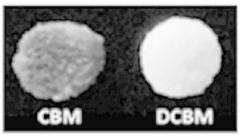
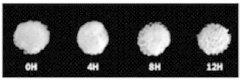
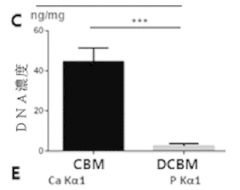
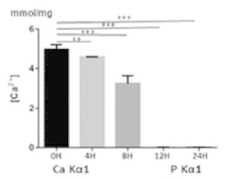
Mineralized ELMs For Bone Tissue Engineering.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mineralized ELMs Background and Objectives
Extracellular matrix-mimicking (ELM) biomaterials have emerged as a promising approach in bone tissue engineering over the past two decades. These materials aim to replicate the complex microenvironment of natural bone tissue, providing both structural support and biological cues necessary for cell adhesion, proliferation, and differentiation. The evolution of mineralized ELMs represents a significant advancement in this field, as they incorporate calcium phosphate minerals similar to those found in native bone tissue.
The development of mineralized ELMs can be traced back to early attempts at creating bioactive ceramics in the 1970s, which evolved into more sophisticated composite materials in the 1990s. The past decade has witnessed remarkable progress in fabrication techniques, allowing for precise control over mineral composition, distribution, and crystallinity within these scaffolds. This technological progression has enabled researchers to more accurately mimic the hierarchical structure of natural bone tissue.
Current trends in mineralized ELM development focus on enhancing bioactivity, mechanical properties, and degradation kinetics to better match those of native bone. Innovations in nanotechnology have facilitated the incorporation of growth factors, stem cells, and other bioactive molecules into these scaffolds, creating multifunctional platforms for bone regeneration. Additionally, advances in 3D printing and electrospinning technologies have enabled the fabrication of patient-specific implants with controlled architecture and porosity.
The primary objective of mineralized ELM research is to develop biomaterials that can effectively promote bone regeneration in critical-sized defects where natural healing processes are insufficient. These materials aim to provide temporary mechanical support while gradually degrading as new bone tissue forms. Specific technical goals include optimizing mineral content and distribution to enhance osteoconductivity, improving mechanical strength to withstand physiological loads, and controlling degradation rates to match the pace of new bone formation.
Another crucial objective is to understand the complex cell-material interactions that govern bone regeneration. This includes elucidating how mineral components influence stem cell differentiation, osteoblast activity, and angiogenesis. Researchers also aim to develop mineralized ELMs that can respond to the local microenvironment, releasing therapeutic agents or changing properties in response to specific biological cues.
Looking forward, the field is moving toward personalized approaches, where mineralized ELMs can be tailored to individual patient needs based on defect size, location, and underlying pathology. The ultimate goal remains the development of off-the-shelf products that can effectively treat a wide range of bone defects with minimal complications and optimal functional outcomes.
The development of mineralized ELMs can be traced back to early attempts at creating bioactive ceramics in the 1970s, which evolved into more sophisticated composite materials in the 1990s. The past decade has witnessed remarkable progress in fabrication techniques, allowing for precise control over mineral composition, distribution, and crystallinity within these scaffolds. This technological progression has enabled researchers to more accurately mimic the hierarchical structure of natural bone tissue.
Current trends in mineralized ELM development focus on enhancing bioactivity, mechanical properties, and degradation kinetics to better match those of native bone. Innovations in nanotechnology have facilitated the incorporation of growth factors, stem cells, and other bioactive molecules into these scaffolds, creating multifunctional platforms for bone regeneration. Additionally, advances in 3D printing and electrospinning technologies have enabled the fabrication of patient-specific implants with controlled architecture and porosity.
The primary objective of mineralized ELM research is to develop biomaterials that can effectively promote bone regeneration in critical-sized defects where natural healing processes are insufficient. These materials aim to provide temporary mechanical support while gradually degrading as new bone tissue forms. Specific technical goals include optimizing mineral content and distribution to enhance osteoconductivity, improving mechanical strength to withstand physiological loads, and controlling degradation rates to match the pace of new bone formation.
Another crucial objective is to understand the complex cell-material interactions that govern bone regeneration. This includes elucidating how mineral components influence stem cell differentiation, osteoblast activity, and angiogenesis. Researchers also aim to develop mineralized ELMs that can respond to the local microenvironment, releasing therapeutic agents or changing properties in response to specific biological cues.
Looking forward, the field is moving toward personalized approaches, where mineralized ELMs can be tailored to individual patient needs based on defect size, location, and underlying pathology. The ultimate goal remains the development of off-the-shelf products that can effectively treat a wide range of bone defects with minimal complications and optimal functional outcomes.
Market Analysis for Bone Tissue Engineering Solutions
The global bone tissue engineering market is experiencing robust growth, valued at approximately $2.7 billion in 2022 and projected to reach $5.4 billion by 2030, representing a compound annual growth rate (CAGR) of 9.1%. This growth is primarily driven by the increasing prevalence of bone disorders, rising geriatric population, and growing incidence of traumatic injuries requiring bone reconstruction.
North America currently dominates the market with about 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about advanced treatment options.
The bone tissue engineering market can be segmented based on material type, application, and end-user. Mineralized extracellular matrix-like materials (ELMs) represent an emerging segment with significant growth potential, currently accounting for approximately 15% of the material market but expected to grow at a CAGR of 12.3% through 2030.
By application, orthopedic and dental applications collectively constitute over 70% of the market. Specifically, applications for critical-sized bone defects represent a high-value segment due to limited alternative treatment options and high clinical need. Hospitals remain the largest end-users (58%), followed by specialty clinics (24%) and research institutions (18%).
Key market drivers include the limitations of traditional bone grafting techniques, increasing demand for personalized medicine, and favorable reimbursement policies in developed countries. The rising incidence of osteoporosis, affecting over 200 million people worldwide, creates substantial demand for effective bone regeneration solutions.
Market restraints include high treatment costs, with average procedures ranging from $15,000 to $50,000, stringent regulatory requirements for novel biomaterials, and limited awareness among healthcare providers about advanced tissue engineering options. Additionally, the long development cycle for bone tissue engineering products (typically 5-7 years) poses challenges for market entrants.
Emerging trends include the integration of 3D printing technologies with mineralized biomaterials, increasing focus on osteoimmunomodulatory properties of scaffolds, and growing interest in drug-eluting mineralized scaffolds for enhanced bone regeneration. The convergence of nanotechnology with mineralized ELMs represents a particularly promising market opportunity, with potential applications extending beyond orthopedics into dental, maxillofacial, and craniofacial reconstruction.
North America currently dominates the market with about 42% share, followed by Europe at 28% and Asia-Pacific at 22%. The Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about advanced treatment options.
The bone tissue engineering market can be segmented based on material type, application, and end-user. Mineralized extracellular matrix-like materials (ELMs) represent an emerging segment with significant growth potential, currently accounting for approximately 15% of the material market but expected to grow at a CAGR of 12.3% through 2030.
By application, orthopedic and dental applications collectively constitute over 70% of the market. Specifically, applications for critical-sized bone defects represent a high-value segment due to limited alternative treatment options and high clinical need. Hospitals remain the largest end-users (58%), followed by specialty clinics (24%) and research institutions (18%).
Key market drivers include the limitations of traditional bone grafting techniques, increasing demand for personalized medicine, and favorable reimbursement policies in developed countries. The rising incidence of osteoporosis, affecting over 200 million people worldwide, creates substantial demand for effective bone regeneration solutions.
Market restraints include high treatment costs, with average procedures ranging from $15,000 to $50,000, stringent regulatory requirements for novel biomaterials, and limited awareness among healthcare providers about advanced tissue engineering options. Additionally, the long development cycle for bone tissue engineering products (typically 5-7 years) poses challenges for market entrants.
Emerging trends include the integration of 3D printing technologies with mineralized biomaterials, increasing focus on osteoimmunomodulatory properties of scaffolds, and growing interest in drug-eluting mineralized scaffolds for enhanced bone regeneration. The convergence of nanotechnology with mineralized ELMs represents a particularly promising market opportunity, with potential applications extending beyond orthopedics into dental, maxillofacial, and craniofacial reconstruction.
Current Challenges in Mineralized ELMs Development
Despite significant advancements in mineralized extracellular matrix-like materials (ELMs) for bone tissue engineering, several critical challenges persist that hinder their widespread clinical application. The primary obstacle remains achieving proper biomineralization that accurately mimics the hierarchical structure and composition of natural bone tissue. Current mineralization techniques often result in homogeneous mineral distribution rather than the heterogeneous patterns observed in native bone, limiting the biomimetic properties of these constructs.
Material stability presents another significant challenge, as many mineralized ELMs exhibit premature degradation or insufficient mechanical integrity under physiological conditions. The balance between material degradation rate and new bone formation remains difficult to optimize, with many scaffolds degrading either too quickly or too slowly relative to the bone regeneration process.
Scalability issues continue to plague the field, with many promising laboratory-scale techniques proving difficult to translate into manufacturing processes capable of producing clinically relevant volumes of consistent materials. This manufacturing gap significantly impedes the transition from research to clinical application.
The biological response to mineralized ELMs remains unpredictable, with variability in cell attachment, proliferation, and differentiation across different material formulations. Controlling the inflammatory response to these materials represents a particular challenge, as excessive inflammation can impair healing while insufficient inflammatory signaling may fail to recruit necessary cells for regeneration.
Vascularization of engineered bone constructs continues to be a critical limitation, with many mineralized ELMs failing to support adequate blood vessel formation. Without proper vascularization, larger bone constructs face nutrient and oxygen limitations that restrict their viability and integration with host tissue.
Regulatory hurdles compound these technical challenges, as the complex composition of mineralized ELMs often complicates their classification and approval pathway. The combination of biological components, synthetic materials, and mineral phases creates regulatory complexity that delays clinical translation.
Integration with existing surgical techniques and healthcare systems presents additional practical challenges. Many promising materials require specialized handling or surgical approaches that limit their adoption by clinicians accustomed to conventional bone grafting materials.
Cost-effectiveness remains a significant barrier, with many advanced mineralization techniques requiring expensive equipment or reagents that make the resulting materials prohibitively costly for routine clinical use, particularly in resource-limited settings.
Material stability presents another significant challenge, as many mineralized ELMs exhibit premature degradation or insufficient mechanical integrity under physiological conditions. The balance between material degradation rate and new bone formation remains difficult to optimize, with many scaffolds degrading either too quickly or too slowly relative to the bone regeneration process.
Scalability issues continue to plague the field, with many promising laboratory-scale techniques proving difficult to translate into manufacturing processes capable of producing clinically relevant volumes of consistent materials. This manufacturing gap significantly impedes the transition from research to clinical application.
The biological response to mineralized ELMs remains unpredictable, with variability in cell attachment, proliferation, and differentiation across different material formulations. Controlling the inflammatory response to these materials represents a particular challenge, as excessive inflammation can impair healing while insufficient inflammatory signaling may fail to recruit necessary cells for regeneration.
Vascularization of engineered bone constructs continues to be a critical limitation, with many mineralized ELMs failing to support adequate blood vessel formation. Without proper vascularization, larger bone constructs face nutrient and oxygen limitations that restrict their viability and integration with host tissue.
Regulatory hurdles compound these technical challenges, as the complex composition of mineralized ELMs often complicates their classification and approval pathway. The combination of biological components, synthetic materials, and mineral phases creates regulatory complexity that delays clinical translation.
Integration with existing surgical techniques and healthcare systems presents additional practical challenges. Many promising materials require specialized handling or surgical approaches that limit their adoption by clinicians accustomed to conventional bone grafting materials.
Cost-effectiveness remains a significant barrier, with many advanced mineralization techniques requiring expensive equipment or reagents that make the resulting materials prohibitively costly for routine clinical use, particularly in resource-limited settings.
Current Mineralization Strategies for ELMs
01 Biomimetic mineralization of extracellular matrix materials
Biomimetic approaches are used to create mineralized extracellular matrix-like materials that mimic natural bone or tissue structures. These processes typically involve the controlled deposition of calcium phosphate or hydroxyapatite onto organic templates that resemble the natural extracellular matrix. The resulting materials exhibit improved mechanical properties and biocompatibility, making them suitable for bone tissue engineering applications.- Biomimetic mineralization of extracellular matrix materials: Biomimetic approaches are used to create mineralized extracellular matrix-like materials that mimic natural tissue mineralization processes. These techniques involve the controlled deposition of calcium phosphate or other mineral phases onto ELM scaffolds under physiological conditions. The resulting materials exhibit hierarchical structures similar to natural mineralized tissues such as bone and dentin, with improved mechanical properties and biocompatibility for tissue engineering applications.
- Polymer-assisted mineralization of ELMs: Polymer additives are incorporated into the mineralization process to control the nucleation, growth, and morphology of mineral crystals on extracellular matrix-like materials. These polymers, including polyacrylic acid, polyaspartic acid, and various synthetic copolymers, can modulate the mineralization kinetics and crystal phase selection. The polymer-mineral interactions lead to the formation of organic-inorganic hybrid materials with enhanced mechanical properties and controlled degradation rates suitable for bone and dental tissue engineering.
- Cell-mediated mineralization of extracellular matrix scaffolds: This approach utilizes cells (such as osteoblasts, odontoblasts, or stem cells) to deposit minerals onto extracellular matrix-like materials. The cells are cultured on ELM scaffolds and stimulated with appropriate biochemical factors to promote mineral deposition. This cell-mediated process results in physiologically relevant mineralization patterns and compositions, creating biomaterials that better mimic the natural tissue microenvironment and support enhanced cellular integration and tissue regeneration.
- Growth factor incorporation in mineralized ELMs: Growth factors and bioactive molecules are incorporated into mineralized extracellular matrix-like materials to enhance their biological functionality. These factors can be bound to the ELM scaffold prior to mineralization or co-precipitated with the mineral phase. The controlled release of these bioactive molecules from the mineralized ELM promotes cell recruitment, proliferation, and differentiation, accelerating tissue regeneration and improving the integration of the implanted material with surrounding tissues.
- Composite mineralized ELMs with enhanced mechanical properties: Advanced composite approaches combine extracellular matrix-like materials with various reinforcing elements during the mineralization process. These reinforcements include nanofibers, nanotubes, ceramic particles, or secondary polymer networks that are integrated with the mineralizing ELM. The resulting composite materials exhibit significantly improved mechanical properties, including enhanced strength, toughness, and fatigue resistance, while maintaining biocompatibility and the ability to support cell attachment and tissue integration.
02 Polymer-based scaffolds for ELM mineralization
Polymer-based scaffolds serve as templates for the mineralization of extracellular matrix-like materials. These scaffolds can be composed of natural polymers like collagen, chitosan, or synthetic polymers that provide structural support for mineral deposition. The polymers are often modified with functional groups that promote nucleation and growth of mineral crystals, resulting in composite materials with enhanced mechanical properties and bioactivity for tissue engineering applications.Expand Specific Solutions03 Controlled mineral deposition techniques for ELMs
Various techniques are employed to control the deposition of minerals onto extracellular matrix-like materials. These include simulated body fluid immersion, electrochemical deposition, and enzyme-mediated mineralization. The controlled mineralization process allows for precise regulation of mineral phase, crystal size, orientation, and distribution, which are critical factors in determining the mechanical and biological properties of the resulting mineralized materials for biomedical applications.Expand Specific Solutions04 Growth factor incorporation in mineralized ELMs
Growth factors and bioactive molecules can be incorporated into mineralized extracellular matrix-like materials to enhance their biological functionality. These factors are either added during the mineralization process or bound to the mineralized matrix afterward. The controlled release of these bioactive molecules from the mineralized ELMs promotes cell adhesion, proliferation, and differentiation, accelerating tissue regeneration and improving the integration of implanted materials with host tissues.Expand Specific Solutions05 Novel composite materials combining ELMs with inorganic components
Innovative composite materials are developed by combining extracellular matrix-like materials with various inorganic components such as bioactive glasses, ceramic particles, or metal oxides. These hybrid materials exhibit synergistic properties including enhanced mechanical strength, improved bioactivity, and controlled degradation rates. The integration of organic and inorganic phases at the nanoscale creates biomimetic structures that better replicate the hierarchical organization of natural tissues for advanced regenerative medicine applications.Expand Specific Solutions
Leading Research Groups and Companies in Mineralized ELMs
The mineralized ELM (Extracellular Matrix) for bone tissue engineering market is in a growth phase, with increasing research activities and commercial applications emerging. The global bone tissue engineering market is projected to reach significant value due to rising orthopedic conditions and aging populations. Technologically, this field shows moderate maturity with key players at different development stages. Academic institutions like University of Michigan, Sichuan University, and Carnegie Mellon lead fundamental research, while commercial entities including Warsaw Orthopedic (Medtronic), Straumann Holding, and Allgens Medical Technology are advancing clinical applications. Chinese companies and universities are increasingly prominent, with Allgens and Maijie Life Sciences developing specialized mineralized collagen products. Western companies like 3D Biotek and InnoTERE focus on 3D scaffolds and innovative calcium phosphate materials, indicating a competitive landscape balancing academic innovation with commercial translation.
The Regents of the University of Michigan
Technical Solution: Michigan大学在矿化细胞外基质(ELMs)骨组织工程领域开发了一种创新的生物材料平台,结合了可控矿化过程与细胞外基质支架。其技术方案基于使用特定生物活性因子诱导细胞产生矿化细胞外基质,然后通过脱细胞处理创建保留天然矿物质和生物信号的支架。这些支架模拟骨组织的层级结构,含有纳米羟基磷灰石晶体与胶原蛋白纤维的复合结构。Michigan的研究人员还开发了可控释放系统,将生长因子(如BMP-2)整合到矿化ELM支架中,实现持续释放以促进骨再生。这种技术在体外实验中显示出优异的成骨细胞附着、增殖和分化能力,在动物模型中也展现出加速骨缺损修复的潜力。
优势:技术方案模拟天然骨形成过程,保留细胞外基质的生物活性成分和三维结构,具有优异的生物相容性和骨诱导能力。支架的机械性能可调,适应不同负重需求。缺点:生产过程复杂,可能面临规模化挑战;细胞来源和质量控制问题可能影响最终产品的一致性。
Univ of Pittsburgh
Technical Solution: 匹兹堡大学在矿化细胞外基质(ELMs)骨组织工程领域开发了一种独特的"细胞指导矿化"技术平台。该方法利用间充质干细胞(MSCs)在特定生化环境下培养,诱导它们分泌并矿化细胞外基质。研究团队采用创新的生物反应器系统,通过施加机械应力和流体剪切力,模拟骨组织的生理微环境,促进更均匀和生理相关的矿化过程。他们还开发了一种专利的脱细胞技术,可在保留矿化基质完整性的同时去除细胞成分,显著降低免疫原性。匹兹堡大学的技术特别关注矿化ELM的微观结构控制,通过调节钙磷比例、晶体大小和排列方式,优化支架的机械性能和生物活性。他们的研究表明,这种矿化ELM支架能够有效促进骨髓干细胞向成骨细胞分化,并在体内实验中展示出优异的骨整合能力和血管化潜力。
优势:技术方案能精确控制矿化过程和矿物质分布,产生具有优化机械性能的支架;支架保留天然生长因子和细胞信号分子,具有卓越的骨诱导能力;脱细胞处理降低了免疫排斥风险。劣势:生产周期较长,成本较高;技术复杂度高,对设备和操作人员要求严格;临床转化还需更多长期安全性数据支持。
Key Patents and Publications on Mineralized ELMs
Method for preparation of gradient mineralized extracellular matrix material of bone
PatentActiveJP2021523749A
Innovation
- A method involving immunogenicity removal and precision gradient demineralization at low temperatures using ultrasonic treatment to prepare a natural tissue-derived gradient mineralized bone extracellular matrix material, maintaining bioactive components and biomechanical properties while promoting regeneration and angiogenesis.
Integral biomaterial for regeneration of bone tissue and fabrication method therefor
PatentWO2018235984A1
Innovation
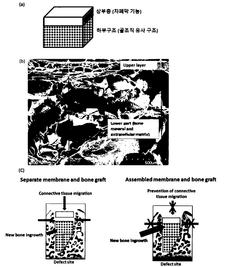
- An integrated biomaterial is developed comprising a lower structure of extracellular matrix proteins and bone minerals, combined with an upper layer of extracellular matrix proteins through physical bonding, eliminating the need for chemical cross-linking agents and ensuring stable adhesion, thereby preventing connective tissue invasion and maximizing bone regeneration.
Regulatory Framework for Bone Tissue Engineering Materials
The regulatory landscape for bone tissue engineering materials, particularly mineralized extracellular matrix-like materials (ELMs), presents a complex framework that developers must navigate to bring innovations from laboratory to clinical application. These regulations vary significantly across global jurisdictions, with the FDA in the United States, the EMA in Europe, and the NMPA in China each maintaining distinct classification systems and approval pathways.
In the United States, mineralized ELMs for bone tissue engineering typically fall under the FDA's combination product category, requiring review by both the Center for Biologics Evaluation and Research (CBER) and the Center for Devices and Radiological Health (CDRH). The regulatory pathway depends on the primary mode of action, with most mineralized scaffolds classified as Class III medical devices requiring Premarket Approval (PMA) due to their implantation in the body and potential risks.
European regulations under the Medical Device Regulation (MDR) and the Advanced Therapy Medicinal Products (ATMP) framework classify mineralized ELMs based on their composition and intended function. Products incorporating viable cells typically face more stringent requirements as ATMPs, while acellular mineralized matrices may be regulated as Class III medical devices under Rule 8 of the MDR classification system.
Quality control standards for mineralized ELMs present unique challenges due to their composite nature. ISO 13485 for quality management systems and ISO 10993 for biocompatibility testing provide baseline requirements, but specialized standards for characterizing mineral components, degradation profiles, and mechanical properties are still evolving. The International Society for Biofabrication has recently proposed standardized testing protocols specifically for mineralized tissue engineering constructs.
Post-market surveillance requirements have become increasingly stringent, with regulatory bodies demanding long-term follow-up studies to monitor potential complications such as ectopic mineralization, inflammatory responses, and integration failures. The FDA's Unique Device Identification (UDI) system and the European Database on Medical Devices (EUDAMED) enhance traceability throughout the product lifecycle.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to standardize requirements across jurisdictions, potentially streamlining the approval process for innovative bone tissue engineering products. However, significant regulatory divergence remains, creating challenges for global commercialization strategies.
Regulatory science in this field continues to evolve, with increasing focus on developing appropriate reference materials, standardized testing methodologies, and predictive models that can better assess the long-term performance and safety of mineralized ELMs in bone tissue engineering applications.
In the United States, mineralized ELMs for bone tissue engineering typically fall under the FDA's combination product category, requiring review by both the Center for Biologics Evaluation and Research (CBER) and the Center for Devices and Radiological Health (CDRH). The regulatory pathway depends on the primary mode of action, with most mineralized scaffolds classified as Class III medical devices requiring Premarket Approval (PMA) due to their implantation in the body and potential risks.
European regulations under the Medical Device Regulation (MDR) and the Advanced Therapy Medicinal Products (ATMP) framework classify mineralized ELMs based on their composition and intended function. Products incorporating viable cells typically face more stringent requirements as ATMPs, while acellular mineralized matrices may be regulated as Class III medical devices under Rule 8 of the MDR classification system.
Quality control standards for mineralized ELMs present unique challenges due to their composite nature. ISO 13485 for quality management systems and ISO 10993 for biocompatibility testing provide baseline requirements, but specialized standards for characterizing mineral components, degradation profiles, and mechanical properties are still evolving. The International Society for Biofabrication has recently proposed standardized testing protocols specifically for mineralized tissue engineering constructs.
Post-market surveillance requirements have become increasingly stringent, with regulatory bodies demanding long-term follow-up studies to monitor potential complications such as ectopic mineralization, inflammatory responses, and integration failures. The FDA's Unique Device Identification (UDI) system and the European Database on Medical Devices (EUDAMED) enhance traceability throughout the product lifecycle.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to standardize requirements across jurisdictions, potentially streamlining the approval process for innovative bone tissue engineering products. However, significant regulatory divergence remains, creating challenges for global commercialization strategies.
Regulatory science in this field continues to evolve, with increasing focus on developing appropriate reference materials, standardized testing methodologies, and predictive models that can better assess the long-term performance and safety of mineralized ELMs in bone tissue engineering applications.
Biocompatibility and Clinical Translation Considerations
The biocompatibility of mineralized elastin-like materials (ELMs) represents a critical factor in their successful application for bone tissue engineering. These biomaterials must demonstrate minimal immunogenicity and toxicity while supporting cellular adhesion, proliferation, and differentiation. Current research indicates that mineralized ELMs generally exhibit favorable biocompatibility profiles due to their protein-based composition and structural similarity to natural extracellular matrix components.
In vitro studies have demonstrated that osteoblasts and mesenchymal stem cells readily adhere to and proliferate on mineralized ELM scaffolds. The calcium phosphate mineral phase incorporated within these materials appears to enhance osteogenic differentiation through both topographical cues and calcium ion signaling pathways. However, the degradation kinetics of these materials must be carefully controlled to ensure that breakdown products do not elicit inflammatory responses or cytotoxicity.
Immune compatibility assessments have shown minimal foreign body reactions to mineralized ELMs in preclinical models. The recombinant nature of these proteins allows for precise control over their amino acid sequence, enabling the removal of potentially immunogenic epitopes. Additionally, the incorporation of specific bioactive sequences, such as RGD peptides, can further improve cell-material interactions and tissue integration.
Translating mineralized ELMs from laboratory research to clinical applications presents several significant challenges. Manufacturing scalability remains a primary concern, as the production of recombinant elastin-like proteins typically yields relatively low quantities at high costs. Recent advances in bioreactor technology and expression system optimization have improved yields, but further refinement is necessary for commercial viability.
Regulatory considerations for mineralized ELMs are complex due to their hybrid nature as both protein-based biomaterials and mineral-containing scaffolds. Classification under existing regulatory frameworks may be challenging, potentially requiring specialized approval pathways. Comprehensive safety and efficacy data from preclinical studies are essential for regulatory submissions, with particular emphasis on long-term stability, degradation profiles, and potential systemic effects.
Sterilization represents another critical challenge for clinical translation. Traditional methods such as autoclaving or gamma irradiation may compromise the structural integrity and bioactivity of the protein component. Alternative approaches like ethylene oxide treatment or supercritical CO2 sterilization show promise but require validation for these specific materials.
Cost-effectiveness analyses suggest that while initial production costs for mineralized ELMs are high, their potential to reduce revision surgeries and improve patient outcomes may justify their implementation in specific clinical scenarios. Strategic partnerships between academic institutions and industry stakeholders will be essential to overcome these translational barriers and bring these promising materials to clinical practice.
In vitro studies have demonstrated that osteoblasts and mesenchymal stem cells readily adhere to and proliferate on mineralized ELM scaffolds. The calcium phosphate mineral phase incorporated within these materials appears to enhance osteogenic differentiation through both topographical cues and calcium ion signaling pathways. However, the degradation kinetics of these materials must be carefully controlled to ensure that breakdown products do not elicit inflammatory responses or cytotoxicity.
Immune compatibility assessments have shown minimal foreign body reactions to mineralized ELMs in preclinical models. The recombinant nature of these proteins allows for precise control over their amino acid sequence, enabling the removal of potentially immunogenic epitopes. Additionally, the incorporation of specific bioactive sequences, such as RGD peptides, can further improve cell-material interactions and tissue integration.
Translating mineralized ELMs from laboratory research to clinical applications presents several significant challenges. Manufacturing scalability remains a primary concern, as the production of recombinant elastin-like proteins typically yields relatively low quantities at high costs. Recent advances in bioreactor technology and expression system optimization have improved yields, but further refinement is necessary for commercial viability.
Regulatory considerations for mineralized ELMs are complex due to their hybrid nature as both protein-based biomaterials and mineral-containing scaffolds. Classification under existing regulatory frameworks may be challenging, potentially requiring specialized approval pathways. Comprehensive safety and efficacy data from preclinical studies are essential for regulatory submissions, with particular emphasis on long-term stability, degradation profiles, and potential systemic effects.
Sterilization represents another critical challenge for clinical translation. Traditional methods such as autoclaving or gamma irradiation may compromise the structural integrity and bioactivity of the protein component. Alternative approaches like ethylene oxide treatment or supercritical CO2 sterilization show promise but require validation for these specific materials.
Cost-effectiveness analyses suggest that while initial production costs for mineralized ELMs are high, their potential to reduce revision surgeries and improve patient outcomes may justify their implementation in specific clinical scenarios. Strategic partnerships between academic institutions and industry stakeholders will be essential to overcome these translational barriers and bring these promising materials to clinical practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!