Encapsulation Techniques For Protecting Cells In Harsh Environments.
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell Encapsulation Background and Objectives
Cell encapsulation technology has evolved significantly over the past four decades since its conceptual introduction in the 1960s. The fundamental principle involves immobilizing biological cells within semi-permeable membranes that protect them from external stressors while allowing nutrient exchange and metabolic function. This technology emerged from the need to shield therapeutic cells from immune rejection in transplantation applications, but has since expanded to numerous fields including bioproduction, environmental remediation, and biosensing.
The evolution of cell encapsulation techniques has been driven by advances in biomaterials science, with early approaches utilizing alginate and other natural polymers giving way to sophisticated synthetic and hybrid materials engineered for specific applications. The trajectory of development has consistently aimed toward creating more biocompatible, mechanically stable, and functionally versatile encapsulation systems capable of withstanding increasingly challenging environments.
Current research focuses on developing encapsulation matrices that can protect cells against extreme pH variations, temperature fluctuations, toxic compounds, mechanical stress, and oxidative damage. These harsh conditions are particularly relevant in industrial biocatalysis, wastewater treatment, and in vivo therapeutic applications where cells must function in non-optimal environments.
The primary technical objectives of modern cell encapsulation research include: enhancing the mechanical stability of capsules to withstand physical stressors; improving selective permeability to allow nutrient diffusion while blocking immunoglobulins or toxins; extending cell viability periods within encapsulated environments; and developing stimuli-responsive encapsulation systems that can adapt to changing environmental conditions.
Beyond these technical goals, researchers aim to scale encapsulation processes for industrial applications while maintaining quality and reproducibility. This includes developing automated, high-throughput encapsulation methods that produce uniform capsules with consistent properties and predictable performance in harsh environments.
The interdisciplinary nature of cell encapsulation research has fostered collaboration between materials scientists, cell biologists, chemical engineers, and medical researchers. This convergence of expertise has accelerated innovation in the field, leading to breakthroughs in encapsulation techniques that combine principles from multiple disciplines to create more effective protection strategies.
As we look toward future developments, the field is increasingly focused on precision engineering of the microenvironment within capsules, creating customized niches that not only protect cells but optimize their functional output. This represents a shift from passive protection strategies to active modulation of cellular behavior through encapsulation design, opening new possibilities for applications in extreme environments previously considered incompatible with biological systems.
The evolution of cell encapsulation techniques has been driven by advances in biomaterials science, with early approaches utilizing alginate and other natural polymers giving way to sophisticated synthetic and hybrid materials engineered for specific applications. The trajectory of development has consistently aimed toward creating more biocompatible, mechanically stable, and functionally versatile encapsulation systems capable of withstanding increasingly challenging environments.
Current research focuses on developing encapsulation matrices that can protect cells against extreme pH variations, temperature fluctuations, toxic compounds, mechanical stress, and oxidative damage. These harsh conditions are particularly relevant in industrial biocatalysis, wastewater treatment, and in vivo therapeutic applications where cells must function in non-optimal environments.
The primary technical objectives of modern cell encapsulation research include: enhancing the mechanical stability of capsules to withstand physical stressors; improving selective permeability to allow nutrient diffusion while blocking immunoglobulins or toxins; extending cell viability periods within encapsulated environments; and developing stimuli-responsive encapsulation systems that can adapt to changing environmental conditions.
Beyond these technical goals, researchers aim to scale encapsulation processes for industrial applications while maintaining quality and reproducibility. This includes developing automated, high-throughput encapsulation methods that produce uniform capsules with consistent properties and predictable performance in harsh environments.
The interdisciplinary nature of cell encapsulation research has fostered collaboration between materials scientists, cell biologists, chemical engineers, and medical researchers. This convergence of expertise has accelerated innovation in the field, leading to breakthroughs in encapsulation techniques that combine principles from multiple disciplines to create more effective protection strategies.
As we look toward future developments, the field is increasingly focused on precision engineering of the microenvironment within capsules, creating customized niches that not only protect cells but optimize their functional output. This represents a shift from passive protection strategies to active modulation of cellular behavior through encapsulation design, opening new possibilities for applications in extreme environments previously considered incompatible with biological systems.
Market Analysis for Protected Cell Applications
The global market for cell encapsulation technologies is experiencing robust growth, driven primarily by increasing applications in biotechnology, pharmaceuticals, and regenerative medicine. Current market valuation stands at approximately 2.3 billion USD, with projections indicating a compound annual growth rate of 8.7% through 2028. This growth trajectory is supported by expanding research activities in cell therapy and tissue engineering, where protected cells serve as critical components in developing novel therapeutic approaches.
The pharmaceutical sector represents the largest application segment, accounting for nearly 40% of the total market share. Within this sector, encapsulated cell systems are increasingly utilized for controlled drug delivery, particularly for chronic conditions requiring sustained release mechanisms. The ability to protect therapeutic cells from immune rejection while allowing nutrient exchange has positioned this technology as a promising alternative to conventional drug administration methods.
Biotechnology applications follow closely, with significant investments directed toward developing encapsulated cell bioreactors for protein production and enzyme immobilization. These systems offer advantages in terms of reusability, stability in varying pH conditions, and protection against proteolytic degradation, making them economically attractive for industrial bioprocessing operations.
Geographically, North America dominates the market with approximately 45% share, attributed to advanced healthcare infrastructure, substantial R&D investments, and favorable regulatory frameworks. Europe follows with 30% market share, while Asia-Pacific represents the fastest-growing region with increasing investments in biotechnology and healthcare sectors, particularly in China, Japan, and South Korea.
Consumer demand patterns reveal growing interest in personalized medicine applications, where encapsulated cells can be tailored to individual patient needs. This trend is particularly evident in diabetes management, where encapsulated islet cells are being developed as potential alternatives to insulin therapy, offering improved quality of life for patients.
Market challenges include high development costs, complex regulatory pathways, and technical limitations related to long-term cell viability within encapsulation matrices. These factors have contributed to relatively high product costs, limiting widespread adoption in cost-sensitive markets and healthcare systems.
Emerging opportunities exist in environmental applications, where protected microbial cells are being developed for bioremediation of harsh industrial environments, wastewater treatment, and soil decontamination. This segment, though currently small, shows promising growth potential as environmental regulations become increasingly stringent globally.
The pharmaceutical sector represents the largest application segment, accounting for nearly 40% of the total market share. Within this sector, encapsulated cell systems are increasingly utilized for controlled drug delivery, particularly for chronic conditions requiring sustained release mechanisms. The ability to protect therapeutic cells from immune rejection while allowing nutrient exchange has positioned this technology as a promising alternative to conventional drug administration methods.
Biotechnology applications follow closely, with significant investments directed toward developing encapsulated cell bioreactors for protein production and enzyme immobilization. These systems offer advantages in terms of reusability, stability in varying pH conditions, and protection against proteolytic degradation, making them economically attractive for industrial bioprocessing operations.
Geographically, North America dominates the market with approximately 45% share, attributed to advanced healthcare infrastructure, substantial R&D investments, and favorable regulatory frameworks. Europe follows with 30% market share, while Asia-Pacific represents the fastest-growing region with increasing investments in biotechnology and healthcare sectors, particularly in China, Japan, and South Korea.
Consumer demand patterns reveal growing interest in personalized medicine applications, where encapsulated cells can be tailored to individual patient needs. This trend is particularly evident in diabetes management, where encapsulated islet cells are being developed as potential alternatives to insulin therapy, offering improved quality of life for patients.
Market challenges include high development costs, complex regulatory pathways, and technical limitations related to long-term cell viability within encapsulation matrices. These factors have contributed to relatively high product costs, limiting widespread adoption in cost-sensitive markets and healthcare systems.
Emerging opportunities exist in environmental applications, where protected microbial cells are being developed for bioremediation of harsh industrial environments, wastewater treatment, and soil decontamination. This segment, though currently small, shows promising growth potential as environmental regulations become increasingly stringent globally.
Current Encapsulation Technologies and Challenges
The field of cell encapsulation has evolved significantly over the past decades, with various technologies developed to protect cells from harsh environmental conditions. Currently, several major encapsulation approaches dominate the landscape, each with distinct advantages and limitations.
Hydrogel-based encapsulation represents one of the most widely adopted techniques, utilizing natural polymers such as alginate, collagen, and chitosan, or synthetic materials like polyethylene glycol (PEG). These materials form porous matrices that allow nutrient diffusion while providing physical protection. However, hydrogels often suffer from mechanical instability in dynamic environments and may degrade unpredictably, leading to premature cell release.
Microencapsulation using layer-by-layer (LbL) assembly has emerged as a versatile approach, enabling precise control over membrane thickness and permeability. This technique involves sequential deposition of oppositely charged polyelectrolytes to form nanoscale protective layers. While offering excellent customization, LbL processes can be time-consuming and may expose cells to harsh conditions during fabrication.
Solid microcapsules, including those made from silica, PLGA, and other synthetic polymers, provide robust mechanical protection but often struggle with limited nutrient diffusion and oxygen transfer. This creates a significant challenge for maintaining long-term cell viability, particularly for metabolically active cell types.
A major technical hurdle across all encapsulation platforms is achieving the delicate balance between protection and functionality. Barriers that effectively shield cells from immune responses, temperature fluctuations, or chemical stressors frequently impede essential biological processes like nutrient exchange and waste removal.
Scale-up challenges persist throughout the field, with many promising laboratory techniques proving difficult to translate to industrial production. Inconsistencies in capsule size, membrane thickness, and permeability often emerge during scale-up attempts, compromising product reliability.
Biocompatibility remains another critical concern, as materials that perform well in vitro may trigger inflammatory responses or fibrosis when implanted. This issue is particularly problematic for long-term applications such as cell-based therapies and bioartificial organs.
Recent advances in smart responsive materials show promise for addressing some of these challenges. These materials can change their properties in response to specific stimuli (pH, temperature, or enzymatic activity), potentially enabling dynamic control over membrane permeability. However, these technologies remain largely experimental, with questions about long-term stability and regulatory approval pathways still unanswered.
The integration of nanotechnology with traditional encapsulation methods represents another frontier, with nanomaterials offering unique properties that may enhance both protection and functionality. Nevertheless, concerns regarding nanotoxicity and environmental impact must be thoroughly addressed before widespread implementation.
Hydrogel-based encapsulation represents one of the most widely adopted techniques, utilizing natural polymers such as alginate, collagen, and chitosan, or synthetic materials like polyethylene glycol (PEG). These materials form porous matrices that allow nutrient diffusion while providing physical protection. However, hydrogels often suffer from mechanical instability in dynamic environments and may degrade unpredictably, leading to premature cell release.
Microencapsulation using layer-by-layer (LbL) assembly has emerged as a versatile approach, enabling precise control over membrane thickness and permeability. This technique involves sequential deposition of oppositely charged polyelectrolytes to form nanoscale protective layers. While offering excellent customization, LbL processes can be time-consuming and may expose cells to harsh conditions during fabrication.
Solid microcapsules, including those made from silica, PLGA, and other synthetic polymers, provide robust mechanical protection but often struggle with limited nutrient diffusion and oxygen transfer. This creates a significant challenge for maintaining long-term cell viability, particularly for metabolically active cell types.
A major technical hurdle across all encapsulation platforms is achieving the delicate balance between protection and functionality. Barriers that effectively shield cells from immune responses, temperature fluctuations, or chemical stressors frequently impede essential biological processes like nutrient exchange and waste removal.
Scale-up challenges persist throughout the field, with many promising laboratory techniques proving difficult to translate to industrial production. Inconsistencies in capsule size, membrane thickness, and permeability often emerge during scale-up attempts, compromising product reliability.
Biocompatibility remains another critical concern, as materials that perform well in vitro may trigger inflammatory responses or fibrosis when implanted. This issue is particularly problematic for long-term applications such as cell-based therapies and bioartificial organs.
Recent advances in smart responsive materials show promise for addressing some of these challenges. These materials can change their properties in response to specific stimuli (pH, temperature, or enzymatic activity), potentially enabling dynamic control over membrane permeability. However, these technologies remain largely experimental, with questions about long-term stability and regulatory approval pathways still unanswered.
The integration of nanotechnology with traditional encapsulation methods represents another frontier, with nanomaterials offering unique properties that may enhance both protection and functionality. Nevertheless, concerns regarding nanotoxicity and environmental impact must be thoroughly addressed before widespread implementation.
State-of-the-Art Encapsulation Solutions
01 Microelectronic device encapsulation
Encapsulation techniques for microelectronic devices provide protection against environmental factors and mechanical stress. These methods involve coating semiconductor components with protective materials such as polymers or resins to shield sensitive electronic elements from moisture, contaminants, and physical damage. Advanced encapsulation approaches may incorporate specialized barrier layers or utilize hermetic sealing to enhance long-term reliability and performance of integrated circuits and other electronic components.- Microelectronic device encapsulation: Encapsulation techniques for microelectronic devices provide protection against environmental factors and mechanical damage. These methods involve coating semiconductor components with protective materials such as epoxy resins or silicone compounds. The encapsulation creates a barrier that shields sensitive electronic components from moisture, contaminants, and physical stress, thereby extending device lifespan and maintaining performance integrity.
- LED packaging protection techniques: Specialized encapsulation methods for light-emitting diodes (LEDs) focus on maintaining optical properties while providing environmental protection. These techniques utilize transparent or translucent encapsulants that protect the LED chip and wire bonds while allowing light transmission. Advanced formulations may include phosphors for color conversion, UV stabilizers to prevent degradation, and thermal management materials to enhance heat dissipation from the light-emitting components.
- Data protection through software encapsulation: Software encapsulation techniques provide protection for digital data and code by creating secure boundaries around information. These methods implement access control mechanisms, data hiding principles, and abstraction layers to prevent unauthorized access. The encapsulation creates logical separations between different components of a system, allowing controlled interactions while maintaining security and integrity of the protected information.
- Nanoparticle encapsulation for material protection: Encapsulation of nanoparticles involves coating small-scale materials with protective shells to enhance stability and functionality. These techniques use core-shell structures where the outer layer shields the inner material from oxidation, aggregation, or chemical degradation. The protective encapsulation can be designed to respond to specific environmental triggers, allowing for controlled release or activation of the encapsulated materials in targeted applications.
- Protective housing encapsulation systems: Physical encapsulation systems provide protection for devices through specialized housing designs. These protective enclosures shield sensitive components from environmental factors such as moisture, dust, and physical impact. Advanced housing systems may incorporate multiple layers of protection, specialized sealing techniques, and materials selected for specific protective properties like electromagnetic shielding or thermal insulation.
02 LED and display protection encapsulation
Specialized encapsulation methods for light-emitting diodes (LEDs) and display technologies focus on maintaining optical properties while providing environmental protection. These techniques often employ transparent or translucent encapsulants that offer high light transmission while protecting against moisture, dust, and mechanical damage. The encapsulation materials may include silicones, epoxies, or advanced composites designed to withstand thermal cycling and prevent degradation of light output or color quality over the device lifetime.Expand Specific Solutions03 Memory and data protection encapsulation
Encapsulation techniques for memory devices and data storage systems focus on both physical protection and security features. These methods incorporate materials and designs that shield memory components from environmental factors while also implementing security measures to prevent unauthorized access to stored data. Advanced encapsulation approaches may include tamper-evident or tamper-resistant features, encryption capabilities, or specialized coatings that obscure internal components from physical inspection or reverse engineering attempts.Expand Specific Solutions04 Multilayer encapsulation barriers
Multilayer encapsulation technologies utilize combinations of different materials to create enhanced protective barriers. These systems typically alternate organic and inorganic layers to provide comprehensive protection against multiple environmental threats. The layered structure creates tortuous paths that impede the penetration of moisture, oxygen, and other contaminants. This approach is particularly valuable for sensitive devices such as organic electronics, where traditional single-layer encapsulation may not provide sufficient protection against degradation factors.Expand Specific Solutions05 Capacitor and energy storage encapsulation
Specialized encapsulation techniques for capacitors and energy storage devices focus on electrical isolation, thermal management, and protection against environmental factors. These methods employ materials with high dielectric strength and thermal stability to ensure safe and reliable operation under various conditions. The encapsulation designs may incorporate features to manage heat dissipation, prevent electrical arcing, and protect against moisture ingress, which could otherwise compromise the performance and safety of high-voltage or high-energy storage components.Expand Specific Solutions
Leading Companies in Cell Encapsulation Industry
The encapsulation techniques for protecting cells in harsh environments market is currently in a growth phase, with increasing applications across medical, energy, and biotechnology sectors. The global market size is estimated to reach $3.5 billion by 2025, driven by demand for advanced cell protection solutions in implantable devices and industrial applications. Technologically, the field shows varying maturity levels, with companies like Surmodics and Encellin leading in biomedical applications through their specialized membrane technologies. BASF and Eastman Chemical are advancing industrial-grade encapsulation materials, while research institutions like National Institute for Materials Science and University of Palermo are developing next-generation solutions. Pharmaceutical players such as Takeda and biotechnology firms like PrimeGen Biotech are exploring therapeutic applications, particularly for cell-based treatments requiring protection from immune responses.
Surmodics, Inc.
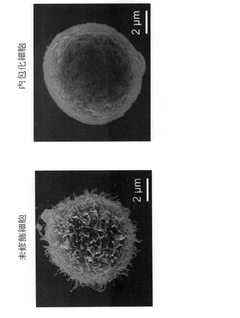
Technical Solution: Surmodics has developed proprietary surface modification and encapsulation technologies specifically designed to protect cells in harsh biological environments. Their core technology platform includes hydrophilic and hydrophobic coatings that can be precisely engineered to control protein adsorption, cell adhesion, and immune recognition. Surmodics' encapsulation systems utilize photoreactive cross-linking chemistry that allows for gentle encapsulation of cells without exposure to harsh solvents or conditions. Their technology creates uniform, defect-free barriers with controlled porosity that permits nutrient diffusion while blocking immune components. The company has demonstrated particular success with their heparin-based encapsulation systems that combine cell protection with anti-inflammatory properties. Surmodics has also developed specialized manufacturing processes that maintain high cell viability during encapsulation, with reported survival rates exceeding 95% in their optimized protocols. Their encapsulation materials have shown stability in vivo for extended periods, with functional protection demonstrated for over 12 months in some applications.
Strengths: Extensive commercial experience in surface modification technologies; established manufacturing capabilities and quality systems; strong intellectual property portfolio in biomaterial interfaces. Weaknesses: Broader focus beyond just cell encapsulation may limit specialization in this specific area; primarily focused on medical device applications rather than cell therapy; potential challenges adapting technologies to different cell types.
Encellin, Inc.
Technical Solution: Encellin has developed a proprietary Cell Encapsulation Device (CED) technology specifically designed to protect therapeutic cells in harsh environments. Their platform utilizes nanoporous membranes made from biocompatible materials that allow for selective permeability - permitting nutrients, oxygen, and therapeutic molecules to pass through while blocking immune system components. The encapsulation system creates a protective microenvironment that shields cells from mechanical stress, immune rejection, and chemical threats while maintaining cell viability and functionality. Encellin's technology has shown particular promise in diabetes treatment, where encapsulated insulin-producing cells can be implanted without triggering immune rejection, potentially eliminating the need for immunosuppression.
Strengths: Highly specialized in cell encapsulation with proprietary nanoporous membrane technology; demonstrated clinical applications in diabetes treatment; provides immunoprotection without compromising cell function. Weaknesses: Limited commercial-scale production experience; relatively narrow focus on specific therapeutic applications compared to broader industry players.
Key Patents and Innovations in Cell Protection
Cell encapsulation method and encapsulated cell
PatentWO2018034097A1
Innovation
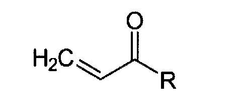
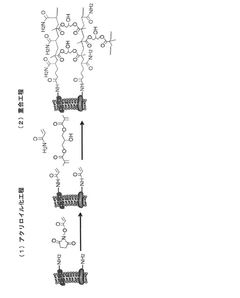
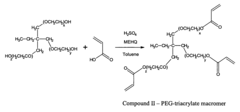
- A method involving acryloylation of the cell membrane followed by polymerization with a water-soluble monomer and crosslinking agent to form a stable, permeable, and non-toxic polymer layer using N-acryloxysuccinimide, dimethyl sulfoxide, glycerol dimethacrylate, and a redox polymerization initiator.
Encapsulated cells using positively-charged initiator polymer
PatentInactiveUS8143043B2
Innovation
- Development of positively-charged polymerization initiator polymers with cationic portions that are soluble in aqueous solutions and specifically interact with biological surfaces, promoting polymerization of macromers using visible light activation, thereby forming a biocompatible polymeric matrix around cells.
Biocompatibility and Safety Considerations
Biocompatibility represents a critical consideration in the development of cell encapsulation technologies for harsh environments. The materials used for encapsulation must not elicit adverse immune responses or inflammatory reactions when in contact with biological systems. This requirement becomes particularly challenging when designing encapsulation systems that must simultaneously protect cells from harsh external conditions while maintaining compatibility with both the encapsulated cells and the host environment.
Current research focuses on natural polymers such as alginate, collagen, and hyaluronic acid due to their inherent biocompatibility. However, these materials often require chemical modifications to enhance their protective capabilities, which can introduce biocompatibility concerns. Synthetic polymers like polyethylene glycol (PEG) and poly(lactic-co-glycolic acid) (PLGA) offer greater control over material properties but present additional challenges regarding degradation products and long-term tissue responses.
The safety profile of encapsulation materials must be evaluated across multiple dimensions. Cytotoxicity testing represents the first level of assessment, examining direct effects on cell viability and function. More comprehensive evaluations include genotoxicity studies, sensitization tests, and assessments of systemic toxicity. For encapsulation technologies intended for medical applications, regulatory frameworks such as ISO 10993 provide standardized methodologies for biocompatibility evaluation.
Degradation characteristics of encapsulation materials present another critical safety consideration. Materials must degrade in a controlled manner without releasing toxic byproducts or causing sudden changes in local pH. The degradation timeline must align with the intended application duration, whether temporary protection during manufacturing processes or long-term implantation for cell therapy applications.
Surface properties of encapsulation materials significantly influence biocompatibility. Parameters such as surface charge, hydrophilicity/hydrophobicity, and topography affect protein adsorption and subsequent cellular interactions. Recent advances in surface modification techniques have enabled the development of "stealth" encapsulation systems that minimize recognition by the immune system while maintaining protective functions.
Scalable manufacturing of biocompatible encapsulation systems presents additional challenges. Production processes must maintain material purity and avoid introduction of toxic residues from crosslinking agents, solvents, or processing aids. Sterilization methods must effectively eliminate microbial contamination without compromising material integrity or generating toxic byproducts.
Regulatory considerations for encapsulated cell products vary significantly based on application context. Cell therapy applications face rigorous regulatory scrutiny, with requirements for extensive preclinical safety data and controlled clinical trials. Industrial applications, such as encapsulated microorganisms for bioremediation, face different regulatory frameworks focused on environmental impact and containment assurance.
Current research focuses on natural polymers such as alginate, collagen, and hyaluronic acid due to their inherent biocompatibility. However, these materials often require chemical modifications to enhance their protective capabilities, which can introduce biocompatibility concerns. Synthetic polymers like polyethylene glycol (PEG) and poly(lactic-co-glycolic acid) (PLGA) offer greater control over material properties but present additional challenges regarding degradation products and long-term tissue responses.
The safety profile of encapsulation materials must be evaluated across multiple dimensions. Cytotoxicity testing represents the first level of assessment, examining direct effects on cell viability and function. More comprehensive evaluations include genotoxicity studies, sensitization tests, and assessments of systemic toxicity. For encapsulation technologies intended for medical applications, regulatory frameworks such as ISO 10993 provide standardized methodologies for biocompatibility evaluation.
Degradation characteristics of encapsulation materials present another critical safety consideration. Materials must degrade in a controlled manner without releasing toxic byproducts or causing sudden changes in local pH. The degradation timeline must align with the intended application duration, whether temporary protection during manufacturing processes or long-term implantation for cell therapy applications.
Surface properties of encapsulation materials significantly influence biocompatibility. Parameters such as surface charge, hydrophilicity/hydrophobicity, and topography affect protein adsorption and subsequent cellular interactions. Recent advances in surface modification techniques have enabled the development of "stealth" encapsulation systems that minimize recognition by the immune system while maintaining protective functions.
Scalable manufacturing of biocompatible encapsulation systems presents additional challenges. Production processes must maintain material purity and avoid introduction of toxic residues from crosslinking agents, solvents, or processing aids. Sterilization methods must effectively eliminate microbial contamination without compromising material integrity or generating toxic byproducts.
Regulatory considerations for encapsulated cell products vary significantly based on application context. Cell therapy applications face rigorous regulatory scrutiny, with requirements for extensive preclinical safety data and controlled clinical trials. Industrial applications, such as encapsulated microorganisms for bioremediation, face different regulatory frameworks focused on environmental impact and containment assurance.
Scalability and Manufacturing Processes
The scalability of cell encapsulation technologies represents a critical factor in their commercial viability and widespread adoption. Current laboratory-scale encapsulation methods often face significant challenges when transitioning to industrial production levels. Microfluidic-based encapsulation systems, while offering precise control over capsule size and composition, typically operate at throughput rates of only 100-1000 capsules per minute, which remains insufficient for large-scale applications such as regenerative medicine or industrial biocatalysis that may require billions of encapsulated cells.
Recent advancements in parallel microfluidic systems have demonstrated promising improvements, with some platforms achieving production rates of up to 10,000 capsules per minute through the implementation of multiple parallel channels. However, these systems often encounter issues with channel clogging and flow rate inconsistencies when operated continuously for extended periods, necessitating further engineering refinements.
Electrospraying and electrospinning techniques offer higher throughput alternatives, capable of producing encapsulated cells at rates exceeding 100,000 particles per minute. These methods benefit from relatively simple equipment requirements and scalable production capabilities, though they typically yield less uniform capsules compared to microfluidic approaches. The trade-off between production volume and capsule uniformity represents a key consideration for commercial applications.
Automated batch processing systems have emerged as a middle-ground solution, incorporating robotics and process control systems to enhance traditional encapsulation methods. These systems can maintain sterility while increasing production capacity, with some commercial platforms now capable of producing encapsulated cell products at scales suitable for early-phase clinical trials.
Manufacturing considerations extend beyond mere production rates to encompass quality control, regulatory compliance, and cost-effectiveness. Continuous monitoring systems utilizing machine vision and artificial intelligence have been developed to assess capsule integrity, size distribution, and cell viability in real-time during production. These systems enable rapid feedback loops for process adjustment, significantly reducing batch failures and improving overall yield.
Cost analysis of various encapsulation manufacturing processes indicates that material selection represents a substantial portion of production expenses. Synthetic polymers typically offer better scalability and batch-to-batch consistency compared to natural materials, though often at higher cost. Recent innovations in biopolymer processing have begun to address this gap, potentially enabling more cost-effective large-scale production using naturally derived encapsulation materials.
Recent advancements in parallel microfluidic systems have demonstrated promising improvements, with some platforms achieving production rates of up to 10,000 capsules per minute through the implementation of multiple parallel channels. However, these systems often encounter issues with channel clogging and flow rate inconsistencies when operated continuously for extended periods, necessitating further engineering refinements.
Electrospraying and electrospinning techniques offer higher throughput alternatives, capable of producing encapsulated cells at rates exceeding 100,000 particles per minute. These methods benefit from relatively simple equipment requirements and scalable production capabilities, though they typically yield less uniform capsules compared to microfluidic approaches. The trade-off between production volume and capsule uniformity represents a key consideration for commercial applications.
Automated batch processing systems have emerged as a middle-ground solution, incorporating robotics and process control systems to enhance traditional encapsulation methods. These systems can maintain sterility while increasing production capacity, with some commercial platforms now capable of producing encapsulated cell products at scales suitable for early-phase clinical trials.
Manufacturing considerations extend beyond mere production rates to encompass quality control, regulatory compliance, and cost-effectiveness. Continuous monitoring systems utilizing machine vision and artificial intelligence have been developed to assess capsule integrity, size distribution, and cell viability in real-time during production. These systems enable rapid feedback loops for process adjustment, significantly reducing batch failures and improving overall yield.
Cost analysis of various encapsulation manufacturing processes indicates that material selection represents a substantial portion of production expenses. Synthetic polymers typically offer better scalability and batch-to-batch consistency compared to natural materials, though often at higher cost. Recent innovations in biopolymer processing have begun to address this gap, potentially enabling more cost-effective large-scale production using naturally derived encapsulation materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!