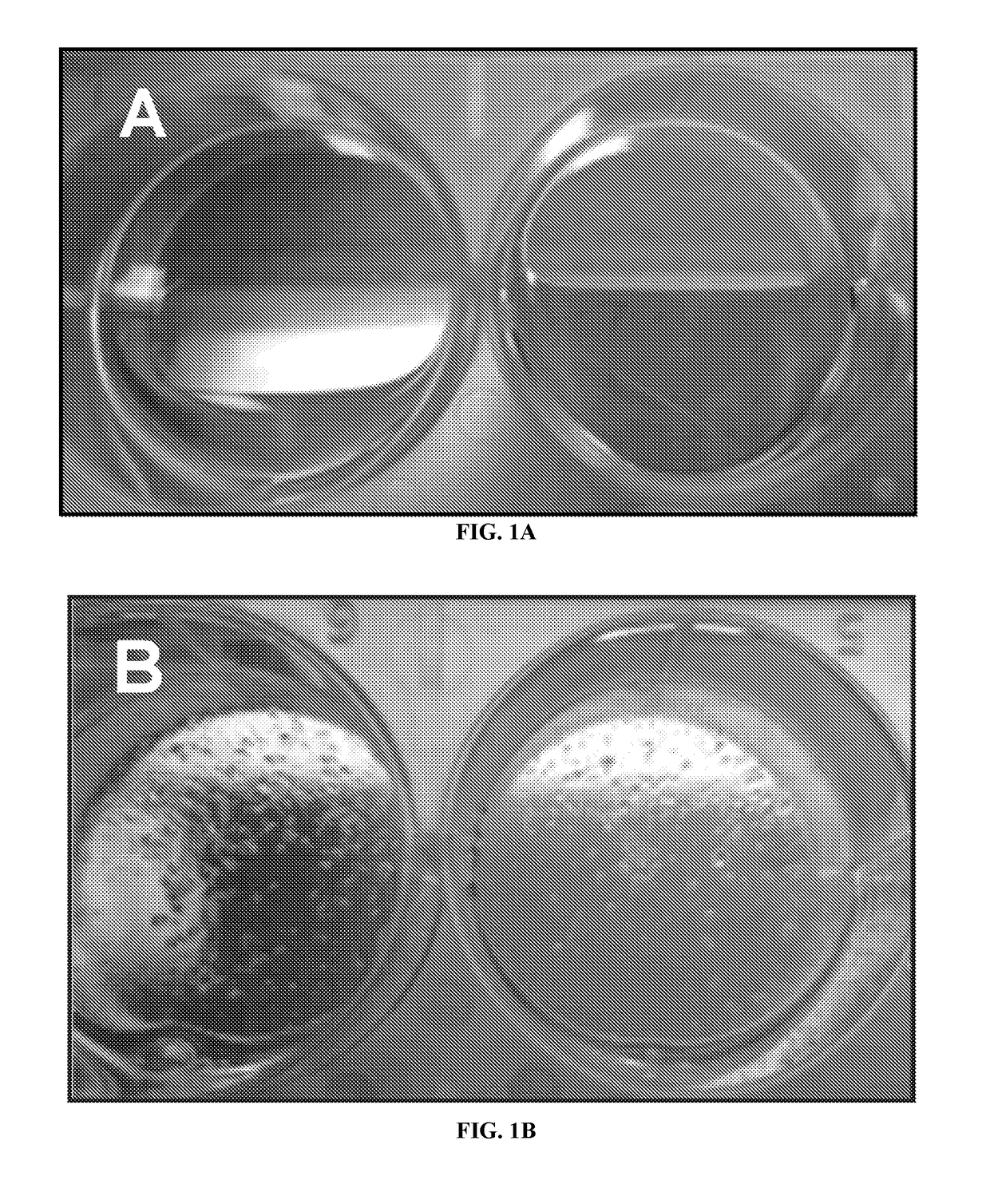
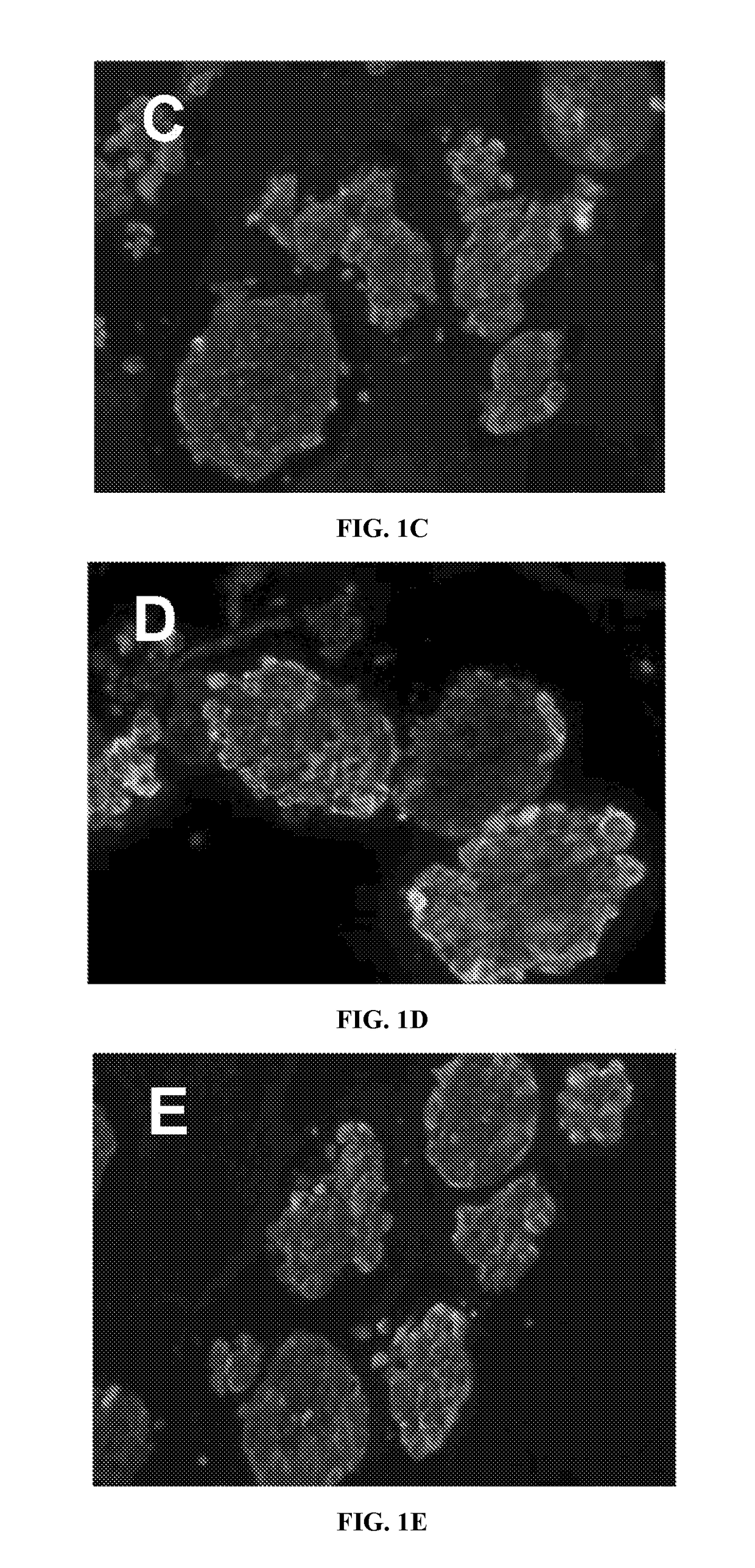
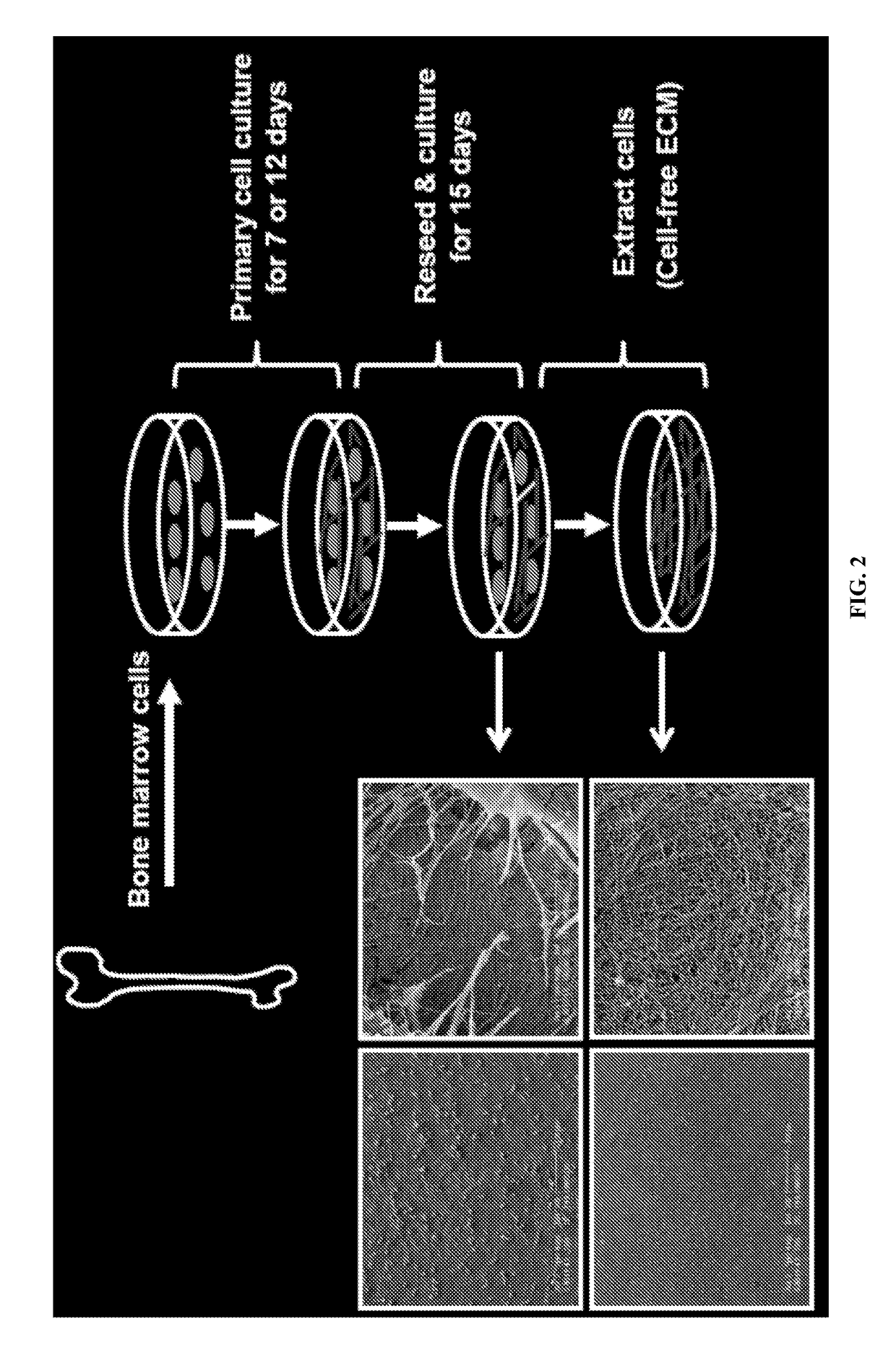
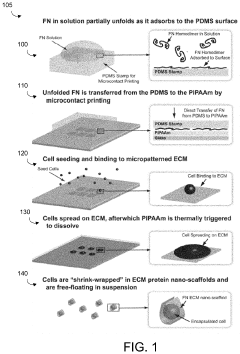
How Do Cell-Scaffold Interactions Dictate ELM Properties?
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-Scaffold Interaction Background and Objectives
The field of extracellular matrix-like materials (ELMs) has evolved significantly over the past decades, transitioning from simple biomaterial scaffolds to complex bioactive structures that mimic the native cellular microenvironment. This evolution reflects our deepening understanding of how cells interact with their surroundings and how these interactions fundamentally influence tissue development, homeostasis, and regeneration processes.
Cell-scaffold interactions represent a complex bidirectional relationship where cells respond to scaffold properties while simultaneously remodeling their environment. Historically, research focused primarily on scaffold mechanical properties, but recent advances have revealed that biochemical composition, topographical features, and degradation kinetics all play crucial roles in dictating cellular behavior and subsequent ELM functionality.
The technological progression in this field has followed several distinct phases: from inert biomaterials designed simply for structural support, to bioactive scaffolds incorporating growth factors, and now toward dynamic, responsive matrices that can adapt to cellular activities. This progression mirrors our evolving understanding of extracellular matrix biology and the complex signaling networks that govern cell-matrix interactions.
Current research trends indicate a shift toward precision-engineered scaffolds with spatiotemporally controlled properties that can direct specific cellular responses. Technologies such as 3D bioprinting, microfluidics, and nanofabrication have enabled unprecedented control over scaffold architecture and composition, allowing researchers to create increasingly sophisticated ELMs that better recapitulate native tissue environments.
The primary objective of current research in this field is to elucidate the fundamental mechanisms by which cell-scaffold interactions dictate ELM properties across multiple scales—from molecular interactions to macroscopic tissue function. This includes understanding how cells sense and respond to mechanical forces, biochemical signals, and topographical cues presented by scaffolds, and how these responses collectively determine tissue formation and function.
Additional research goals include developing predictive models of cell-scaffold interactions, establishing design principles for application-specific ELMs, and creating standardized methods for characterizing these complex interactions. The ultimate aim is to translate this knowledge into clinically relevant technologies for tissue engineering, regenerative medicine, and disease modeling applications.
The interdisciplinary nature of this field necessitates collaboration across multiple domains, including materials science, cell biology, biophysics, and computational modeling. Recent technological convergence has accelerated progress, with advances in one domain often catalyzing breakthroughs in others, creating a positive feedback loop that continues to drive innovation in ELM development.
Cell-scaffold interactions represent a complex bidirectional relationship where cells respond to scaffold properties while simultaneously remodeling their environment. Historically, research focused primarily on scaffold mechanical properties, but recent advances have revealed that biochemical composition, topographical features, and degradation kinetics all play crucial roles in dictating cellular behavior and subsequent ELM functionality.
The technological progression in this field has followed several distinct phases: from inert biomaterials designed simply for structural support, to bioactive scaffolds incorporating growth factors, and now toward dynamic, responsive matrices that can adapt to cellular activities. This progression mirrors our evolving understanding of extracellular matrix biology and the complex signaling networks that govern cell-matrix interactions.
Current research trends indicate a shift toward precision-engineered scaffolds with spatiotemporally controlled properties that can direct specific cellular responses. Technologies such as 3D bioprinting, microfluidics, and nanofabrication have enabled unprecedented control over scaffold architecture and composition, allowing researchers to create increasingly sophisticated ELMs that better recapitulate native tissue environments.
The primary objective of current research in this field is to elucidate the fundamental mechanisms by which cell-scaffold interactions dictate ELM properties across multiple scales—from molecular interactions to macroscopic tissue function. This includes understanding how cells sense and respond to mechanical forces, biochemical signals, and topographical cues presented by scaffolds, and how these responses collectively determine tissue formation and function.
Additional research goals include developing predictive models of cell-scaffold interactions, establishing design principles for application-specific ELMs, and creating standardized methods for characterizing these complex interactions. The ultimate aim is to translate this knowledge into clinically relevant technologies for tissue engineering, regenerative medicine, and disease modeling applications.
The interdisciplinary nature of this field necessitates collaboration across multiple domains, including materials science, cell biology, biophysics, and computational modeling. Recent technological convergence has accelerated progress, with advances in one domain often catalyzing breakthroughs in others, creating a positive feedback loop that continues to drive innovation in ELM development.
Market Analysis for ELM Technologies
The Engineered Living Materials (ELM) market is experiencing significant growth driven by advancements in understanding cell-scaffold interactions. Current market valuation stands at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 14.7% over the next five years. This growth trajectory is primarily fueled by increasing applications in biomedical engineering, tissue regeneration, and sustainable materials development.
Healthcare applications currently dominate the ELM market landscape, accounting for nearly 65% of total market share. Within this segment, tissue engineering and regenerative medicine represent the most substantial revenue streams, as healthcare providers and research institutions invest heavily in technologies that can reduce transplant waiting times and improve patient outcomes through personalized tissue constructs.
The pharmaceutical industry has emerged as another significant market segment, with companies leveraging ELM technologies for drug screening and development. This application reduces the need for animal testing while providing more physiologically relevant models, thereby accelerating the drug development pipeline and reducing associated costs by an estimated 30%.
Regional analysis reveals North America as the current market leader with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing research investments and favorable government policies supporting biotechnology advancement in countries like China, Japan, and South Korea.
Consumer demand patterns indicate a growing preference for sustainable and biocompatible materials across industries. This trend has opened new market opportunities for ELM technologies in sectors previously dominated by synthetic materials, including consumer goods, construction, and environmental remediation. The sustainability aspect of ELMs has attracted significant venture capital funding, with investments increasing by 27% year-over-year.
Key market challenges include regulatory hurdles, with approval processes for ELM-based products typically taking 18-24 months longer than conventional materials. Additionally, production scalability remains a significant barrier to widespread commercial adoption, with current manufacturing processes often limited to laboratory or small-batch production scales.
Market forecasts suggest that breakthroughs in understanding cell-scaffold interactions will be a primary driver of future market expansion. Companies that can develop proprietary technologies optimizing these interactions are positioned to capture substantial market share, particularly as applications expand beyond traditional biomedical fields into emerging sectors like biofabrication and smart living architecture.
Healthcare applications currently dominate the ELM market landscape, accounting for nearly 65% of total market share. Within this segment, tissue engineering and regenerative medicine represent the most substantial revenue streams, as healthcare providers and research institutions invest heavily in technologies that can reduce transplant waiting times and improve patient outcomes through personalized tissue constructs.
The pharmaceutical industry has emerged as another significant market segment, with companies leveraging ELM technologies for drug screening and development. This application reduces the need for animal testing while providing more physiologically relevant models, thereby accelerating the drug development pipeline and reducing associated costs by an estimated 30%.
Regional analysis reveals North America as the current market leader with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing research investments and favorable government policies supporting biotechnology advancement in countries like China, Japan, and South Korea.
Consumer demand patterns indicate a growing preference for sustainable and biocompatible materials across industries. This trend has opened new market opportunities for ELM technologies in sectors previously dominated by synthetic materials, including consumer goods, construction, and environmental remediation. The sustainability aspect of ELMs has attracted significant venture capital funding, with investments increasing by 27% year-over-year.
Key market challenges include regulatory hurdles, with approval processes for ELM-based products typically taking 18-24 months longer than conventional materials. Additionally, production scalability remains a significant barrier to widespread commercial adoption, with current manufacturing processes often limited to laboratory or small-batch production scales.
Market forecasts suggest that breakthroughs in understanding cell-scaffold interactions will be a primary driver of future market expansion. Companies that can develop proprietary technologies optimizing these interactions are positioned to capture substantial market share, particularly as applications expand beyond traditional biomedical fields into emerging sectors like biofabrication and smart living architecture.
Current Challenges in Cell-Scaffold Interactions
Despite significant advancements in engineered living materials (ELMs), the field faces substantial challenges in understanding and controlling cell-scaffold interactions. The fundamental mechanisms governing how cells interact with scaffolding materials remain incompletely characterized, creating a significant knowledge gap that impedes progress. Researchers struggle to predict how specific scaffold properties—such as stiffness, porosity, surface chemistry, and topography—influence cellular behavior including adhesion, migration, proliferation, and differentiation.
A major technical hurdle involves the development of standardized methods to quantitatively assess these interactions across different material systems. Current analytical techniques often provide only partial insights, failing to capture the dynamic nature of cell-scaffold interactions that evolve over time. This limitation makes it difficult to establish reliable cause-effect relationships between scaffold modifications and resulting cellular responses.
The biological complexity presents another significant challenge. Cells respond to scaffolds through multiple signaling pathways simultaneously, creating intricate feedback loops that are difficult to isolate and study. Additionally, cell populations exhibit heterogeneous responses to identical scaffold conditions, introducing variability that complicates experimental reproducibility and data interpretation.
Scale-up issues further complicate the field, as interactions observed in laboratory settings often change dramatically when implemented at production scales. The transition from controlled experimental environments to practical applications frequently reveals unforeseen complications in cell-scaffold dynamics that weren't apparent in smaller-scale studies.
Interdisciplinary barriers also impede progress, as effective solutions require expertise spanning materials science, cell biology, mechanical engineering, and computational modeling. The lack of standardized terminology and methodologies across these disciplines creates communication challenges that slow collaborative advancement.
Regulatory considerations add another layer of complexity, particularly for ELMs intended for biomedical applications. Demonstrating consistent and predictable cell-scaffold interactions is essential for regulatory approval, yet current limitations in understanding these interactions make such demonstrations difficult.
The time-dependent nature of these interactions poses additional challenges. As cells remodel their surrounding environment, the initial scaffold properties change, creating a continuously evolving system that is difficult to characterize and control. This dynamic relationship between cells and scaffolds means that properties measured at one timepoint may not predict future material behavior, complicating long-term performance predictions for ELM technologies.
A major technical hurdle involves the development of standardized methods to quantitatively assess these interactions across different material systems. Current analytical techniques often provide only partial insights, failing to capture the dynamic nature of cell-scaffold interactions that evolve over time. This limitation makes it difficult to establish reliable cause-effect relationships between scaffold modifications and resulting cellular responses.
The biological complexity presents another significant challenge. Cells respond to scaffolds through multiple signaling pathways simultaneously, creating intricate feedback loops that are difficult to isolate and study. Additionally, cell populations exhibit heterogeneous responses to identical scaffold conditions, introducing variability that complicates experimental reproducibility and data interpretation.
Scale-up issues further complicate the field, as interactions observed in laboratory settings often change dramatically when implemented at production scales. The transition from controlled experimental environments to practical applications frequently reveals unforeseen complications in cell-scaffold dynamics that weren't apparent in smaller-scale studies.
Interdisciplinary barriers also impede progress, as effective solutions require expertise spanning materials science, cell biology, mechanical engineering, and computational modeling. The lack of standardized terminology and methodologies across these disciplines creates communication challenges that slow collaborative advancement.
Regulatory considerations add another layer of complexity, particularly for ELMs intended for biomedical applications. Demonstrating consistent and predictable cell-scaffold interactions is essential for regulatory approval, yet current limitations in understanding these interactions make such demonstrations difficult.
The time-dependent nature of these interactions poses additional challenges. As cells remodel their surrounding environment, the initial scaffold properties change, creating a continuously evolving system that is difficult to characterize and control. This dynamic relationship between cells and scaffolds means that properties measured at one timepoint may not predict future material behavior, complicating long-term performance predictions for ELM technologies.
Current Methodologies for Studying Cell-Scaffold Interactions
01 Extracellular Matrix (ECM) Properties for Cell Scaffolds
Scaffolds designed to mimic natural extracellular matrix properties provide optimal environments for cell adhesion, proliferation, and differentiation. These biomaterials incorporate specific ECM components or structural features that enhance cell-scaffold interactions. By replicating the biochemical and mechanical properties of native ECM, these scaffolds can better support cellular functions and tissue regeneration processes.- Extracellular Matrix (ECM) composition for cell-scaffold interactions: The composition of extracellular matrix components in scaffolds significantly influences cell behavior and tissue development. Specific ECM proteins like collagen, fibronectin, and laminin can be incorporated into scaffolds to enhance cell adhesion, proliferation, and differentiation. These biomimetic scaffolds provide natural binding sites for cells, improving the biological performance of tissue engineering constructs and promoting better integration with host tissues.
- Mechanical properties of scaffolds affecting cellular response: The mechanical properties of scaffolds, including elasticity, stiffness, and porosity, play crucial roles in regulating cell behavior. Cells can sense and respond to the mechanical environment through mechanotransduction pathways. Scaffolds with optimized mechanical properties that match native tissue characteristics can guide stem cell differentiation, promote proper tissue formation, and enhance functional outcomes in tissue engineering applications.
- Surface modifications to enhance cell-scaffold interactions: Surface modifications of scaffolds can significantly improve cell attachment and subsequent cellular functions. Techniques such as plasma treatment, chemical functionalization, and biomolecule immobilization can alter surface chemistry, topography, and wettability. These modifications create bioactive surfaces that present specific ligands or growth factors to cells, enhancing cell adhesion, migration, proliferation, and differentiation in tissue engineering applications.
- Dynamic scaffolds with responsive properties: Advanced scaffolds with stimuli-responsive or dynamic properties can better mimic the natural extracellular environment. These smart materials can change their properties in response to external stimuli such as temperature, pH, light, or electrical signals. Dynamic scaffolds allow for temporal control over cell-material interactions, enabling the regulation of cell behavior at different stages of tissue development and providing improved outcomes in tissue regeneration applications.
- 3D architecture and topographical cues for cellular guidance: The three-dimensional architecture and topographical features of scaffolds provide important physical cues that guide cell behavior. Micro- and nano-scale topographical patterns can influence cell alignment, morphology, migration, and differentiation. Precisely designed scaffold architectures with controlled pore size, interconnectivity, and spatial organization can facilitate nutrient transport, waste removal, and cellular infiltration, leading to improved tissue formation and functionality.
02 Mechanical and Topographical Scaffold Features
The physical properties of scaffolds, including stiffness, elasticity, porosity, and surface topography, significantly influence cell behavior and fate. Engineered scaffolds with specific mechanical properties can direct stem cell differentiation toward desired lineages. Surface modifications and micro/nano-scale topographical features can enhance cell attachment, migration, and organization, leading to improved tissue formation and functionality.Expand Specific Solutions03 Bioactive Molecules and Growth Factors in Scaffolds
Incorporation of bioactive molecules and growth factors into scaffolds creates instructive microenvironments that guide cellular responses. These bioactive components can be immobilized or released in controlled patterns to regulate cell adhesion, proliferation, migration, and differentiation. Such functionalized scaffolds enhance cell-material interactions and promote specific tissue development pathways, improving overall regenerative outcomes.Expand Specific Solutions04 Smart and Responsive Scaffold Materials
Advanced scaffold materials with stimuli-responsive properties can dynamically interact with cells and adapt to changing microenvironments. These smart materials respond to physical, chemical, or biological stimuli by altering their properties, such as stiffness, degradation rate, or presentation of bioactive cues. This dynamic behavior enables temporal control over cell-scaffold interactions, better mimicking the natural tissue development process.Expand Specific Solutions05 Computational Modeling of Cell-Scaffold Interactions
Computational approaches are increasingly used to predict and optimize cell-scaffold interactions. These models simulate how cells respond to various scaffold properties, including material composition, mechanical characteristics, and degradation kinetics. By integrating experimental data with theoretical frameworks, researchers can design scaffolds with precise properties tailored for specific tissue engineering applications, reducing development time and improving functional outcomes.Expand Specific Solutions
Key Research Groups and Industry Players
The cell-scaffold interaction field is currently in a growth phase, with increasing market demand driven by tissue engineering and regenerative medicine applications. The global market is expanding rapidly as research translates into clinical applications. Technologically, the field shows varying maturity levels across different applications. Leading academic institutions like MIT, Harvard, and Washington University are advancing fundamental research, while companies such as Becton Dickinson, Nordic Bioscience, and Xylyx Bio are commercializing technologies. Research organizations like Purdue Research Foundation and Suzhou Institute of Nano-Tech & Nano-Bionics are bridging fundamental science with applications. The competitive landscape features collaboration between academic institutions and industry partners, with increasing focus on developing biomimetic scaffolds that accurately replicate extracellular matrix properties for improved cell-material interactions.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to cell-scaffold interactions in ELM properties centers on their pioneering organ-on-chip technology and advanced biomaterial engineering. Their researchers have developed microfluidic devices that precisely control the mechanical and biochemical microenvironment to study cell-ECM interactions. Harvard's platform enables real-time monitoring of cellular responses to varying scaffold properties including stiffness, porosity, and ligand presentation. Their technology incorporates programmable mechanical actuation systems that can apply physiologically relevant forces to cell-laden scaffolds, allowing researchers to observe how mechanical stimuli influence cell behavior and subsequent ELM characteristics. Harvard has also developed synthetic hydrogel systems with independently tunable mechanical and biochemical properties, enabling systematic investigation of how specific scaffold parameters dictate cell fate decisions and matrix remodeling. Their research has demonstrated that scaffold architecture significantly influences stem cell differentiation through mechanotransduction pathways involving YAP/TAZ signaling and cytoskeletal tension[1][2].
Strengths: Exceptional interdisciplinary approach combining microfluidics, materials science, and cell biology; access to cutting-edge analytical tools for comprehensive characterization of cell-scaffold interactions. Weaknesses: Their highly sophisticated systems may be difficult to translate to large-scale manufacturing processes, and the complexity of their models sometimes makes it challenging to isolate individual variables affecting ELM properties.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a comprehensive technological approach to understanding cell-scaffold interactions in ELM properties through their innovative biomaterial platforms. Their research teams have engineered dynamic hydrogel systems with spatiotemporally controlled mechanical and biochemical properties that mimic the native extracellular matrix. These smart scaffolds incorporate responsive elements that can undergo programmed changes in stiffness, degradability, and ligand presentation in response to specific cellular activities. Michigan researchers have pioneered the use of 3D bioprinting techniques to create precisely defined scaffold architectures with controlled porosity gradients and anisotropic mechanical properties. Their technology enables the systematic investigation of how scaffold topography, stiffness, and biochemical composition collectively influence cell adhesion, migration, proliferation, and differentiation. The university has also developed advanced imaging platforms that allow real-time visualization of cell-matrix interactions at the molecular level, revealing how cells sense and respond to their microenvironment through focal adhesion dynamics and cytoskeletal reorganization[3][4].
Strengths: Exceptional capabilities in creating biomimetic scaffolds with precisely controlled properties; strong integration of computational modeling with experimental approaches to predict cell-scaffold interactions. Weaknesses: Some of their more advanced scaffold systems require specialized equipment and expertise, potentially limiting widespread adoption; their focus on fundamental mechanisms sometimes results in systems that are not immediately translatable to clinical applications.
Critical Mechanisms Governing ELM Properties
Three-dimensional scaffold culture system of functional pancreatic islets
PatentInactiveUS20180051255A1
Innovation
- A cell culture system using a silk fibroid scaffold coated with fibronectin, combined with a bone marrow extracellular matrix, which supports the growth of pancreatic cells in three-dimensional aggregates, retaining morphological and functional features of pancreatic tissue, including insulin production and secretion.
Extracellular Matrix Scaffolds
PatentPendingUS20220333063A1
Innovation
- The method involves forming 2D micro-tissues on a thermoresponsive substrate coated with an extracellular matrix compound, allowing cells to maintain structure and phenotype, and then releasing these micro-tissues to fold around the cells, providing protection and attachment sites for tissue repair.
Biomaterial Selection Criteria
The selection of appropriate biomaterials is critical for engineering extracellular matrix-like (ELM) structures that effectively support cell-scaffold interactions. When evaluating potential biomaterials for ELM applications, several key criteria must be considered to ensure optimal performance and cellular response.
Biocompatibility represents the foremost consideration, as materials must not elicit adverse immune responses or cytotoxicity when interacting with cells. Materials should support cell adhesion, proliferation, and differentiation without triggering inflammatory cascades that could compromise scaffold integrity or cellular function. This compatibility extends beyond mere tolerance to actively promoting beneficial cell behaviors.
Mechanical properties of selected biomaterials must closely mimic those of the native tissue being replaced or augmented. Elasticity, stiffness, and tensile strength significantly influence cell behavior through mechanotransduction pathways. Research has demonstrated that substrate stiffness alone can direct stem cell lineage commitment, with softer matrices promoting neurogenic differentiation while stiffer substrates favor osteogenic outcomes.
Degradation kinetics present another crucial parameter, as biomaterials should degrade at rates complementary to tissue regeneration. Ideally, scaffold degradation should occur concurrently with ECM deposition by resident cells, maintaining structural integrity throughout the regenerative process. Degradation byproducts must also be non-toxic and readily metabolized or excreted.
Surface topography and chemistry significantly impact cell-scaffold interactions at the micro and nanoscale. Features such as porosity, fiber alignment, and surface roughness guide cell orientation, migration, and morphology. Chemical functionalization with bioactive molecules, including cell adhesion motifs like RGD peptides, can enhance specific cellular responses and improve integration with surrounding tissues.
Processability considerations ensure that biomaterials can be fabricated into architectures that recapitulate the complex structural organization of native ECM. Materials should be amenable to various manufacturing techniques such as electrospinning, 3D bioprinting, or self-assembly processes that create hierarchical structures supporting cellular organization.
Cost-effectiveness and scalability factors cannot be overlooked, particularly when considering translation to clinical applications. Materials that require prohibitively expensive processing or that cannot be consistently manufactured at scale present significant barriers to widespread implementation, regardless of their performance in laboratory settings.
Regulatory compliance represents the final critical criterion, as biomaterials must meet established safety standards for their intended applications. Materials with established regulatory approval pathways offer advantages in accelerating clinical translation compared to novel compounds requiring extensive safety validation.
Biocompatibility represents the foremost consideration, as materials must not elicit adverse immune responses or cytotoxicity when interacting with cells. Materials should support cell adhesion, proliferation, and differentiation without triggering inflammatory cascades that could compromise scaffold integrity or cellular function. This compatibility extends beyond mere tolerance to actively promoting beneficial cell behaviors.
Mechanical properties of selected biomaterials must closely mimic those of the native tissue being replaced or augmented. Elasticity, stiffness, and tensile strength significantly influence cell behavior through mechanotransduction pathways. Research has demonstrated that substrate stiffness alone can direct stem cell lineage commitment, with softer matrices promoting neurogenic differentiation while stiffer substrates favor osteogenic outcomes.
Degradation kinetics present another crucial parameter, as biomaterials should degrade at rates complementary to tissue regeneration. Ideally, scaffold degradation should occur concurrently with ECM deposition by resident cells, maintaining structural integrity throughout the regenerative process. Degradation byproducts must also be non-toxic and readily metabolized or excreted.
Surface topography and chemistry significantly impact cell-scaffold interactions at the micro and nanoscale. Features such as porosity, fiber alignment, and surface roughness guide cell orientation, migration, and morphology. Chemical functionalization with bioactive molecules, including cell adhesion motifs like RGD peptides, can enhance specific cellular responses and improve integration with surrounding tissues.
Processability considerations ensure that biomaterials can be fabricated into architectures that recapitulate the complex structural organization of native ECM. Materials should be amenable to various manufacturing techniques such as electrospinning, 3D bioprinting, or self-assembly processes that create hierarchical structures supporting cellular organization.
Cost-effectiveness and scalability factors cannot be overlooked, particularly when considering translation to clinical applications. Materials that require prohibitively expensive processing or that cannot be consistently manufactured at scale present significant barriers to widespread implementation, regardless of their performance in laboratory settings.
Regulatory compliance represents the final critical criterion, as biomaterials must meet established safety standards for their intended applications. Materials with established regulatory approval pathways offer advantages in accelerating clinical translation compared to novel compounds requiring extensive safety validation.
Regulatory Considerations for ELM Applications
The regulatory landscape for Engineered Living Materials (ELMs) presents a complex framework that developers must navigate carefully. As cell-scaffold interactions fundamentally determine ELM properties, regulatory bodies have established specific guidelines addressing these biological interfaces. The FDA, EMA, and other international regulatory agencies have developed classification systems that categorize ELMs based on their cellular components, scaffold materials, and intended applications, with particular emphasis on how cell-scaffold interactions influence material behavior.
Risk assessment protocols for ELMs require comprehensive documentation of cell-scaffold interaction mechanisms, including adhesion dynamics, mechanotransduction pathways, and cellular response to scaffold topography. Regulatory submissions must demonstrate how these interactions contribute to material stability, functionality, and safety profiles. The FDA's guidance for combination products provides a foundation for ELM regulation, though specialized frameworks continue to evolve as the technology advances.
Quality control standards for ELMs focus heavily on reproducibility of cell-scaffold interactions, with requirements for validated assays that quantify cellular attachment, migration, and differentiation within scaffold environments. Manufacturers must implement robust testing protocols that assess how variations in scaffold properties affect cellular behavior and resultant material characteristics, ensuring batch-to-batch consistency in final product performance.
Biocompatibility testing requirements are particularly stringent for ELMs, with regulatory bodies mandating extensive in vitro and in vivo studies to evaluate how cell-scaffold interactions might trigger immune responses or toxicity. These assessments must account for dynamic changes in material properties as living cells modify their scaffold environment over time, presenting unique challenges not encountered with traditional biomaterials.
International harmonization efforts are underway to standardize ELM regulatory approaches, with initiatives like the International Medical Device Regulators Forum (IMDRF) developing consensus documents addressing cell-scaffold technologies. These collaborative efforts aim to establish common terminology, testing methodologies, and safety thresholds specific to engineered living materials, facilitating global market access while maintaining rigorous safety standards.
Regulatory pathways for ELMs often require post-market surveillance strategies that monitor long-term stability of cell-scaffold interactions in real-world applications. Manufacturers must implement systems to track how environmental factors influence cellular behavior within scaffolds over extended timeframes, with particular attention to potential drift in material properties that might affect safety or performance.
Risk assessment protocols for ELMs require comprehensive documentation of cell-scaffold interaction mechanisms, including adhesion dynamics, mechanotransduction pathways, and cellular response to scaffold topography. Regulatory submissions must demonstrate how these interactions contribute to material stability, functionality, and safety profiles. The FDA's guidance for combination products provides a foundation for ELM regulation, though specialized frameworks continue to evolve as the technology advances.
Quality control standards for ELMs focus heavily on reproducibility of cell-scaffold interactions, with requirements for validated assays that quantify cellular attachment, migration, and differentiation within scaffold environments. Manufacturers must implement robust testing protocols that assess how variations in scaffold properties affect cellular behavior and resultant material characteristics, ensuring batch-to-batch consistency in final product performance.
Biocompatibility testing requirements are particularly stringent for ELMs, with regulatory bodies mandating extensive in vitro and in vivo studies to evaluate how cell-scaffold interactions might trigger immune responses or toxicity. These assessments must account for dynamic changes in material properties as living cells modify their scaffold environment over time, presenting unique challenges not encountered with traditional biomaterials.
International harmonization efforts are underway to standardize ELM regulatory approaches, with initiatives like the International Medical Device Regulators Forum (IMDRF) developing consensus documents addressing cell-scaffold technologies. These collaborative efforts aim to establish common terminology, testing methodologies, and safety thresholds specific to engineered living materials, facilitating global market access while maintaining rigorous safety standards.
Regulatory pathways for ELMs often require post-market surveillance strategies that monitor long-term stability of cell-scaffold interactions in real-world applications. Manufacturers must implement systems to track how environmental factors influence cellular behavior within scaffolds over extended timeframes, with particular attention to potential drift in material properties that might affect safety or performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!