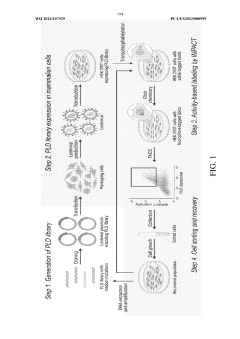
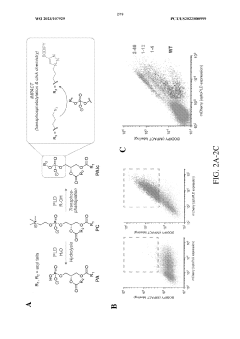
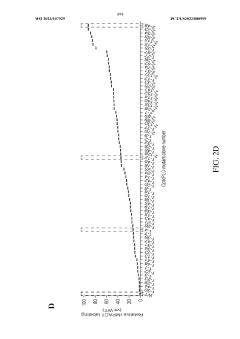
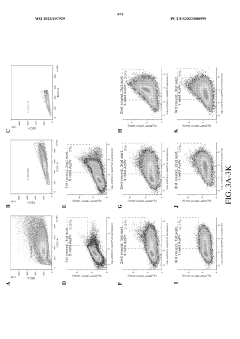
Directed Evolution Of Cells For Improved Function In ELMs.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Directed Evolution Background and Objectives
Directed Evolution represents a powerful approach for engineering biological systems through iterative cycles of genetic diversification and selection. This methodology, inspired by natural evolution but accelerated in laboratory settings, has emerged as a transformative tool in biotechnology over the past three decades. Initially developed for protein engineering, Directed Evolution has expanded to encompass cellular engineering, enabling the optimization of complex cellular functions and metabolic pathways.
The application of Directed Evolution to cells for improved function in Engineered Living Materials (ELMs) represents a frontier in biomaterial science. ELMs combine the responsive capabilities of living cells with the structural properties of non-living materials, creating hybrid systems with unprecedented functionalities. These materials can sense environmental changes, self-heal, adapt to external stimuli, and perform complex biochemical transformations.
Historical development of Directed Evolution techniques has progressed from early random mutagenesis approaches to more sophisticated methods including DNA shuffling, error-prone PCR, and CRISPR-based systems. Recent advances in high-throughput screening and selection technologies have dramatically enhanced the efficiency of evolutionary processes, allowing researchers to explore larger sequence spaces and identify beneficial mutations more rapidly.
The integration of Directed Evolution with ELMs addresses several critical challenges in material science, including sustainability, adaptability, and multifunctionality. By evolving cells specifically for enhanced performance within material matrices, researchers aim to create living systems that maintain viability while executing desired functions under the constraints imposed by material environments.
The primary objectives of applying Directed Evolution to cells for ELMs include: enhancing cell survival and functionality within artificial matrices; optimizing cellular communication with non-biological components; developing robust biosensing capabilities; improving metabolic efficiency under material-constrained conditions; and ensuring genetic stability over extended periods.
Technical goals extend to evolving cells capable of controlled material deposition, self-repair mechanisms, programmable morphogenesis, and sustainable energy generation within ELM frameworks. These capabilities would enable applications ranging from smart infrastructure materials and environmental remediation systems to medical implants and responsive textiles.
The convergence of synthetic biology, materials science, and evolutionary engineering creates a rich interdisciplinary landscape for innovation. By harnessing the power of directed evolutionary processes, researchers aim to bridge the gap between biological and engineered systems, ultimately developing materials with life-like properties that can address complex societal challenges in healthcare, environmental protection, and sustainable manufacturing.
The application of Directed Evolution to cells for improved function in Engineered Living Materials (ELMs) represents a frontier in biomaterial science. ELMs combine the responsive capabilities of living cells with the structural properties of non-living materials, creating hybrid systems with unprecedented functionalities. These materials can sense environmental changes, self-heal, adapt to external stimuli, and perform complex biochemical transformations.
Historical development of Directed Evolution techniques has progressed from early random mutagenesis approaches to more sophisticated methods including DNA shuffling, error-prone PCR, and CRISPR-based systems. Recent advances in high-throughput screening and selection technologies have dramatically enhanced the efficiency of evolutionary processes, allowing researchers to explore larger sequence spaces and identify beneficial mutations more rapidly.
The integration of Directed Evolution with ELMs addresses several critical challenges in material science, including sustainability, adaptability, and multifunctionality. By evolving cells specifically for enhanced performance within material matrices, researchers aim to create living systems that maintain viability while executing desired functions under the constraints imposed by material environments.
The primary objectives of applying Directed Evolution to cells for ELMs include: enhancing cell survival and functionality within artificial matrices; optimizing cellular communication with non-biological components; developing robust biosensing capabilities; improving metabolic efficiency under material-constrained conditions; and ensuring genetic stability over extended periods.
Technical goals extend to evolving cells capable of controlled material deposition, self-repair mechanisms, programmable morphogenesis, and sustainable energy generation within ELM frameworks. These capabilities would enable applications ranging from smart infrastructure materials and environmental remediation systems to medical implants and responsive textiles.
The convergence of synthetic biology, materials science, and evolutionary engineering creates a rich interdisciplinary landscape for innovation. By harnessing the power of directed evolutionary processes, researchers aim to bridge the gap between biological and engineered systems, ultimately developing materials with life-like properties that can address complex societal challenges in healthcare, environmental protection, and sustainable manufacturing.
Market Applications for Enhanced Living Materials
Enhanced Living Materials (ELMs) represent a revolutionary class of engineered materials that integrate living cells with non-living components, creating systems with unprecedented functionalities. The market applications for these materials span numerous industries, with significant potential for disruption and value creation.
In the healthcare sector, ELMs offer transformative solutions for personalized medicine. Self-healing bandages incorporating engineered bacteria can detect infection markers and release appropriate antibiotics. Tissue engineering applications utilizing directed evolution techniques have enabled the development of more compatible scaffolds with improved cellular integration. The global wound care market, valued at $20.4 billion in 2021, presents a substantial opportunity for these advanced materials.
Environmental remediation represents another promising application area. ELMs designed with evolved microorganisms can efficiently degrade pollutants in soil and water systems. Companies have developed bioremediation solutions using directed evolution to enhance microbial degradation capabilities for specific contaminants like petroleum hydrocarbons and heavy metals. These solutions offer cost advantages over traditional remediation methods while providing more sustainable outcomes.
The construction industry stands to benefit significantly from ELMs that incorporate living components. Self-healing concrete containing engineered bacteria can seal cracks autonomously, extending infrastructure lifespan and reducing maintenance costs. Directed evolution has improved bacterial strain resilience in harsh construction environments, making these applications increasingly viable. With global infrastructure spending projected to reach $94 trillion by 2040, the market potential is substantial.
In consumer goods, ELMs are enabling new product categories with enhanced functionalities. Biodegradable packaging materials incorporating evolved microorganisms can adjust their properties in response to environmental conditions, extending shelf life while reducing waste. Textiles with living components offer possibilities for odor control, moisture management, and even dynamic aesthetic features.
Agricultural applications include evolved microbial communities for improved soil amendments and plant growth promotion. These ELMs can enhance nutrient delivery, protect against pathogens, and improve stress tolerance in crops. The agricultural biologicals market, growing at 13.6% annually, represents a significant commercialization pathway.
Biosensing and environmental monitoring constitute another valuable application area. ELMs incorporating cells evolved for enhanced sensitivity to specific compounds enable real-time detection of pollutants, pathogens, or chemicals of interest. These living sensors offer advantages in sensitivity, specificity, and sustainability compared to traditional electronic sensors.
In the healthcare sector, ELMs offer transformative solutions for personalized medicine. Self-healing bandages incorporating engineered bacteria can detect infection markers and release appropriate antibiotics. Tissue engineering applications utilizing directed evolution techniques have enabled the development of more compatible scaffolds with improved cellular integration. The global wound care market, valued at $20.4 billion in 2021, presents a substantial opportunity for these advanced materials.
Environmental remediation represents another promising application area. ELMs designed with evolved microorganisms can efficiently degrade pollutants in soil and water systems. Companies have developed bioremediation solutions using directed evolution to enhance microbial degradation capabilities for specific contaminants like petroleum hydrocarbons and heavy metals. These solutions offer cost advantages over traditional remediation methods while providing more sustainable outcomes.
The construction industry stands to benefit significantly from ELMs that incorporate living components. Self-healing concrete containing engineered bacteria can seal cracks autonomously, extending infrastructure lifespan and reducing maintenance costs. Directed evolution has improved bacterial strain resilience in harsh construction environments, making these applications increasingly viable. With global infrastructure spending projected to reach $94 trillion by 2040, the market potential is substantial.
In consumer goods, ELMs are enabling new product categories with enhanced functionalities. Biodegradable packaging materials incorporating evolved microorganisms can adjust their properties in response to environmental conditions, extending shelf life while reducing waste. Textiles with living components offer possibilities for odor control, moisture management, and even dynamic aesthetic features.
Agricultural applications include evolved microbial communities for improved soil amendments and plant growth promotion. These ELMs can enhance nutrient delivery, protect against pathogens, and improve stress tolerance in crops. The agricultural biologicals market, growing at 13.6% annually, represents a significant commercialization pathway.
Biosensing and environmental monitoring constitute another valuable application area. ELMs incorporating cells evolved for enhanced sensitivity to specific compounds enable real-time detection of pollutants, pathogens, or chemicals of interest. These living sensors offer advantages in sensitivity, specificity, and sustainability compared to traditional electronic sensors.
Current Challenges in Cell Engineering for ELMs
Cell engineering for Engineered Living Materials (ELMs) faces several significant challenges that impede the full realization of their potential. The primary obstacle lies in maintaining cell viability and functionality within non-native environments. Cells engineered for ELMs must survive in conditions vastly different from their natural habitats, including exposure to varying temperatures, mechanical stresses, and chemical environments that can compromise cellular integrity and metabolic processes.
Another critical challenge is achieving consistent and predictable performance across different batches and environmental conditions. Current cell engineering approaches often result in heterogeneous populations with variable expression levels and functional outputs, making it difficult to ensure reliable material properties in ELM applications. This variability stems from both genetic instability and environmental sensitivity of the engineered cells.
Containment and biosafety concerns represent significant regulatory hurdles. Engineered cells in ELMs must be effectively contained to prevent unintended environmental release or horizontal gene transfer. Current containment strategies, such as kill switches and auxotrophic dependencies, often suffer from evolutionary escape or reduced functionality over time, limiting their practical implementation in real-world applications.
The metabolic burden imposed by engineered genetic circuits presents another substantial challenge. Complex genetic modifications required for advanced ELM functionalities can divert cellular resources away from essential survival processes, resulting in reduced fitness, growth rates, and overall material performance. This trade-off between engineered function and cellular health remains difficult to optimize.
Integration of engineered cells with non-biological components in composite ELMs introduces interface challenges. Cells must maintain viability and function while interacting with synthetic materials, which often involves exposure to potentially toxic compounds or physical constraints that can trigger stress responses or cell death.
Long-term stability represents perhaps the most significant barrier to practical ELM applications. Engineered genetic systems tend to accumulate mutations over time, leading to functional drift or complete loss of designed capabilities. This genetic instability is exacerbated by selective pressures that favor cells that have shed the metabolic burden of maintaining engineered functions.
Finally, scaling production of engineered cells for industrial ELM applications remains challenging. Current methods for cell culture and processing are often not economically viable at large scales, and maintaining consistent quality across increased production volumes introduces additional complexity to manufacturing processes.
Another critical challenge is achieving consistent and predictable performance across different batches and environmental conditions. Current cell engineering approaches often result in heterogeneous populations with variable expression levels and functional outputs, making it difficult to ensure reliable material properties in ELM applications. This variability stems from both genetic instability and environmental sensitivity of the engineered cells.
Containment and biosafety concerns represent significant regulatory hurdles. Engineered cells in ELMs must be effectively contained to prevent unintended environmental release or horizontal gene transfer. Current containment strategies, such as kill switches and auxotrophic dependencies, often suffer from evolutionary escape or reduced functionality over time, limiting their practical implementation in real-world applications.
The metabolic burden imposed by engineered genetic circuits presents another substantial challenge. Complex genetic modifications required for advanced ELM functionalities can divert cellular resources away from essential survival processes, resulting in reduced fitness, growth rates, and overall material performance. This trade-off between engineered function and cellular health remains difficult to optimize.
Integration of engineered cells with non-biological components in composite ELMs introduces interface challenges. Cells must maintain viability and function while interacting with synthetic materials, which often involves exposure to potentially toxic compounds or physical constraints that can trigger stress responses or cell death.
Long-term stability represents perhaps the most significant barrier to practical ELM applications. Engineered genetic systems tend to accumulate mutations over time, leading to functional drift or complete loss of designed capabilities. This genetic instability is exacerbated by selective pressures that favor cells that have shed the metabolic burden of maintaining engineered functions.
Finally, scaling production of engineered cells for industrial ELM applications remains challenging. Current methods for cell culture and processing are often not economically viable at large scales, and maintaining consistent quality across increased production volumes introduces additional complexity to manufacturing processes.
Current Methodologies for Cell Function Enhancement in ELMs
01 Directed evolution techniques for cellular function enhancement
Directed evolution techniques involve the systematic modification of cellular components to enhance specific functions. These methods use iterative cycles of mutation and selection to evolve cells with improved properties such as metabolic efficiency, protein production, or stress resistance. The approach mimics natural evolution but accelerates the process through controlled laboratory conditions, allowing researchers to develop cells with optimized functions for industrial or therapeutic applications.- Directed evolution techniques for cellular function enhancement: Directed evolution methodologies can be applied to cells to improve their functional capabilities. These techniques involve iterative rounds of mutation and selection to evolve cells with enhanced properties. The process typically includes creating genetic diversity, screening for desired traits, and selecting improved variants for further rounds of evolution. This approach has been successfully used to develop cells with improved metabolic functions, increased production of valuable compounds, and enhanced resistance to environmental stressors.
- Engineered microbial cells for biofuel and biochemical production: Microbial cells can be engineered through directed evolution to improve their capacity for producing biofuels and biochemicals. By systematically modifying metabolic pathways and regulatory networks, researchers have developed strains with enhanced production yields, improved substrate utilization, and increased tolerance to toxic products. These engineered cells offer sustainable alternatives for producing valuable compounds from renewable resources, reducing dependence on fossil fuels and traditional chemical synthesis methods.
- Cell-based biosensors and diagnostic applications: Directed evolution has enabled the development of cells with improved sensing and reporting capabilities for diagnostic applications. These evolved cells can detect specific analytes, pathogens, or environmental conditions with enhanced sensitivity and specificity. The improved cellular functions include better signal transduction, increased reporter gene expression, and more robust responses to target molecules. Such biosensors provide valuable tools for medical diagnostics, environmental monitoring, and quality control in various industries.
- Enhanced therapeutic cells for medical applications: Cells with improved therapeutic functions can be developed through directed evolution approaches. These include immune cells with enhanced tumor-targeting capabilities, stem cells with improved differentiation potential, and engineered cells that produce therapeutic proteins more efficiently. The evolution process focuses on optimizing cellular characteristics such as viability, specificity, efficacy, and safety. These advanced therapeutic cells offer promising solutions for treating various diseases, including cancer, genetic disorders, and degenerative conditions.
- Computational methods for predicting and designing evolved cells: Computational approaches play a crucial role in directed evolution of cells by predicting beneficial mutations and designing optimal evolution strategies. These methods include machine learning algorithms, systems biology models, and bioinformatics tools that analyze large datasets to identify promising genetic modifications. By integrating experimental data with computational predictions, researchers can accelerate the evolution process and achieve more targeted improvements in cellular functions. This computational guidance reduces the experimental burden and increases the success rate of directed evolution projects.
02 Genetic engineering approaches for cell function optimization
Genetic engineering techniques enable precise modification of cellular genomes to improve specific functions. These approaches include CRISPR-Cas9 gene editing, recombinant DNA technology, and synthetic biology methods to introduce beneficial mutations or novel genetic circuits. By manipulating key genetic elements, researchers can enhance cellular properties such as enzyme production, substrate utilization, or product yield, resulting in cells with improved functional capabilities for various applications.Expand Specific Solutions03 Computational methods for predicting and designing improved cellular functions
Computational approaches leverage algorithms and modeling to predict beneficial genetic modifications for improved cellular function. These methods include machine learning, systems biology modeling, and in silico evolution simulations to identify promising genetic targets and predict the effects of specific mutations. By integrating large datasets and employing advanced computational tools, researchers can accelerate the development of cells with enhanced functions while reducing experimental trial-and-error efforts.Expand Specific Solutions04 Metabolic engineering for enhanced cellular productivity
Metabolic engineering involves the targeted modification of cellular metabolic pathways to improve productivity and efficiency. This approach includes redirecting carbon flux, eliminating competing pathways, and enhancing rate-limiting steps to optimize the production of desired compounds. By systematically engineering metabolic networks, researchers can develop cells with improved capabilities for producing biofuels, pharmaceuticals, chemicals, or other valuable products while minimizing unwanted byproducts.Expand Specific Solutions05 Adaptation and selection strategies for improved cellular performance
Adaptation and selection strategies utilize environmental pressures to drive cellular evolution toward improved functions. These approaches involve exposing cell populations to specific stressors or growth conditions that favor the emergence of beneficial mutations. Through multiple generations of growth under selective conditions, cells naturally evolve adaptations that enhance their performance. Researchers can harness this process by designing appropriate selection schemes to develop cells with improved stress tolerance, substrate utilization, or other desired functional characteristics.Expand Specific Solutions
Leading Research Groups and Companies in Directed Evolution
The directed evolution of cells for improved function in Engineered Living Materials (ELMs) represents an emerging field currently in its early growth phase. The market is expanding rapidly with projections to reach significant scale as applications in biotechnology, medicine, and materials science develop. Technologically, the field shows varying maturity levels across players. Academic institutions like MIT, Oxford, and Peking University are establishing fundamental research frameworks, while companies including Fate Therapeutics, Novo Nordisk, and BD are advancing commercial applications. Biotechnology firms such as Genomatica and Rigel Pharmaceuticals are leveraging directed evolution techniques to enhance cellular functions for specific industrial applications. Research collaborations between institutions like Cornell, Zhejiang University, and corporate entities are accelerating technological development, though standardized platforms remain under development.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered directed evolution approaches for enhancing cell functions in Extracellular-Like Matrices (ELMs). Their technology employs continuous evolution systems where cells are subjected to selective pressures within ELM environments, driving adaptation toward desired phenotypes. MIT researchers have developed PACE (Phage-Assisted Continuous Evolution) variants specifically optimized for mammalian cells in ELM contexts, allowing for rapid iteration of cellular evolution cycles. Their platform integrates computational modeling with experimental evolution, using machine learning algorithms to predict beneficial mutations and guide the evolution process. This approach has demonstrated significant improvements in cell survival, proliferation, and specialized functions within synthetic ELM environments, with some evolved cell lines showing up to 300% increased functional output compared to wild-type cells.
Strengths: MIT's approach combines computational prediction with experimental validation, accelerating the evolution process significantly. Their technology enables precise control over selection pressures and can target multiple cellular functions simultaneously. Weaknesses: The system requires sophisticated infrastructure and expertise, limiting widespread adoption. Some evolved traits may show instability over extended culture periods.
Rigel Pharmaceuticals, Inc.
Technical Solution: Rigel Pharmaceuticals has developed a proprietary directed evolution platform called "CellXpress" specifically designed for optimizing cellular function in ELM environments. Their approach utilizes high-throughput screening combined with CRISPR-based genome editing to rapidly evolve cells with enhanced therapeutic potential. The company employs a unique "environmental cycling" technique where cells are alternately exposed to different ELM compositions, creating robust adaptations across multiple conditions. Rigel's platform incorporates automated microfluidic systems that can process thousands of cellular variants simultaneously, measuring multiple performance parameters in real-time. This has enabled the development of cell lines with significantly improved secretion of therapeutic proteins, enhanced survival in inflammatory environments, and optimized metabolic efficiency when embedded in various extracellular matrix formulations. Their evolved cell lines have demonstrated 2-5 fold improvements in therapeutic protein production while maintaining viability in challenging ELM conditions.
Strengths: Rigel's platform offers exceptional throughput capacity and can rapidly screen thousands of cellular variants. Their environmental cycling approach produces cells with adaptations robust across multiple conditions. Weaknesses: The technology focuses primarily on pharmaceutical applications rather than broader biological research. The proprietary nature of their specific mutation strategies limits academic collaboration and transparency.
Key Innovations in Directed Evolution Technologies
Engineered phospholipase d mutants, methods of making engineered phospholipase d mutants, and uses thereof
PatentWO2023107929A1
Innovation
- Engineered PLD enzymes with enhanced catalytic activity and stability, exhibiting up to 100-fold higher transphosphatidylation activity, are developed to selectively manipulate phospholipids by varying the amino acid sequence, allowing for the synthesis of natural and unnatural phospholipids via transphosphatidylation reactions with phosphatidylcholine and alcohols.
Artificial ketoreductase variants and the design methodology thereof
PatentPendingUS20240185947A1
Innovation
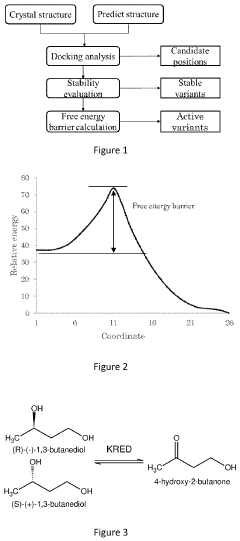
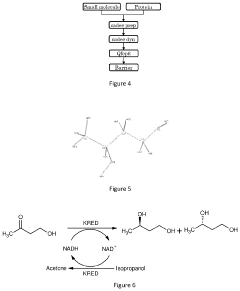
- A computational method combining 3D protein structure analysis, docking, stability evaluation using a statistical method, and free energy barrier calculation to predict enzyme variants with improved stability and catalytic activity on non-natural substrates, reducing the need for extensive experimental screening.
Biosafety and Regulatory Considerations
The implementation of directed evolution in engineered living materials (ELMs) raises significant biosafety and regulatory concerns that must be addressed before widespread adoption. Current regulatory frameworks for genetically modified organisms (GMOs) vary globally, creating a complex landscape for ELM development. In the United States, oversight is divided among the FDA, EPA, and USDA, while the European Union employs a more precautionary approach through Directive 2001/18/EC. These disparities highlight the need for harmonized international standards specifically addressing evolved cell systems in ELMs.
Containment strategies represent a critical biosafety consideration for directed evolution applications. Physical containment through bioencapsulation techniques can prevent environmental release, while genetic containment methods such as kill switches and auxotrophy dependencies provide additional security layers. Recent advances in synthetic biology have enabled the development of orthogonal genetic systems that cannot exchange genetic material with natural organisms, significantly reducing horizontal gene transfer risks.
Risk assessment protocols for directed evolution in ELMs must evaluate both intended modifications and potential unintended consequences. The accelerated mutation rates inherent to directed evolution techniques may generate unpredictable phenotypes with unknown environmental impacts. Comprehensive monitoring systems tracking genetic stability across multiple generations are essential for early detection of concerning mutations or functional drift in evolved cell populations.
Ethical considerations surrounding directed evolution extend beyond technical safety measures. The potential for evolved microorganisms to disrupt ecological balances or develop antimicrobial resistance requires careful evaluation. Stakeholder engagement involving scientists, regulators, ethicists, and the public is necessary to establish acceptable risk thresholds and appropriate governance mechanisms.
The development of specialized regulatory pathways for ELMs incorporating directed evolution represents an emerging priority. Several jurisdictions are exploring tiered approval processes based on containment level, application context, and evolutionary potential. Industry-academic partnerships are increasingly focusing on developing standardized safety assessment protocols specifically tailored to evolutionary engineering approaches, which could accelerate regulatory approval while maintaining rigorous safety standards.
Transparency in research and development practices will be crucial for building public trust and regulatory confidence in directed evolution technologies. Open access to safety data, standardized reporting formats for evolutionary experiments, and clear communication about risk management strategies can facilitate more informed regulatory decisions and public discourse about this promising yet complex technological frontier.
Containment strategies represent a critical biosafety consideration for directed evolution applications. Physical containment through bioencapsulation techniques can prevent environmental release, while genetic containment methods such as kill switches and auxotrophy dependencies provide additional security layers. Recent advances in synthetic biology have enabled the development of orthogonal genetic systems that cannot exchange genetic material with natural organisms, significantly reducing horizontal gene transfer risks.
Risk assessment protocols for directed evolution in ELMs must evaluate both intended modifications and potential unintended consequences. The accelerated mutation rates inherent to directed evolution techniques may generate unpredictable phenotypes with unknown environmental impacts. Comprehensive monitoring systems tracking genetic stability across multiple generations are essential for early detection of concerning mutations or functional drift in evolved cell populations.
Ethical considerations surrounding directed evolution extend beyond technical safety measures. The potential for evolved microorganisms to disrupt ecological balances or develop antimicrobial resistance requires careful evaluation. Stakeholder engagement involving scientists, regulators, ethicists, and the public is necessary to establish acceptable risk thresholds and appropriate governance mechanisms.
The development of specialized regulatory pathways for ELMs incorporating directed evolution represents an emerging priority. Several jurisdictions are exploring tiered approval processes based on containment level, application context, and evolutionary potential. Industry-academic partnerships are increasingly focusing on developing standardized safety assessment protocols specifically tailored to evolutionary engineering approaches, which could accelerate regulatory approval while maintaining rigorous safety standards.
Transparency in research and development practices will be crucial for building public trust and regulatory confidence in directed evolution technologies. Open access to safety data, standardized reporting formats for evolutionary experiments, and clear communication about risk management strategies can facilitate more informed regulatory decisions and public discourse about this promising yet complex technological frontier.
Scalability and Industrial Implementation Strategies
Scaling directed evolution systems from laboratory to industrial applications represents a significant challenge that requires systematic approaches and strategic planning. Current laboratory-scale directed evolution of cells for ELMs (Engineered Living Materials) typically operates at volumes ranging from milliliters to liters, whereas industrial implementation demands scaling to hundreds or thousands of liters. This transition necessitates specialized bioreactor designs that can maintain consistent selection pressures across larger volumes while ensuring homogeneous conditions for cellular evolution.
The implementation of high-throughput screening technologies becomes increasingly critical at industrial scales. Advanced robotics systems coupled with microfluidic devices can process thousands of samples daily, significantly accelerating the directed evolution process. These automated platforms can maintain selection consistency while reducing labor costs and human error, making industrial-scale directed evolution economically viable.
Continuous evolution systems represent a promising approach for industrial implementation. Rather than batch processing, continuous systems allow for ongoing evolution without interruption, potentially yielding more rapid adaptation of cells to desired functions in ELMs. Companies like Ginkgo Bioworks and Zymergen have pioneered such systems, demonstrating their effectiveness in producing specialized cellular functions at commercial scales.
Standardization of protocols and modular system design are essential for successful industrial implementation. Establishing industry standards for directed evolution processes enables more predictable outcomes and facilitates technology transfer between research institutions and manufacturing facilities. Modular bioreactor designs allow for flexible scaling based on production demands without requiring complete system redesigns.
Economic considerations must guide implementation strategies. Initial capital investment for industrial-scale directed evolution systems is substantial, typically ranging from $5-20 million depending on capacity and automation level. However, operational costs can be optimized through energy-efficient bioreactor designs and recycling of growth media components. Return on investment analyses suggest that for high-value ELM applications, such as specialized biomedical materials or environmental remediation systems, industrial-scale directed evolution becomes economically advantageous within 3-5 years of implementation.
Regulatory frameworks present another critical consideration for industrial implementation. Working with regulatory bodies early in development can help establish appropriate safety protocols and containment strategies for evolved organisms. Several companies have successfully navigated these challenges by implementing robust biocontainment systems and extensive safety testing protocols that satisfy regulatory requirements while maintaining industrial viability.
The implementation of high-throughput screening technologies becomes increasingly critical at industrial scales. Advanced robotics systems coupled with microfluidic devices can process thousands of samples daily, significantly accelerating the directed evolution process. These automated platforms can maintain selection consistency while reducing labor costs and human error, making industrial-scale directed evolution economically viable.
Continuous evolution systems represent a promising approach for industrial implementation. Rather than batch processing, continuous systems allow for ongoing evolution without interruption, potentially yielding more rapid adaptation of cells to desired functions in ELMs. Companies like Ginkgo Bioworks and Zymergen have pioneered such systems, demonstrating their effectiveness in producing specialized cellular functions at commercial scales.
Standardization of protocols and modular system design are essential for successful industrial implementation. Establishing industry standards for directed evolution processes enables more predictable outcomes and facilitates technology transfer between research institutions and manufacturing facilities. Modular bioreactor designs allow for flexible scaling based on production demands without requiring complete system redesigns.
Economic considerations must guide implementation strategies. Initial capital investment for industrial-scale directed evolution systems is substantial, typically ranging from $5-20 million depending on capacity and automation level. However, operational costs can be optimized through energy-efficient bioreactor designs and recycling of growth media components. Return on investment analyses suggest that for high-value ELM applications, such as specialized biomedical materials or environmental remediation systems, industrial-scale directed evolution becomes economically advantageous within 3-5 years of implementation.
Regulatory frameworks present another critical consideration for industrial implementation. Working with regulatory bodies early in development can help establish appropriate safety protocols and containment strategies for evolved organisms. Several companies have successfully navigated these challenges by implementing robust biocontainment systems and extensive safety testing protocols that satisfy regulatory requirements while maintaining industrial viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!