs remain unclear, creating uncertainty for clinical translation.
Decellularized extracellular matrix (dECM) bio-inks represent a significant advancement, preserving the native tissue microenvironment and biochemical cues essential for cell differentiation and function. These bio-inks are derived from specific tissues through decellularization processes that remove cellular components while maintaining the structural integrity of the extracellular matrix.
Composite bio-inks combine multiple materials to overcome limitations of single-component systems. For example, integrating nanoparticles or fibers into hydrogels can enhance mechanical properties without compromising biocompatibility. Similarly, hybrid bio-inks incorporating both synthetic and natural polymers aim to balance printability with biological functionality.
Despite these advances, current bio-ink technologies face several critical limitations. Printability versus biocompatibility remains a fundamental challenge - materials with optimal printing properties often compromise cell viability, while highly biocompatible materials frequently lack the mechanical stability required for precise printing. This trade-off necessitates careful formulation adjustments that often result in suboptimal performance in either category.
Mechanical properties present another significant hurdle. Many bio-inks lack sufficient strength to maintain structural integrity during and after printing, particularly for load-bearing applications. Additionally, achieving tissue-specific mechanical properties that mimic native tissue elasticity and stiffness remains difficult with current formulations.
Vascularization represents perhaps the most critical limitation in bio-printed constructs. Current bio-ink technologies struggle to create functional vascular networks necessary for nutrient delivery and waste removal in tissues exceeding 200 micrometers in thickness. This constraint severely limits the size and complexity of viable printed tissues.
Scalability and standardization issues also persist across the field. Batch-to-batch variability in natural materials, complex processing requirements, and limited shelf-life impede widespread adoption and commercialization. Furthermore, regulatory pathways for bio-printed constructs remain unclear, creating uncertainty for clinical translation.
Contemporary Bio-Ink Formulations and Methodologies
01 Composition of bio-inks for 3D printing
Bio-inks can be formulated with various biomaterials to achieve specific printing capabilities. These compositions typically include natural polymers (like alginate, gelatin, or collagen), synthetic polymers, and crosslinking agents that provide structural integrity. The formulation determines key printing parameters such as viscosity, gelation behavior, and mechanical properties of the printed construct, which are essential for successful bioprinting applications.- Biocompatible polymer-based bio-inks: Biocompatible polymers are essential components in bio-inks for 3D bioprinting applications. These polymers provide structural support and mimic the extracellular matrix to enhance cell viability and functionality. Various natural and synthetic polymers such as alginate, gelatin, collagen, and hyaluronic acid can be formulated into printable bio-inks with tunable mechanical properties. The polymer composition can be adjusted to achieve optimal viscosity, gelation behavior, and post-printing stability required for different tissue engineering applications.
- Cell-laden bio-ink formulations: Cell-laden bio-inks incorporate living cells directly into the printing material, enabling the fabrication of complex tissue constructs. These formulations must balance printability with cell viability, providing appropriate rheological properties while maintaining a cell-friendly environment. The bio-inks typically contain cells suspended in hydrogels with nutrients and growth factors to support cellular functions during and after printing. Different cell types require specific bio-ink compositions to maintain their phenotype and promote proper tissue development after printing.
- Stimuli-responsive bio-ink systems: Stimuli-responsive bio-inks can change their physical properties in response to external triggers such as temperature, light, pH, or ionic strength. These smart materials enable precise control over the printing process and post-printing maturation of tissue constructs. Thermosensitive bio-inks that solidify at physiological temperature allow for seamless transition from printing to culture conditions. Photo-crosslinkable bio-inks provide spatial and temporal control over the gelation process, enabling the creation of complex structures with high resolution and fidelity.
- Multi-material bio-printing techniques: Multi-material bio-printing involves the simultaneous or sequential deposition of different bio-inks to create heterogeneous tissue constructs with region-specific properties. This approach enables the fabrication of complex tissues with multiple cell types and varying mechanical properties. Advanced printing systems can precisely control the deposition of different bio-inks to create gradients and interfaces that mimic native tissue architecture. The integration of support materials and sacrificial inks allows for the creation of hollow structures and vasculature within printed constructs.
- Nanocomposite bio-inks for enhanced functionality: Nanocomposite bio-inks incorporate nanomaterials such as nanoparticles, nanofibers, or nanosheets to enhance the mechanical, electrical, or biological properties of printed constructs. These advanced formulations can improve printability, structural integrity, and functionality of the resulting tissues. Conductive nanomaterials like graphene or carbon nanotubes can be incorporated to create bio-inks suitable for neural or cardiac tissue engineering. Nanoparticles can also serve as delivery vehicles for growth factors or drugs, providing sustained release of bioactive molecules within the printed construct to guide tissue development.
02 Cell-laden bio-ink technologies
Advanced bio-inks incorporate living cells to create functional tissue constructs. These specialized formulations maintain cell viability during the printing process while providing appropriate mechanical support. The bio-inks must balance printability with a cell-friendly environment, including proper nutrient diffusion, pH regulation, and protection from shear stress during extrusion. This technology enables the creation of complex tissue structures with living components for regenerative medicine applications.Expand Specific Solutions03 Multi-material bio-printing capabilities
Multi-material bio-printing systems allow for the simultaneous or sequential deposition of different bio-inks to create heterogeneous tissue constructs. This capability enables the fabrication of complex structures with varying mechanical properties, cell types, and biological functions within a single printed construct. Advanced printing platforms incorporate multiple printheads or nozzles with precise control systems to achieve spatial control of different materials.Expand Specific Solutions04 Printability enhancement techniques
Various methods are employed to enhance the printability of bio-inks while maintaining their biological functionality. These include rheological modifiers to control flow behavior, temperature-responsive components for controlled gelation, and support bath techniques for printing soft materials. Other approaches involve dual-crosslinking mechanisms, where initial printing stability is provided by one mechanism while long-term structural integrity is ensured by another.Expand Specific Solutions05 Post-printing stabilization methods
After bio-ink deposition, various stabilization methods are employed to enhance the mechanical properties and durability of printed constructs. These include photo-crosslinking using UV or visible light, ionic crosslinking with multivalent ions, thermal treatments, and chemical crosslinking agents. These post-printing processes are crucial for maintaining the shape fidelity of printed structures while providing appropriate mechanical properties for the intended application.Expand Specific Solutions
Leading Companies and Research Institutions in Bioprinting
The bio-ink market for printing living materials is currently in an early growth phase, characterized by significant research activity but limited commercial maturity. The global market size is expanding rapidly, estimated to reach several hundred million dollars by 2025, driven by applications in tissue engineering and regenerative medicine. From a technological maturity perspective, the field shows varied development levels across different players. Academic institutions like Sun Yat-Sen University, Tsinghua University, and National Taiwan University are advancing fundamental research, while commercial entities such as BICO Group AB (formerly CELLINK) and 3-D Matrix are developing proprietary bio-ink formulations and printing systems. Government research organizations including CNRS and Naval Research Laboratory contribute through specialized applications development. The ecosystem demonstrates a collaborative innovation model where academic discoveries increasingly transition to commercial applications through industry partnerships.
3-D Matrix, Inc.
Technical Solution: 3-D Matrix, Inc. has developed innovative self-assembling peptide-based bio-inks that form nanofibrous hydrogels mimicking the natural extracellular matrix. Their flagship technology utilizes PuraMatrix, a synthetic peptide hydrogel system composed of 16-amino acid sequences that spontaneously assemble into nanofibers upon exposure to physiological conditions[3]. This technology enables the creation of defined 3D microenvironments with tunable mechanical properties and excellent transparency for imaging applications. The company's bio-inks are particularly notable for their ability to encapsulate cells without requiring potentially harmful crosslinking agents or UV exposure, maintaining high cell viability throughout the printing process. 3-D Matrix has engineered their peptide sequences to incorporate cell-binding domains that promote cell adhesion, migration, and differentiation, making them suitable for various tissue engineering applications including neural, cardiac, and hepatic tissues[4]. Their bio-inks can be modified with growth factors and other bioactive molecules to create tissue-specific microenvironments.
Strengths: Exceptional biocompatibility with minimal immunogenicity, tunable mechanical properties without chemical crosslinkers, and ability to support complex cellular behaviors including differentiation and network formation. Weaknesses: Higher cost compared to natural polymer-based bio-inks, more limited printability for complex structures requiring high shape fidelity, and shorter shelf life compared to some synthetic alternatives.
BICO Group AB
Technical Solution: BICO Group AB (formerly CELLINK) has developed a comprehensive range of bio-inks specifically designed for 3D bioprinting of living tissues. Their technology focuses on creating biomimetic environments that support cell viability and functionality. BICO's bio-inks are primarily composed of modified gelatin methacrylate (GelMA), alginate, and cellulose nanofibrils, offering tunable mechanical properties and excellent printability[1]. The company has pioneered temperature-sensitive bio-inks that remain liquid during printing at controlled temperatures but solidify at physiological conditions, enabling precise spatial positioning of cells. Their CELLINK series bio-inks incorporate crosslinking mechanisms that can be activated by UV light, ionic interactions, or thermal transitions, providing versatility for different tissue engineering applications[2]. BICO has also developed tissue-specific formulations optimized for cartilage, bone, and soft tissue regeneration with appropriate biochemical cues and mechanical properties matching native tissues.
Strengths: Excellent biocompatibility with high cell viability (>90% post-printing), precise control over mechanical properties, and established commercial availability with standardized quality. Weaknesses: Higher cost compared to basic research-grade materials, proprietary formulations limiting customization for specialized applications, and some formulations require specialized equipment for optimal printing results.
Critical Patents and Publications in Bio-Ink Technology
Bioink set and applications thereof for three-dimensional printing of cells
PatentActiveUS20190225824A1
Innovation
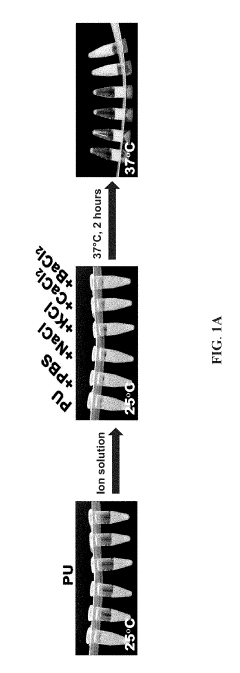
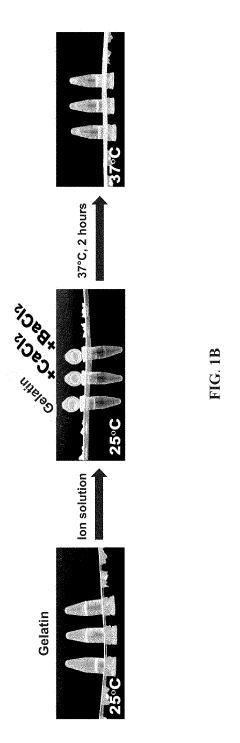
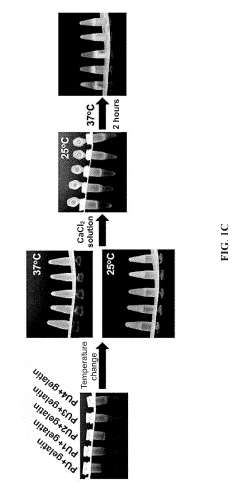
- A bioink set comprising biodegradable polyurethane and biopolymers like gelatin, agar, or alginate, combined with a divalent metal ion solution, which enhances structural stability and biocompatibility, allowing for high-resolution and long-term 3D printing without impairing cell viability.
Biogum and botanical gum hydrogel bioinks for physiological 3D bioprinting of tissue constructs for in vitro culture and transplantation
PatentInactiveJP2023090746A
Innovation
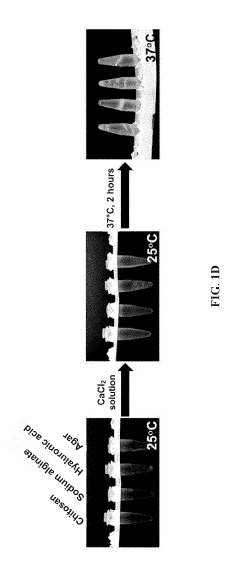
- Development of bioink compositions combining hydrogels with microbial-, fungal-, or plant-produced polysaccharides that demonstrate excellent printability while maintaining cell function and viability.
- Formulation of bioinks that can be supplemented with auxiliary proteins and growth factors including extracellular matrix components, laminins, super affinity growth factors, and morphogens to enhance cellular function.
- Creation of bioink compositions that can be used under physiological conditions with cytocompatible 3D bioprinting parameters, improving cell engraftment for transplantation applications.
Regulatory Framework for Bioprinted Materials
The regulatory landscape for bioprinted materials represents a complex and evolving framework that spans multiple jurisdictions and oversight bodies. Currently, bioprinted materials exist in a regulatory gray area between medical devices, biological products, and advanced therapy medicinal products (ATMPs). In the United States, the Food and Drug Administration (FDA) has established a tiered regulatory approach based on risk classification, with bioprinted materials typically falling under the Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on their intended use and mechanism of action.
The European Medicines Agency (EMA) classifies most bioprinted materials as ATMPs, subject to centralized authorization procedures under Regulation (EC) No 1394/2007. This framework requires extensive preclinical and clinical data demonstrating safety, efficacy, and quality before market approval. Japan has implemented an accelerated approval pathway through its Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approval for regenerative medicine products, potentially accelerating bioprinted materials' market entry.
Quality control standards present significant challenges for bioprinted materials due to their inherent variability and complexity. The International Organization for Standardization (ISO) has begun developing specific standards for bioprinting processes (ISO/TC 276 Biotechnology), though comprehensive standardization remains incomplete. Current good manufacturing practices (cGMP) requirements necessitate stringent process validation, which is particularly challenging for personalized bioprinted constructs.
Ethical considerations further complicate the regulatory landscape, particularly regarding cell sourcing, informed consent, and potential applications. The International Society for Stem Cell Research (ISSCR) guidelines provide ethical frameworks for cellular materials, while national ethics committees often provide additional oversight for novel bioprinting applications.
Long-term safety monitoring represents another regulatory challenge, with requirements for post-market surveillance varying significantly across jurisdictions. The lack of harmonized international standards creates regulatory uncertainty for developers and potentially impedes global commercialization efforts. Several regulatory agencies have established innovation pathways to address these challenges, including the FDA's Tissue Reference Group and the EMA's Innovation Task Force, which provide early dialogue opportunities for bioprinting innovators.
As the field advances, regulatory frameworks are expected to evolve toward more specific guidance for bioprinted materials. Industry stakeholders, academic researchers, and regulatory bodies are increasingly collaborating through initiatives like the TERMIS (Tissue Engineering and Regenerative Medicine International Society) working groups to develop consensus standards and best practices that balance innovation with patient safety considerations.
The European Medicines Agency (EMA) classifies most bioprinted materials as ATMPs, subject to centralized authorization procedures under Regulation (EC) No 1394/2007. This framework requires extensive preclinical and clinical data demonstrating safety, efficacy, and quality before market approval. Japan has implemented an accelerated approval pathway through its Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approval for regenerative medicine products, potentially accelerating bioprinted materials' market entry.
Quality control standards present significant challenges for bioprinted materials due to their inherent variability and complexity. The International Organization for Standardization (ISO) has begun developing specific standards for bioprinting processes (ISO/TC 276 Biotechnology), though comprehensive standardization remains incomplete. Current good manufacturing practices (cGMP) requirements necessitate stringent process validation, which is particularly challenging for personalized bioprinted constructs.
Ethical considerations further complicate the regulatory landscape, particularly regarding cell sourcing, informed consent, and potential applications. The International Society for Stem Cell Research (ISSCR) guidelines provide ethical frameworks for cellular materials, while national ethics committees often provide additional oversight for novel bioprinting applications.
Long-term safety monitoring represents another regulatory challenge, with requirements for post-market surveillance varying significantly across jurisdictions. The lack of harmonized international standards creates regulatory uncertainty for developers and potentially impedes global commercialization efforts. Several regulatory agencies have established innovation pathways to address these challenges, including the FDA's Tissue Reference Group and the EMA's Innovation Task Force, which provide early dialogue opportunities for bioprinting innovators.
As the field advances, regulatory frameworks are expected to evolve toward more specific guidance for bioprinted materials. Industry stakeholders, academic researchers, and regulatory bodies are increasingly collaborating through initiatives like the TERMIS (Tissue Engineering and Regenerative Medicine International Society) working groups to develop consensus standards and best practices that balance innovation with patient safety considerations.
Biocompatibility and Cell Viability Considerations
Biocompatibility and Cell Viability Considerations represent critical factors in the development and application of bio-inks for printing living materials. The interaction between printed cells and the surrounding bio-ink matrix fundamentally determines the success of bioprinting applications, particularly in tissue engineering and regenerative medicine contexts.
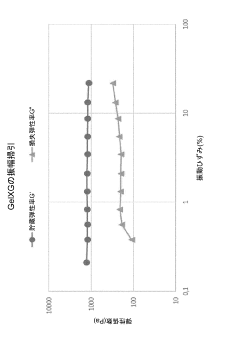
The primary biocompatibility requirement for bio-inks is the absence of cytotoxicity during both the printing process and subsequent culture periods. Materials must not release harmful byproducts during degradation or crosslinking that could compromise cell function or viability. Recent studies have demonstrated that natural polymers like alginate, gelatin, and hyaluronic acid generally exhibit superior biocompatibility compared to synthetic alternatives, though modifications to synthetic polymers continue to improve their performance.
Cell viability assessment typically involves both immediate post-printing evaluation and long-term survival monitoring. Standard protocols include live/dead staining, metabolic activity assays (MTT/MTS/Alamar Blue), and DNA quantification methods. Research indicates that printing parameters significantly impact cell survival rates, with extrusion pressure, nozzle diameter, and printing speed being particularly influential factors. Studies by Blaeser et al. (2020) demonstrated that reducing extrusion pressure below 30 kPa while maintaining printability can increase cell viability by up to 25% in certain cell types.
The microenvironment created by bio-inks plays a crucial role in maintaining cellular function. Optimal bio-inks should provide appropriate mechanical support while facilitating nutrient diffusion, waste removal, and cell-cell communication. The porosity of crosslinked structures has emerged as a critical parameter, with ideal pore sizes ranging from 100-300 μm depending on the target tissue type. Recent innovations include the incorporation of oxygen-releasing compounds and growth factors to enhance the cellular microenvironment during the critical post-printing period.
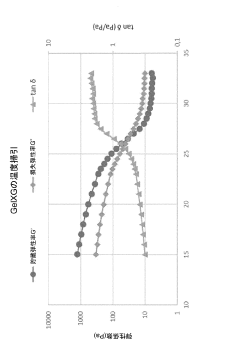
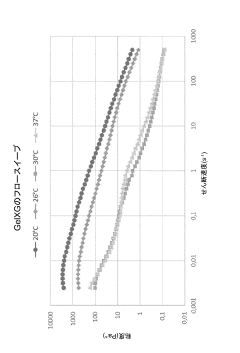
Temperature sensitivity presents another significant consideration, as temperature fluctuations during printing can trigger heat shock responses in cells. Bio-inks with printing temperatures close to physiological conditions (35-37°C) demonstrate superior cell viability outcomes. The development of room-temperature printable bio-inks has shown promising results in preserving sensitive cell types like stem cells and primary hepatocytes.
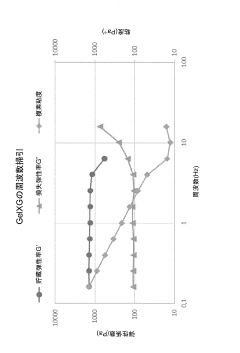
Shear stress during the extrusion process remains a major challenge for maintaining cell viability. Rheological properties of bio-inks must be carefully balanced to achieve both printability and cell protection. Recent advances in shear-thinning hydrogels have demonstrated improved cell survival rates by reducing mechanical forces during extrusion while maintaining structural integrity post-printing.
The primary biocompatibility requirement for bio-inks is the absence of cytotoxicity during both the printing process and subsequent culture periods. Materials must not release harmful byproducts during degradation or crosslinking that could compromise cell function or viability. Recent studies have demonstrated that natural polymers like alginate, gelatin, and hyaluronic acid generally exhibit superior biocompatibility compared to synthetic alternatives, though modifications to synthetic polymers continue to improve their performance.
Cell viability assessment typically involves both immediate post-printing evaluation and long-term survival monitoring. Standard protocols include live/dead staining, metabolic activity assays (MTT/MTS/Alamar Blue), and DNA quantification methods. Research indicates that printing parameters significantly impact cell survival rates, with extrusion pressure, nozzle diameter, and printing speed being particularly influential factors. Studies by Blaeser et al. (2020) demonstrated that reducing extrusion pressure below 30 kPa while maintaining printability can increase cell viability by up to 25% in certain cell types.
The microenvironment created by bio-inks plays a crucial role in maintaining cellular function. Optimal bio-inks should provide appropriate mechanical support while facilitating nutrient diffusion, waste removal, and cell-cell communication. The porosity of crosslinked structures has emerged as a critical parameter, with ideal pore sizes ranging from 100-300 μm depending on the target tissue type. Recent innovations include the incorporation of oxygen-releasing compounds and growth factors to enhance the cellular microenvironment during the critical post-printing period.
Temperature sensitivity presents another significant consideration, as temperature fluctuations during printing can trigger heat shock responses in cells. Bio-inks with printing temperatures close to physiological conditions (35-37°C) demonstrate superior cell viability outcomes. The development of room-temperature printable bio-inks has shown promising results in preserving sensitive cell types like stem cells and primary hepatocytes.
Shear stress during the extrusion process remains a major challenge for maintaining cell viability. Rheological properties of bio-inks must be carefully balanced to achieve both printability and cell protection. Recent advances in shear-thinning hydrogels have demonstrated improved cell survival rates by reducing mechanical forces during extrusion while maintaining structural integrity post-printing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!