The Role Of Extracellular Matrix In Functional ELMs.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ECM in ELMs: Background and Objectives
Extracellular Matrix (ECM) has emerged as a critical component in the development and functionality of Engineered Living Materials (ELMs), representing a significant intersection between materials science and synthetic biology. The evolution of ECM research in biological systems dates back to the early 20th century, with substantial advancements occurring in the past three decades as analytical techniques have improved. This technological progression has enabled researchers to better understand the complex composition and dynamic nature of ECM in various tissues and organisms.
The integration of ECM principles into ELM design represents a paradigm shift from traditional material engineering approaches. Initially, ELMs were primarily focused on cellular components and their genetic modifications, with limited attention to the surrounding matrix. However, as the field has matured, researchers have recognized that ECM provides not only structural support but also crucial biochemical and biomechanical cues that influence cell behavior, differentiation, and overall material functionality.
Current technological trends indicate a growing emphasis on biomimetic approaches that replicate natural ECM properties in synthetic systems. This includes the development of composite materials that incorporate both living cells and ECM-like components, as well as the genetic engineering of cells to produce specific ECM proteins or structures. The convergence of these approaches aims to create more sophisticated ELMs with enhanced functionality, stability, and responsiveness to environmental stimuli.
The primary technical objectives in this field include: (1) elucidating the mechanisms by which ECM components influence cellular behavior within ELMs; (2) developing methods to precisely control ECM composition and architecture in engineered systems; (3) creating responsive ECM-like materials that can adapt to changing environmental conditions; and (4) establishing standardized protocols for characterizing ECM-cell interactions in complex ELM systems.
Additionally, researchers aim to bridge the gap between laboratory demonstrations and practical applications by addressing scalability challenges and regulatory considerations. This includes developing manufacturing processes that maintain ECM integrity during scale-up and establishing safety profiles for ECM-containing living materials intended for medical, environmental, or industrial applications.
The interdisciplinary nature of this field necessitates collaboration between experts in materials science, synthetic biology, tissue engineering, and computational modeling. Recent advances in high-throughput screening technologies and machine learning approaches are accelerating the discovery of optimal ECM compositions for specific ELM applications, potentially reducing development timelines and enhancing material performance.
As the field continues to evolve, understanding the fundamental role of ECM in functional ELMs will be essential for realizing the full potential of these innovative materials across diverse sectors including healthcare, environmental remediation, and sustainable manufacturing.
The integration of ECM principles into ELM design represents a paradigm shift from traditional material engineering approaches. Initially, ELMs were primarily focused on cellular components and their genetic modifications, with limited attention to the surrounding matrix. However, as the field has matured, researchers have recognized that ECM provides not only structural support but also crucial biochemical and biomechanical cues that influence cell behavior, differentiation, and overall material functionality.
Current technological trends indicate a growing emphasis on biomimetic approaches that replicate natural ECM properties in synthetic systems. This includes the development of composite materials that incorporate both living cells and ECM-like components, as well as the genetic engineering of cells to produce specific ECM proteins or structures. The convergence of these approaches aims to create more sophisticated ELMs with enhanced functionality, stability, and responsiveness to environmental stimuli.
The primary technical objectives in this field include: (1) elucidating the mechanisms by which ECM components influence cellular behavior within ELMs; (2) developing methods to precisely control ECM composition and architecture in engineered systems; (3) creating responsive ECM-like materials that can adapt to changing environmental conditions; and (4) establishing standardized protocols for characterizing ECM-cell interactions in complex ELM systems.
Additionally, researchers aim to bridge the gap between laboratory demonstrations and practical applications by addressing scalability challenges and regulatory considerations. This includes developing manufacturing processes that maintain ECM integrity during scale-up and establishing safety profiles for ECM-containing living materials intended for medical, environmental, or industrial applications.
The interdisciplinary nature of this field necessitates collaboration between experts in materials science, synthetic biology, tissue engineering, and computational modeling. Recent advances in high-throughput screening technologies and machine learning approaches are accelerating the discovery of optimal ECM compositions for specific ELM applications, potentially reducing development timelines and enhancing material performance.
As the field continues to evolve, understanding the fundamental role of ECM in functional ELMs will be essential for realizing the full potential of these innovative materials across diverse sectors including healthcare, environmental remediation, and sustainable manufacturing.
Market Analysis for ECM-Enhanced ELMs
The global market for Engineered Living Materials (ELMs) enhanced with Extracellular Matrix (ECM) components is experiencing significant growth, driven by increasing applications in regenerative medicine, tissue engineering, and sustainable materials development. Current market estimates value the broader biomaterials sector at approximately $107 billion as of 2022, with ECM-specific applications representing a rapidly expanding segment projected to grow at a compound annual growth rate of 12.8% through 2030.
Healthcare applications dominate the current market landscape, with wound healing, tissue regeneration, and implantable medical devices representing the primary commercial opportunities. The integration of ECM components into ELMs has created particularly strong demand in orthopedics, where these materials offer superior biocompatibility and tissue integration compared to traditional synthetic alternatives.
Pharmaceutical and biotechnology companies are increasingly investing in ECM-enhanced ELMs for drug discovery and development platforms. These living materials provide more physiologically relevant testing environments than traditional cell culture systems, potentially reducing drug development timelines and costs while improving predictive accuracy for human responses.
Beyond healthcare, emerging applications in sustainable manufacturing and environmental remediation are opening new market segments. ECM-enhanced ELMs designed for biodegradable packaging materials, environmental sensors, and bioremediation systems are attracting significant venture capital investment, with funding increasing by 45% in the past two years alone.
Regional analysis reveals North America currently leads the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 23%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade due to increasing healthcare expenditure, expanding biotechnology sectors in China and South Korea, and supportive government initiatives.
Key market challenges include high production costs, regulatory uncertainties, and scalability issues. The complex nature of ECM components and their integration into living materials systems presents manufacturing challenges that currently limit widespread commercial adoption outside specialized applications.
Consumer and stakeholder acceptance represents another market consideration, particularly for applications involving genetically modified organisms. Public perception studies indicate growing acceptance of these technologies when benefits are clearly communicated, though regulatory frameworks continue to evolve at different rates globally.
Market forecasts suggest ECM-enhanced ELMs will experience accelerated commercialization between 2025-2030 as manufacturing processes mature and regulatory pathways become better established, potentially reaching mainstream adoption in medical applications first, followed by consumer and industrial applications.
Healthcare applications dominate the current market landscape, with wound healing, tissue regeneration, and implantable medical devices representing the primary commercial opportunities. The integration of ECM components into ELMs has created particularly strong demand in orthopedics, where these materials offer superior biocompatibility and tissue integration compared to traditional synthetic alternatives.
Pharmaceutical and biotechnology companies are increasingly investing in ECM-enhanced ELMs for drug discovery and development platforms. These living materials provide more physiologically relevant testing environments than traditional cell culture systems, potentially reducing drug development timelines and costs while improving predictive accuracy for human responses.
Beyond healthcare, emerging applications in sustainable manufacturing and environmental remediation are opening new market segments. ECM-enhanced ELMs designed for biodegradable packaging materials, environmental sensors, and bioremediation systems are attracting significant venture capital investment, with funding increasing by 45% in the past two years alone.
Regional analysis reveals North America currently leads the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 23%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade due to increasing healthcare expenditure, expanding biotechnology sectors in China and South Korea, and supportive government initiatives.
Key market challenges include high production costs, regulatory uncertainties, and scalability issues. The complex nature of ECM components and their integration into living materials systems presents manufacturing challenges that currently limit widespread commercial adoption outside specialized applications.
Consumer and stakeholder acceptance represents another market consideration, particularly for applications involving genetically modified organisms. Public perception studies indicate growing acceptance of these technologies when benefits are clearly communicated, though regulatory frameworks continue to evolve at different rates globally.
Market forecasts suggest ECM-enhanced ELMs will experience accelerated commercialization between 2025-2030 as manufacturing processes mature and regulatory pathways become better established, potentially reaching mainstream adoption in medical applications first, followed by consumer and industrial applications.
Current ECM Technology Status and Challenges
The field of Engineered Living Materials (ELMs) has witnessed significant advancements globally, yet the integration and optimization of Extracellular Matrix (ECM) components remain challenging. Current ECM technologies in functional ELMs face several limitations despite their promising potential. Research institutions across North America, Europe, and Asia have made substantial progress, but a comprehensive solution remains elusive.
The primary challenge lies in achieving precise control over ECM composition and architecture within ELMs. While traditional tissue engineering approaches have established methods for ECM manipulation, translating these techniques to living, self-sustaining materials presents unique difficulties. Current technologies struggle to maintain consistent ECM production and organization over extended periods, limiting the functional longevity of ELMs.
Biocompatibility issues represent another significant hurdle. The immune response to engineered ECM components can compromise material functionality and integration with host tissues. Recent studies from MIT and Stanford have demonstrated improved biocompatibility through modified ECM proteins, but complete immune tolerance remains difficult to achieve, particularly in complex in vivo environments.
Scalability presents a third major challenge. Laboratory-scale production of ECM-rich ELMs has shown promising results, but industrial-scale manufacturing faces substantial technical barriers. Current bioreactor technologies cannot consistently maintain the microenvironmental conditions necessary for uniform ECM deposition across large material volumes.
Geographically, ECM technology development shows distinct patterns. North American institutions lead in fundamental research and patent applications, with particular strength in genetically engineered ECM components. European research centers excel in biomimetic approaches and sustainable production methods. Asian institutions, particularly in Japan and Singapore, have made notable advances in ECM-based sensing applications and miniaturization technologies.
The regulatory landscape adds another layer of complexity. Current regulatory frameworks were not designed with living materials in mind, creating uncertainty for commercial development. This has resulted in a fragmented approach to ECM technology development, with different regions pursuing divergent regulatory strategies.
Material stability represents a persistent technical challenge. ECM components in ELMs are subject to degradation and remodeling processes that can be difficult to predict and control. Current stabilization technologies, including chemical crosslinking and engineered degradation resistance, often compromise the living components of ELMs or restrict their adaptive capabilities.
Despite these challenges, recent breakthroughs in synthetic biology and materials science offer promising directions. CRISPR-based gene editing has enabled more precise control over ECM production, while advanced imaging techniques have improved our understanding of ECM organization and dynamics within living materials.
The primary challenge lies in achieving precise control over ECM composition and architecture within ELMs. While traditional tissue engineering approaches have established methods for ECM manipulation, translating these techniques to living, self-sustaining materials presents unique difficulties. Current technologies struggle to maintain consistent ECM production and organization over extended periods, limiting the functional longevity of ELMs.
Biocompatibility issues represent another significant hurdle. The immune response to engineered ECM components can compromise material functionality and integration with host tissues. Recent studies from MIT and Stanford have demonstrated improved biocompatibility through modified ECM proteins, but complete immune tolerance remains difficult to achieve, particularly in complex in vivo environments.
Scalability presents a third major challenge. Laboratory-scale production of ECM-rich ELMs has shown promising results, but industrial-scale manufacturing faces substantial technical barriers. Current bioreactor technologies cannot consistently maintain the microenvironmental conditions necessary for uniform ECM deposition across large material volumes.
Geographically, ECM technology development shows distinct patterns. North American institutions lead in fundamental research and patent applications, with particular strength in genetically engineered ECM components. European research centers excel in biomimetic approaches and sustainable production methods. Asian institutions, particularly in Japan and Singapore, have made notable advances in ECM-based sensing applications and miniaturization technologies.
The regulatory landscape adds another layer of complexity. Current regulatory frameworks were not designed with living materials in mind, creating uncertainty for commercial development. This has resulted in a fragmented approach to ECM technology development, with different regions pursuing divergent regulatory strategies.
Material stability represents a persistent technical challenge. ECM components in ELMs are subject to degradation and remodeling processes that can be difficult to predict and control. Current stabilization technologies, including chemical crosslinking and engineered degradation resistance, often compromise the living components of ELMs or restrict their adaptive capabilities.
Despite these challenges, recent breakthroughs in synthetic biology and materials science offer promising directions. CRISPR-based gene editing has enabled more precise control over ECM production, while advanced imaging techniques have improved our understanding of ECM organization and dynamics within living materials.
Current ECM-ELM Integration Approaches
01 ECM components in engineered living materials (ELMs)
Extracellular matrix components play a crucial role in engineered living materials by providing structural support and biological functionality. These components, including collagens, fibronectins, and proteoglycans, can be incorporated into ELMs to mimic natural tissue environments. The integration of ECM components enhances cell adhesion, proliferation, and differentiation within the engineered constructs, leading to improved functionality and biocompatibility of the resulting materials.- ECM components in engineered living materials (ELMs): Extracellular matrix components play a crucial role in engineered living materials by providing structural support and biological functionality. These components, including collagen, fibronectin, and laminin, can be incorporated into ELMs to mimic natural tissue environments. The integration of ECM components enhances cell adhesion, proliferation, and differentiation within the engineered constructs, leading to improved functionality and biocompatibility of the resulting materials.
- ECM-based scaffolds for functional ELMs: ECM-based scaffolds serve as templates for cell growth and organization in functional engineered living materials. These scaffolds can be derived from decellularized tissues or synthesized using biomimetic approaches. The three-dimensional architecture of ECM scaffolds provides mechanical support while facilitating nutrient diffusion and waste removal. By controlling the composition and structure of these scaffolds, researchers can guide cellular behavior and enhance the overall functionality of ELMs for various applications.
- Cell-ECM interactions in ELM functionality: The interactions between cells and the extracellular matrix significantly influence the functionality of engineered living materials. Cells respond to ECM cues through mechanotransduction pathways, which affect their behavior, including migration, proliferation, and differentiation. By engineering specific cell-ECM interactions, researchers can control cellular responses and enhance the performance of ELMs. These interactions can be modulated through surface modifications, incorporation of bioactive molecules, or alteration of matrix stiffness to achieve desired functional outcomes.
- Dynamic ECM remodeling in living materials: Dynamic remodeling of the extracellular matrix is essential for maintaining the functionality of engineered living materials over time. Cells within ELMs continuously modify their surrounding matrix through the secretion of enzymes like matrix metalloproteinases and deposition of new ECM components. This remodeling process allows ELMs to adapt to changing environmental conditions and maintain their structural integrity. By understanding and controlling ECM remodeling, researchers can develop ELMs with enhanced durability, self-healing capabilities, and responsive properties.
- ECM-inspired synthetic matrices for ELMs: Synthetic matrices inspired by natural extracellular matrix components offer precise control over the properties of engineered living materials. These matrices can be designed with specific mechanical properties, degradation rates, and bioactive functionalities to support cell growth and tissue formation. By incorporating ECM-mimetic peptides, growth factors, or other bioactive molecules, synthetic matrices can provide instructive cues to guide cellular behavior. These biomimetic approaches enable the development of functional ELMs with tailored properties for specific applications in tissue engineering, drug delivery, and biosensing.
02 ECM-based scaffolds for functional ELMs
ECM-based scaffolds serve as templates for cell growth and tissue development in functional ELMs. These scaffolds can be derived from decellularized tissues or synthesized using biomimetic approaches. The three-dimensional architecture of ECM scaffolds provides mechanical support while facilitating nutrient diffusion and waste removal. By incorporating specific ECM proteins and growth factors, these scaffolds can be tailored to support various cell types and functionalities, enhancing the overall performance of engineered living materials.Expand Specific Solutions03 Cell-ECM interactions in ELM functionality
The interactions between cells and the extracellular matrix significantly influence the functionality of engineered living materials. Cells respond to ECM cues through mechanotransduction pathways, which affect their behavior, including migration, proliferation, and differentiation. By engineering specific cell-ECM interactions, researchers can control cellular responses and direct tissue formation within ELMs. These interactions can be modulated through surface modifications, incorporation of adhesion molecules, and adjustment of matrix stiffness to achieve desired functional outcomes.Expand Specific Solutions04 Dynamic ECM remodeling in responsive ELMs
Dynamic remodeling of the extracellular matrix enables responsive functionality in engineered living materials. Cells within ELMs continuously modify their surrounding matrix through secretion of enzymes like matrix metalloproteinases and deposition of new ECM components. This remodeling process allows ELMs to adapt to changing environmental conditions and functional demands. By controlling the balance between ECM degradation and synthesis, researchers can develop self-healing materials and tissues with the ability to maintain homeostasis over extended periods.Expand Specific Solutions05 ECM-derived bioactive factors for enhanced ELM performance
Bioactive factors derived from the extracellular matrix can be incorporated into engineered living materials to enhance their performance. These factors include growth factors, cytokines, and small peptides that regulate cellular processes such as proliferation, differentiation, and matrix production. By strategically incorporating these bioactive molecules, researchers can guide tissue development and improve the functional properties of ELMs. Controlled release systems can be designed to deliver these factors in a spatiotemporally regulated manner, optimizing their effects on cellular behavior and material functionality.Expand Specific Solutions
Key Players in ECM and ELM Research
The extracellular matrix (ECM) in functional engineered liver microsystems (ELMs) represents an emerging field at the intersection of tissue engineering and regenerative medicine. The market is currently in an early growth phase, with an estimated global value of $2-3 billion and projected annual growth of 15-20%. Key players demonstrate varying levels of technical maturity: established companies like Corning and Boston Scientific bring advanced materials expertise; Emulate and T&R Biofab lead in organ-on-chip technologies; while research institutions including SINANO, Harvard, and Johns Hopkins drive fundamental innovations. The competitive landscape features strategic collaborations between commercial entities (Nordic Bioscience, Ethicon) and academic centers, creating an ecosystem where intellectual property and translational capabilities determine market positioning. Technical challenges in ECM composition and functionality remain significant barriers to widespread clinical adoption.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed advanced biomaterial platforms for studying ECM roles in functional ELMs. Their approach utilizes decellularized ECM combined with synthetic hydrogels to create biomimetic microenvironments with tunable mechanical and biochemical properties. The Wyss Institute's technology incorporates 3D bioprinting of precise ECM protein patterns to guide cell organization and function in engineered tissues. Their research has demonstrated that specific ECM compositions can direct stem cell differentiation pathways and maintain tissue-specific functions in ELMs. Harvard's platforms enable systematic investigation of ECM-mediated mechanotransduction through controlled presentation of adhesion ligands and matrix stiffness gradients[2][5]. Recent innovations include photopatternable ECM hydrogels that allow dynamic remodeling studies and ECM-integrated microfluidic systems that recapitulate tissue-specific vascular interfaces with controlled permeability properties.
Strengths: Cutting-edge research combining synthetic and natural ECM components; sophisticated spatial patterning capabilities; strong interdisciplinary collaboration between engineering and biological sciences. Weaknesses: Many technologies remain in research phase rather than commercial development; complex fabrication techniques may limit widespread adoption; higher variability between experimental batches compared to fully synthetic systems.
Emulate, Inc.
Technical Solution: Emulate has pioneered Organ-on-Chip technology that incorporates extracellular matrix (ECM) components to create functional engineered living microsystems (ELMs). Their platform uses microfluidic devices with porous membranes coated with specific ECM proteins to recreate tissue-tissue interfaces. The company's Human Emulation System integrates mechanical forces and ECM compositions tailored to each organ type, allowing cells to maintain physiological functions in vitro. Their proprietary ECM formulations include organ-specific combinations of laminin, collagen, fibronectin, and proteoglycans that support cellular differentiation and tissue organization. Emulate's technology enables the study of ECM-dependent cellular behaviors in controlled microenvironments, with demonstrated applications in drug development, toxicity testing, and disease modeling[1][3]. Recent advancements include tunable ECM stiffness parameters to model fibrosis and other pathological conditions.
Strengths: Highly controlled microenvironment allowing precise manipulation of ECM components; commercial platform with standardized protocols; ability to incorporate mechanical forces alongside ECM interactions. Weaknesses: Limited to simplified ECM compositions compared to native tissues; challenges in scaling to higher-throughput applications; relatively high cost per experimental unit compared to traditional cell culture systems.
Critical ECM Components for ELM Functionality
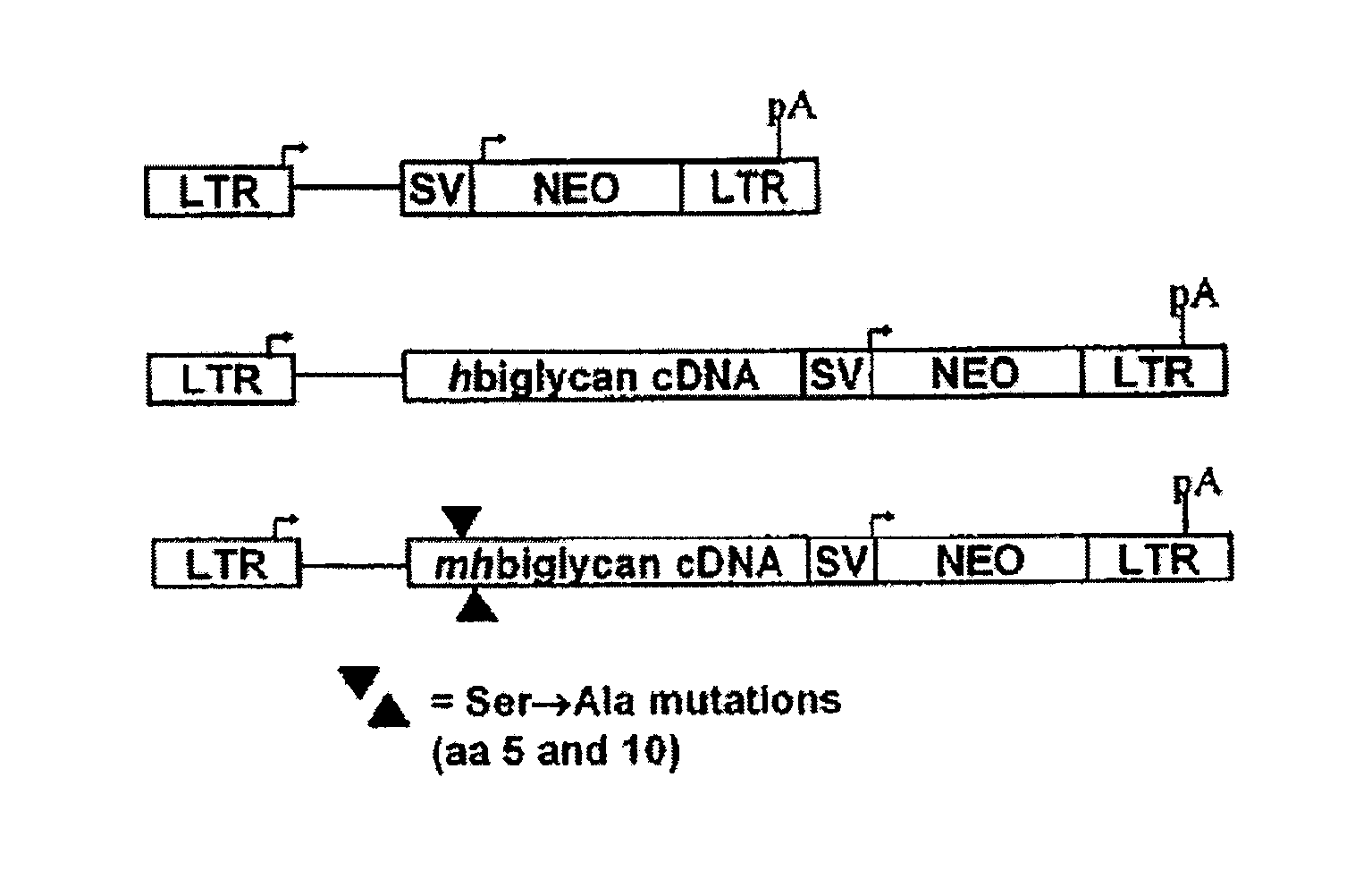
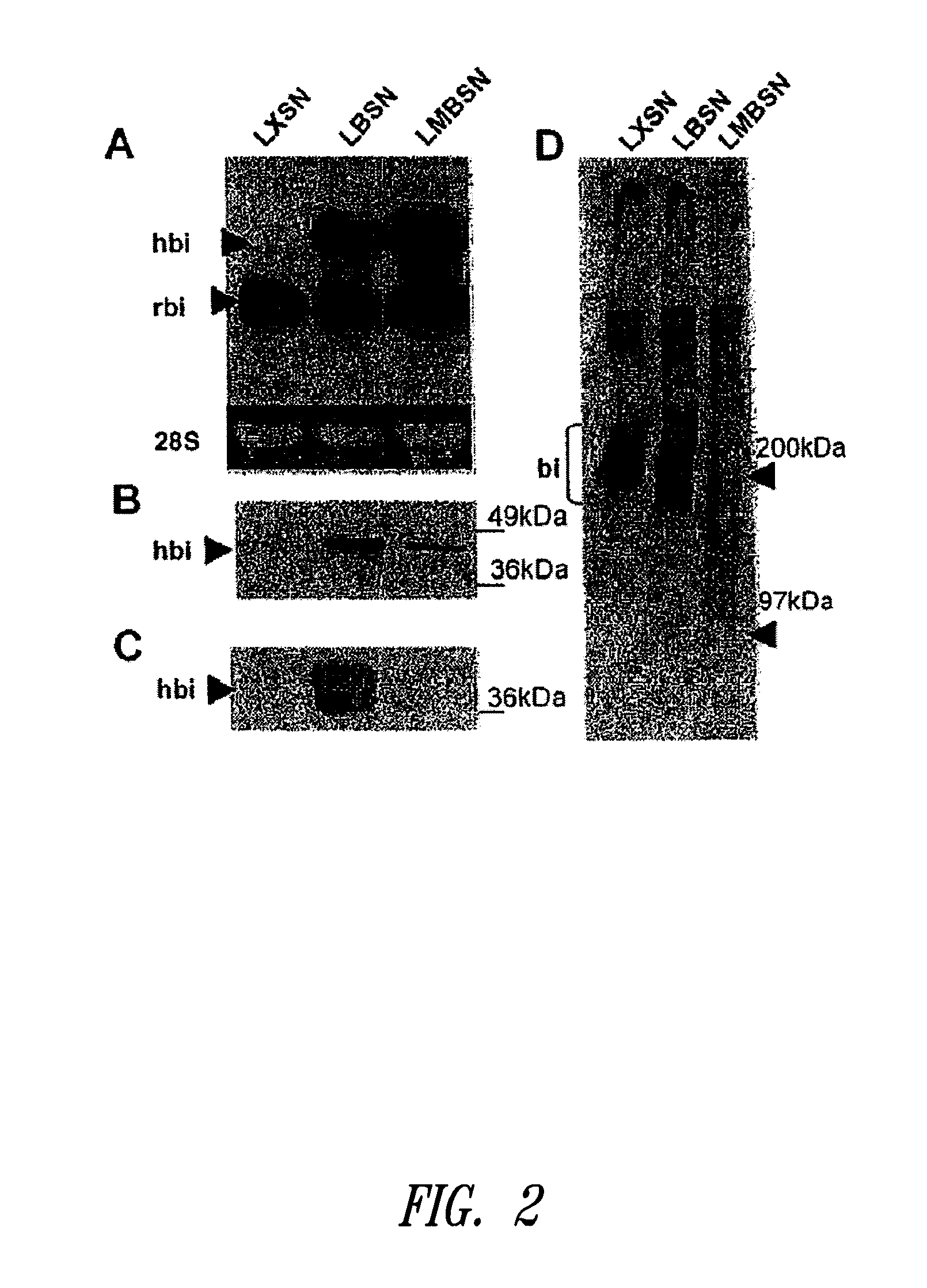
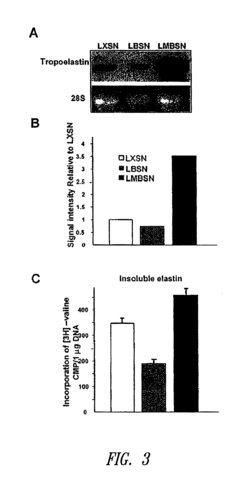
Compositions and methods for altering elastogenesis
PatentInactiveUS20100197563A1
Innovation
- The use of mutant biglycan polypeptides and engineered versican V3-derived polypeptides, along with metastatin proteoglycans, to interfere with anti-elastogenic mechanisms and promote elastogenesis by altering glycosaminoglycan attachment sites and binding interactions, thereby facilitating the assembly of elastic fibers.
Pharmaceutical composition for treating diabetes, comprising pancreatic islet cells and elastin-like artificial extracellular matrix
PatentActiveUS20180318357A1
Innovation
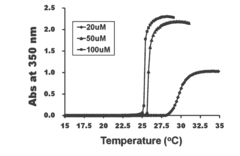
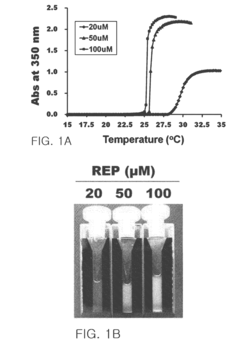
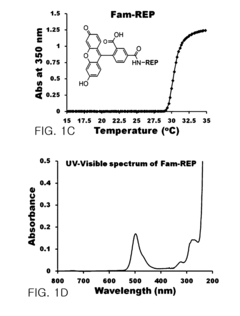
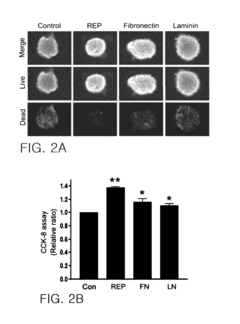
- A pharmaceutical composition comprising pancreatic islet cells reacted with an elastin-like artificial extracellular matrix (REP) prepared through repeated fusion of elastin-like polypeptides and ligands, such as VGVPG and RGD, which maintains cell viability and activity, thereby enhancing transplantation success rates.
Biocompatibility and Safety Considerations
The integration of extracellular matrix (ECM) components in engineered living materials (ELMs) necessitates rigorous assessment of biocompatibility and safety profiles. ECM-based ELMs interact directly with host tissues, making their safety evaluation paramount for clinical translation and commercial viability. Current biocompatibility testing frameworks for ECM in functional ELMs follow ISO 10993 standards, evaluating cytotoxicity, sensitization, irritation, and systemic toxicity through both in vitro and in vivo models.
Immunogenicity remains a critical concern when incorporating natural ECM components into ELMs. While decellularized ECM materials generally exhibit reduced immunogenicity compared to cellular tissues, residual DNA, cell debris, or processing agents can trigger adverse immune responses. Advanced decellularization protocols utilizing enzymatic treatments and detergent washes have significantly improved the safety profile of ECM-based materials, though batch-to-batch variability continues to present challenges for standardization.
Long-term safety considerations for ECM-based ELMs include degradation kinetics and metabolite toxicity. The degradation products of ECM components must be non-toxic and readily cleared from the body. Recent studies have demonstrated that controlled degradation rates can be engineered by modifying crosslinking density or incorporating specific enzymatic cleavage sites, allowing for predictable material behavior in vivo and reducing potential complications from premature degradation or persistent foreign material.
Regulatory pathways for ECM-incorporated ELMs remain complex due to their hybrid nature combining biological and engineered components. The FDA and EMA typically classify these materials as combination products, requiring comprehensive safety data packages addressing both the ECM components and the engineered living elements. Recent regulatory precedents with approved ECM-based medical devices provide valuable frameworks for navigating these requirements.
Emerging safety assessment technologies include advanced imaging techniques for tracking ECM degradation in vivo, metabolomic profiling of degradation products, and immunophenotyping approaches to characterize host responses. These methods provide more nuanced understanding of safety profiles beyond traditional biocompatibility testing. Additionally, the development of humanized animal models and organ-on-chip platforms offers more predictive preclinical safety assessment tools specifically relevant to ECM-ELM interactions.
Standardization efforts through international consortia are currently addressing the need for ECM-specific safety protocols in the context of living materials. These initiatives aim to establish reference materials, validated testing methodologies, and harmonized reporting standards to facilitate regulatory approval and ensure consistent safety evaluation across different ECM-based ELM technologies.
Immunogenicity remains a critical concern when incorporating natural ECM components into ELMs. While decellularized ECM materials generally exhibit reduced immunogenicity compared to cellular tissues, residual DNA, cell debris, or processing agents can trigger adverse immune responses. Advanced decellularization protocols utilizing enzymatic treatments and detergent washes have significantly improved the safety profile of ECM-based materials, though batch-to-batch variability continues to present challenges for standardization.
Long-term safety considerations for ECM-based ELMs include degradation kinetics and metabolite toxicity. The degradation products of ECM components must be non-toxic and readily cleared from the body. Recent studies have demonstrated that controlled degradation rates can be engineered by modifying crosslinking density or incorporating specific enzymatic cleavage sites, allowing for predictable material behavior in vivo and reducing potential complications from premature degradation or persistent foreign material.
Regulatory pathways for ECM-incorporated ELMs remain complex due to their hybrid nature combining biological and engineered components. The FDA and EMA typically classify these materials as combination products, requiring comprehensive safety data packages addressing both the ECM components and the engineered living elements. Recent regulatory precedents with approved ECM-based medical devices provide valuable frameworks for navigating these requirements.
Emerging safety assessment technologies include advanced imaging techniques for tracking ECM degradation in vivo, metabolomic profiling of degradation products, and immunophenotyping approaches to characterize host responses. These methods provide more nuanced understanding of safety profiles beyond traditional biocompatibility testing. Additionally, the development of humanized animal models and organ-on-chip platforms offers more predictive preclinical safety assessment tools specifically relevant to ECM-ELM interactions.
Standardization efforts through international consortia are currently addressing the need for ECM-specific safety protocols in the context of living materials. These initiatives aim to establish reference materials, validated testing methodologies, and harmonized reporting standards to facilitate regulatory approval and ensure consistent safety evaluation across different ECM-based ELM technologies.
Scalability and Manufacturing Challenges
The scaling of Engineered Living Materials (ELMs) incorporating extracellular matrix (ECM) components presents significant manufacturing challenges that must be addressed for commercial viability. Current laboratory-scale production methods often rely on specialized equipment and highly controlled environments that are difficult to translate to industrial settings. The complex interplay between living cells and ECM components requires precise control over environmental parameters such as temperature, pH, and nutrient availability, making consistent large-scale production particularly challenging.
Manufacturing ELMs with functional ECM components faces bottlenecks in standardization and reproducibility. The biological variability inherent in living systems creates inconsistencies in ECM production and composition, resulting in functional properties that may differ between batches. This variability becomes more pronounced at larger scales, where maintaining homogeneous conditions throughout bioreactors becomes increasingly difficult. The development of robust quality control metrics specifically for ECM characteristics in ELMs remains underdeveloped compared to traditional materials manufacturing.
Cost-effectiveness represents another major hurdle in scaling ECM-containing ELMs. The specialized growth media, purification processes, and sterile manufacturing environments required significantly increase production costs. Current estimates suggest that ECM-rich ELMs may cost 10-100 times more per unit area than conventional materials, limiting their market penetration to high-value applications. Reducing these costs will require innovations in bioprocessing technology and potentially the development of synthetic alternatives that mimic key ECM functions.
Regulatory frameworks present additional complexity for manufacturing scale-up. The hybrid nature of ELMs—combining living components with ECM materials—creates uncertainty regarding which regulatory pathways apply. This regulatory ambiguity increases development timelines and costs, particularly for applications in medical devices or food-contact materials. Companies must navigate different regulatory requirements across global markets, further complicating international manufacturing and distribution strategies.
Supply chain considerations also impact scalability, as consistent sources of cells and ECM components must be secured. The shelf-life limitations of living materials necessitate innovations in preservation technologies and distribution logistics. Some manufacturers are exploring lyophilization and encapsulation techniques to extend viability, though these approaches may alter the functional properties of the ECM components and require extensive validation.
Manufacturing ELMs with functional ECM components faces bottlenecks in standardization and reproducibility. The biological variability inherent in living systems creates inconsistencies in ECM production and composition, resulting in functional properties that may differ between batches. This variability becomes more pronounced at larger scales, where maintaining homogeneous conditions throughout bioreactors becomes increasingly difficult. The development of robust quality control metrics specifically for ECM characteristics in ELMs remains underdeveloped compared to traditional materials manufacturing.
Cost-effectiveness represents another major hurdle in scaling ECM-containing ELMs. The specialized growth media, purification processes, and sterile manufacturing environments required significantly increase production costs. Current estimates suggest that ECM-rich ELMs may cost 10-100 times more per unit area than conventional materials, limiting their market penetration to high-value applications. Reducing these costs will require innovations in bioprocessing technology and potentially the development of synthetic alternatives that mimic key ECM functions.
Regulatory frameworks present additional complexity for manufacturing scale-up. The hybrid nature of ELMs—combining living components with ECM materials—creates uncertainty regarding which regulatory pathways apply. This regulatory ambiguity increases development timelines and costs, particularly for applications in medical devices or food-contact materials. Companies must navigate different regulatory requirements across global markets, further complicating international manufacturing and distribution strategies.
Supply chain considerations also impact scalability, as consistent sources of cells and ECM components must be secured. The shelf-life limitations of living materials necessitate innovations in preservation technologies and distribution logistics. Some manufacturers are exploring lyophilization and encapsulation techniques to extend viability, though these approaches may alter the functional properties of the ECM components and require extensive validation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!