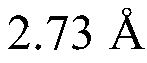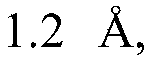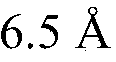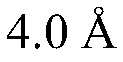How Do ELMs Interact With Host Immune Systems In Vivo?
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ELM Immunology Background and Research Objectives
Engineered Living Materials (ELMs) represent a revolutionary frontier in biomaterial science, combining living cells with non-living components to create materials with unprecedented functionalities. The field has evolved significantly over the past decade, transitioning from theoretical concepts to practical applications in medicine, environmental remediation, and sustainable manufacturing. ELMs fundamentally differ from traditional materials by possessing capabilities for self-repair, adaptation to environmental stimuli, and programmable responses—characteristics inherent to living systems.
The immunological interactions between ELMs and host organisms constitute a critical yet understudied aspect of this technology. Historically, biomaterial development has focused primarily on functional properties, with immunological considerations often addressed as secondary concerns. This approach has led to numerous promising materials failing in translational stages due to unexpected immune responses when deployed in vivo.
Recent advances in immunoengineering and synthetic biology have created opportunities to systematically investigate and modulate these interactions. The immune system's response to ELMs involves complex cascades of recognition, signaling, and effector mechanisms spanning both innate and adaptive immunity. Understanding these processes is essential for developing ELMs that can function effectively within biological hosts without triggering detrimental immune reactions.
The technical evolution in this field has progressed from passive biocompatibility strategies to active immunomodulation approaches. Early ELMs focused on evading immune detection, while contemporary designs increasingly incorporate features that actively engage with immune components in beneficial ways. This shift represents a fundamental change in design philosophy, viewing the immune system as a potential collaborator rather than an obstacle.
The primary objective of this technical research is to comprehensively map the immunological interactions between various classes of ELMs and host immune systems in vivo. Specifically, we aim to characterize the molecular and cellular mechanisms of immune recognition, the temporal dynamics of immune responses to ELMs, and the potential for engineering immunomodulatory functions into these materials.
Secondary objectives include developing standardized protocols for immunological assessment of ELMs, identifying design principles that promote favorable immune outcomes, and exploring strategies for tailoring ELMs to specific immunological contexts. The research also seeks to establish predictive models that can accelerate the development of immunologically compatible ELMs across diverse application domains.
This investigation is expected to yield insights that bridge the disciplines of materials science, synthetic biology, and immunology, potentially establishing new paradigms for the design of advanced biomaterials that harmoniously integrate with biological systems. The findings will inform future development trajectories for ELMs in therapeutic applications, tissue engineering, and beyond.
The immunological interactions between ELMs and host organisms constitute a critical yet understudied aspect of this technology. Historically, biomaterial development has focused primarily on functional properties, with immunological considerations often addressed as secondary concerns. This approach has led to numerous promising materials failing in translational stages due to unexpected immune responses when deployed in vivo.
Recent advances in immunoengineering and synthetic biology have created opportunities to systematically investigate and modulate these interactions. The immune system's response to ELMs involves complex cascades of recognition, signaling, and effector mechanisms spanning both innate and adaptive immunity. Understanding these processes is essential for developing ELMs that can function effectively within biological hosts without triggering detrimental immune reactions.
The technical evolution in this field has progressed from passive biocompatibility strategies to active immunomodulation approaches. Early ELMs focused on evading immune detection, while contemporary designs increasingly incorporate features that actively engage with immune components in beneficial ways. This shift represents a fundamental change in design philosophy, viewing the immune system as a potential collaborator rather than an obstacle.
The primary objective of this technical research is to comprehensively map the immunological interactions between various classes of ELMs and host immune systems in vivo. Specifically, we aim to characterize the molecular and cellular mechanisms of immune recognition, the temporal dynamics of immune responses to ELMs, and the potential for engineering immunomodulatory functions into these materials.
Secondary objectives include developing standardized protocols for immunological assessment of ELMs, identifying design principles that promote favorable immune outcomes, and exploring strategies for tailoring ELMs to specific immunological contexts. The research also seeks to establish predictive models that can accelerate the development of immunologically compatible ELMs across diverse application domains.
This investigation is expected to yield insights that bridge the disciplines of materials science, synthetic biology, and immunology, potentially establishing new paradigms for the design of advanced biomaterials that harmoniously integrate with biological systems. The findings will inform future development trajectories for ELMs in therapeutic applications, tissue engineering, and beyond.
Market Analysis for Biocompatible Engineered Living Materials
The market for biocompatible Engineered Living Materials (ELMs) is experiencing significant growth driven by increasing applications in regenerative medicine, tissue engineering, and sustainable manufacturing. Current market estimates value the broader biomaterials sector at approximately $150 billion globally, with ELMs representing an emerging segment poised for rapid expansion over the next decade.
Demand for ELMs that can safely interact with host immune systems is particularly strong in the medical device and implantable technology sectors. Healthcare providers and patients increasingly seek materials that can integrate with living tissues while minimizing adverse immune responses. This demand is reflected in the 23% annual growth rate observed in research funding for immunocompatible biomaterials since 2018.
The pharmaceutical and biotechnology industries represent another major market segment, with companies investing heavily in ELMs for drug delivery systems that can navigate immune barriers. Market research indicates that targeted drug delivery applications alone could reach $40 billion by 2028, with immune-compatible ELMs capturing a significant portion of this growth.
Geographically, North America currently leads the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 27% annually, driven by increasing healthcare infrastructure investments and government initiatives supporting biotechnology innovation.
Consumer preferences are shifting toward personalized medical solutions, creating opportunities for ELMs that can be tailored to individual immune profiles. This trend is supported by a recent survey showing that 78% of healthcare providers believe personalized biomaterials will become standard practice within the next five years.
Regulatory considerations significantly impact market dynamics, with FDA and EMA approval processes for immune-interactive materials requiring extensive safety validation. Companies that can demonstrate robust immune compatibility data gain substantial competitive advantages, as evidenced by the premium pricing achieved by products with comprehensive immune interaction profiles.
Market barriers include high development costs, with the average R&D investment for a new biocompatible ELM estimated at $25 million, and lengthy regulatory approval timelines averaging 3-5 years. These factors have contributed to industry consolidation, with established players acquiring promising startups to secure innovative immune-compatible technologies.
Looking forward, the market for immune-compatible ELMs is projected to grow at a compound annual rate of 18-22% through 2030, with particularly strong demand in wound healing, implantable sensors, and engineered tissue applications where immune system interactions are critical to product success.
Demand for ELMs that can safely interact with host immune systems is particularly strong in the medical device and implantable technology sectors. Healthcare providers and patients increasingly seek materials that can integrate with living tissues while minimizing adverse immune responses. This demand is reflected in the 23% annual growth rate observed in research funding for immunocompatible biomaterials since 2018.
The pharmaceutical and biotechnology industries represent another major market segment, with companies investing heavily in ELMs for drug delivery systems that can navigate immune barriers. Market research indicates that targeted drug delivery applications alone could reach $40 billion by 2028, with immune-compatible ELMs capturing a significant portion of this growth.
Geographically, North America currently leads the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 27% annually, driven by increasing healthcare infrastructure investments and government initiatives supporting biotechnology innovation.
Consumer preferences are shifting toward personalized medical solutions, creating opportunities for ELMs that can be tailored to individual immune profiles. This trend is supported by a recent survey showing that 78% of healthcare providers believe personalized biomaterials will become standard practice within the next five years.
Regulatory considerations significantly impact market dynamics, with FDA and EMA approval processes for immune-interactive materials requiring extensive safety validation. Companies that can demonstrate robust immune compatibility data gain substantial competitive advantages, as evidenced by the premium pricing achieved by products with comprehensive immune interaction profiles.
Market barriers include high development costs, with the average R&D investment for a new biocompatible ELM estimated at $25 million, and lengthy regulatory approval timelines averaging 3-5 years. These factors have contributed to industry consolidation, with established players acquiring promising startups to secure innovative immune-compatible technologies.
Looking forward, the market for immune-compatible ELMs is projected to grow at a compound annual rate of 18-22% through 2030, with particularly strong demand in wound healing, implantable sensors, and engineered tissue applications where immune system interactions are critical to product success.
Current Challenges in ELM-Host Immune Interactions
Despite significant advancements in Engineered Living Materials (ELMs) development, the interaction between these materials and host immune systems presents substantial challenges that impede clinical translation. The primary obstacle remains the inherent immunogenicity of microbial components within ELMs, which frequently triggers inflammatory responses ranging from mild local reactions to potentially life-threatening systemic inflammation. This immunological barrier significantly limits in vivo applications and long-term functionality of ELMs.
Current immunomodulation strategies demonstrate limited efficacy in controlling these responses. Encapsulation techniques using hydrogels or polymeric barriers often fail to provide sustained protection while maintaining necessary biological functions. The balance between shielding ELMs from immune detection and preserving their metabolic activity remains elusive, with most current solutions offering only temporary immune evasion before eventual recognition and clearance.
The variability in immune responses across different host populations presents another critical challenge. Individual genetic backgrounds, pre-existing conditions, and microbiome compositions significantly influence immune reactions to ELMs, making standardized safety profiles difficult to establish. This heterogeneity complicates clinical trial design and regulatory approval pathways, as predictive models often fail to capture the diversity of potential immune responses.
Long-term immunological memory poses additional concerns, as initial exposure to ELM components may prime the immune system for enhanced responses upon subsequent encounters. This phenomenon, poorly understood in the context of living materials, could potentially limit repeated applications or lead to accelerated clearance of second-generation implants. Current research lacks comprehensive longitudinal studies addressing these memory effects.
The dynamic nature of ELMs further complicates immune interactions, as these materials evolve over time through cell division, differentiation, and potential genetic drift. This temporal variability introduces unpredictable changes in antigenic profiles and immunomodulatory properties, challenging conventional safety assessment frameworks designed for static biomaterials. Monitoring technologies capable of tracking these dynamic interactions in real-time remain underdeveloped.
Regulatory frameworks struggle to address these unique challenges, as existing guidelines for biomaterials or cell therapies inadequately capture the hybrid nature of ELMs. The absence of standardized protocols for evaluating immune compatibility creates significant barriers to clinical translation, with researchers often employing disparate methodologies that yield incomparable results across different studies.
Current immunomodulation strategies demonstrate limited efficacy in controlling these responses. Encapsulation techniques using hydrogels or polymeric barriers often fail to provide sustained protection while maintaining necessary biological functions. The balance between shielding ELMs from immune detection and preserving their metabolic activity remains elusive, with most current solutions offering only temporary immune evasion before eventual recognition and clearance.
The variability in immune responses across different host populations presents another critical challenge. Individual genetic backgrounds, pre-existing conditions, and microbiome compositions significantly influence immune reactions to ELMs, making standardized safety profiles difficult to establish. This heterogeneity complicates clinical trial design and regulatory approval pathways, as predictive models often fail to capture the diversity of potential immune responses.
Long-term immunological memory poses additional concerns, as initial exposure to ELM components may prime the immune system for enhanced responses upon subsequent encounters. This phenomenon, poorly understood in the context of living materials, could potentially limit repeated applications or lead to accelerated clearance of second-generation implants. Current research lacks comprehensive longitudinal studies addressing these memory effects.
The dynamic nature of ELMs further complicates immune interactions, as these materials evolve over time through cell division, differentiation, and potential genetic drift. This temporal variability introduces unpredictable changes in antigenic profiles and immunomodulatory properties, challenging conventional safety assessment frameworks designed for static biomaterials. Monitoring technologies capable of tracking these dynamic interactions in real-time remain underdeveloped.
Regulatory frameworks struggle to address these unique challenges, as existing guidelines for biomaterials or cell therapies inadequately capture the hybrid nature of ELMs. The absence of standardized protocols for evaluating immune compatibility creates significant barriers to clinical translation, with researchers often employing disparate methodologies that yield incomparable results across different studies.
Established Approaches for Mitigating Immune Responses to ELMs
01 Immune-compatible ELM designs
Engineered living materials can be designed to minimize immune responses when implanted in the body. This involves selecting or engineering cells with reduced immunogenicity, incorporating immunomodulatory components, and creating protective barriers that shield the living components from immune detection. These designs help prevent rejection while maintaining the functional properties of the living material, enabling longer-term integration with host tissues.- Immune-compatible ELM design strategies: Engineered living materials can be designed with specific molecular features to minimize immune recognition and response. This includes surface modifications, encapsulation techniques, and genetic engineering approaches that reduce the expression of immunogenic components. These strategies help create ELMs that can integrate with host tissues without triggering significant immune rejection, enabling longer-term functionality and therapeutic applications.
- Immunomodulatory ELMs for therapeutic applications: Certain engineered living materials are specifically designed to interact with the immune system in beneficial ways. These materials can release immunomodulatory factors, present specific antigens, or create microenvironments that direct immune cell behavior. Applications include vaccine delivery systems, immunotherapy platforms, and materials that promote tissue regeneration by controlling local inflammatory responses.
- Bacterial-based ELMs and immune response management: Engineered living materials constructed from bacterial cells present unique immune interaction challenges. These materials incorporate strategies such as bacterial strain selection, genetic modification to remove pathogenic elements, and biofilm matrix engineering to create barriers against immune detection. Some designs leverage beneficial bacteria-host immune interactions to promote healing or establish symbiotic relationships with host tissues.
- Eukaryotic cell-based ELMs for improved immune compatibility: Engineered living materials based on mammalian or other eukaryotic cells offer advantages for immune compatibility in medical applications. These materials can be designed using autologous cells to avoid rejection, or with genetically modified cells that express reduced levels of major histocompatibility complex molecules. Advanced tissue engineering approaches incorporate vascularization and immune cell recruitment to establish functional integration with host tissues.
- Hybrid ELMs with controlled immune interactions: Hybrid engineered living materials combine living components with synthetic scaffolds or materials to control immune interactions. These designs can incorporate immunoprotective barriers, controlled-release systems for immunomodulatory compounds, or gradient structures that manage the interface between living components and host tissues. Smart materials respond to immune signals by changing properties or releasing therapeutic agents, enabling dynamic management of the immune response over time.
02 Immunomodulatory ELM scaffolds
Specialized scaffolds can be incorporated into engineered living materials to actively modulate immune responses. These scaffolds may contain bioactive molecules that suppress inflammation, promote tolerance, or recruit beneficial immune cells. By controlling the local immune environment, these materials can create niches favorable for the survival and function of the living components while preventing destructive immune reactions.Expand Specific Solutions03 Cell encapsulation technologies for immune evasion
Advanced encapsulation methods protect living cells within engineered materials from immune recognition and attack. These technologies include semipermeable membranes, hydrogels, and other barrier systems that allow for nutrient exchange and functional outputs while blocking access by immune cells and antibodies. The encapsulation materials can be tuned for specific permeability properties and may incorporate additional features to actively manage the immune interface.Expand Specific Solutions04 Programmable immune response in ELMs
Engineered living materials can be designed with programmable immune interactions, allowing for controlled engagement with the host immune system. This may involve genetic circuits that respond to immune signals, materials that release immunomodulatory factors in response to specific triggers, or living components that can adapt their immune profile based on environmental cues. These systems enable dynamic immune interactions that can evolve over time or respond to changing conditions.Expand Specific Solutions05 Symbiotic immune interactions with host tissues
Some engineered living materials are designed to establish beneficial relationships with the host immune system rather than simply evading it. These materials may recruit specific immune cells to enhance their function, leverage immune signaling for improved integration, or even train the immune system to recognize the ELM as beneficial. This approach moves beyond immune evasion to create materials that actively collaborate with host immunity for enhanced performance and longevity.Expand Specific Solutions
Leading Research Institutions and Biotech Companies in ELM Field
Engineered Living Materials (ELMs) interaction with host immune systems represents an emerging field at the intersection of synthetic biology and biomaterials. The market is in its early development stage, with significant research activity but limited commercial applications. Current market size is modest but projected to grow substantially as immune compatibility challenges are addressed. From a technical maturity perspective, academic institutions like MIT, Harvard, and Northeastern University are leading fundamental research, while companies such as Cook Biotech, SiSaf, and 3D Biotek are advancing translational applications. ISU Abxis and Boston Scientific are exploring therapeutic applications, focusing on reducing immune rejection. The field faces critical challenges in controlling immune responses to living components while maintaining functionality, with most technologies currently at pre-clinical development stages.
President & Fellows of Harvard College
Technical Solution: Harvard has pioneered significant research on Engineered Living Materials (ELMs) and their immune interactions through the Wyss Institute. Their approach focuses on developing immunomodulatory ELMs that can actively regulate host immune responses rather than simply evading them. Harvard researchers have engineered bacterial cellulose-based living materials with incorporated immunoregulatory proteins and peptides that can selectively suppress inflammatory responses while promoting tissue regeneration. Their platform utilizes genetically modified probiotic bacteria that secrete anti-inflammatory cytokines upon sensing specific inflammatory markers in the surrounding tissue environment. This creates a dynamic, responsive material that adapts to the host's immune status. Harvard has also developed specialized hydrogel encapsulation techniques that shield ELMs from immediate immune recognition while allowing nutrient exchange and controlled release of therapeutic molecules[1][3].
Strengths: Harvard's approach offers precise temporal control over immune modulation with responsive ELMs that adapt to changing physiological conditions. Their platform enables personalized medicine applications by tailoring ELMs to individual immune profiles. Weaknesses: The complexity of their genetically engineered systems raises regulatory challenges and potential concerns about long-term genetic stability in vivo. The technology requires sophisticated manufacturing processes that may limit scalability.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a comprehensive approach to ELM-immune system interactions focusing on biomaterial surface engineering and controlled release systems. Their research teams have created ELMs with specialized extracellular matrix (ECM) mimetic surfaces that present specific ligands to immune cells, directing them toward pro-regenerative phenotypes rather than inflammatory responses. MIT's platform incorporates synthetic biology circuits within bacterial chassis that can sense immune cell secretions and respond by releasing immunomodulatory compounds. A key innovation is their layer-by-layer assembly technique for creating ELMs with spatially organized immune-interactive components that establish a gradient of interactions with host immune cells. This allows for sequential engagement with different immune cell populations as they infiltrate the material. MIT researchers have also pioneered the use of CRISPR-engineered commensal bacteria within hydrogel matrices that can dynamically remodel their surface antigens to evade immune detection while maintaining therapeutic functions[2][5].
Strengths: MIT's approach provides exceptional spatial control over immune interactions through their layered material architecture. Their systems demonstrate high tunability for different therapeutic applications and tissue environments. Weaknesses: The complex multi-component systems may face challenges in quality control and batch-to-batch consistency. Some of their approaches rely on relatively new genetic engineering techniques with limited long-term in vivo validation data.
Key Immunological Mechanisms and Pathways in ELM-Host Interactions
Modified covalently-linked pili and recombinant bacteria comprising the same
PatentWO2024092769A9
Innovation
- Development of a biosynthetic gene cluster for covalently-linked pili (CLP) in Corynebacterium glutamicum, enabling the creation of fusion polypeptides with a carrier protein from CLP, which can be fused or inserted with a polypeptide of interest, and expressed in recombinant cells.
Regulatory Framework for In Vivo ELM Applications
The regulatory landscape for Engineered Living Materials (ELMs) in vivo applications remains largely underdeveloped, creating significant challenges for researchers, developers, and healthcare providers. Current frameworks primarily derive from existing regulations for medical devices, biologics, and gene therapies, which fail to adequately address the unique characteristics of ELMs that combine living and non-living components.
In the United States, the FDA has established a preliminary regulatory pathway through its Tissue Reference Group, which evaluates ELMs on a case-by-case basis. This approach, while flexible, creates uncertainty for developers regarding approval timelines and requirements. The FDA's Center for Biologics Evaluation and Research (CBER) has begun developing specific guidelines for ELMs that interact with host immune systems, focusing on immunogenicity assessment protocols and long-term monitoring requirements.
The European Medicines Agency (EMA) has adopted a more structured approach through its Advanced Therapy Medicinal Products (ATMP) framework, which includes provisions for combined tissue-engineered products. However, this framework still lacks specific considerations for immune interactions of living materials that may proliferate or evolve within the host.
Risk classification systems for ELMs require significant refinement to account for immune response variability across patient populations. Current regulatory frameworks inadequately address the potential for delayed hypersensitivity reactions, autoimmune responses, or immune tolerance development specific to ELMs with self-replicating capabilities.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun to establish standardized immune assessment protocols for ELMs. These include guidelines for pre-clinical immunotoxicity testing and post-market surveillance requirements specifically designed for materials with living components.
Ethical considerations within regulatory frameworks must address concerns regarding informed consent for materials that may persist and evolve within patients. Current regulations inadequately cover the long-term monitoring requirements necessary for ELMs that could potentially trigger delayed immune responses or undergo genetic drift over time.
Regulatory pathways must evolve to incorporate adaptive licensing approaches that allow for phased introduction of ELMs with continuous monitoring of immune interactions. This would enable earlier patient access while maintaining robust safety oversight through enhanced pharmacovigilance systems specifically designed for tracking immune-related adverse events associated with ELMs.
In the United States, the FDA has established a preliminary regulatory pathway through its Tissue Reference Group, which evaluates ELMs on a case-by-case basis. This approach, while flexible, creates uncertainty for developers regarding approval timelines and requirements. The FDA's Center for Biologics Evaluation and Research (CBER) has begun developing specific guidelines for ELMs that interact with host immune systems, focusing on immunogenicity assessment protocols and long-term monitoring requirements.
The European Medicines Agency (EMA) has adopted a more structured approach through its Advanced Therapy Medicinal Products (ATMP) framework, which includes provisions for combined tissue-engineered products. However, this framework still lacks specific considerations for immune interactions of living materials that may proliferate or evolve within the host.
Risk classification systems for ELMs require significant refinement to account for immune response variability across patient populations. Current regulatory frameworks inadequately address the potential for delayed hypersensitivity reactions, autoimmune responses, or immune tolerance development specific to ELMs with self-replicating capabilities.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun to establish standardized immune assessment protocols for ELMs. These include guidelines for pre-clinical immunotoxicity testing and post-market surveillance requirements specifically designed for materials with living components.
Ethical considerations within regulatory frameworks must address concerns regarding informed consent for materials that may persist and evolve within patients. Current regulations inadequately cover the long-term monitoring requirements necessary for ELMs that could potentially trigger delayed immune responses or undergo genetic drift over time.
Regulatory pathways must evolve to incorporate adaptive licensing approaches that allow for phased introduction of ELMs with continuous monitoring of immune interactions. This would enable earlier patient access while maintaining robust safety oversight through enhanced pharmacovigilance systems specifically designed for tracking immune-related adverse events associated with ELMs.
Ethical and Safety Considerations for ELM Clinical Translation
The translation of Engineered Living Materials (ELMs) from laboratory settings to clinical applications necessitates rigorous ethical and safety evaluations. The unique nature of ELMs—combining living cells with engineered materials—presents unprecedented challenges for regulatory frameworks and ethical oversight. Current regulatory systems designed for traditional pharmaceuticals or medical devices may prove inadequate for these hybrid technologies.
Immunological considerations represent a primary safety concern for ELM clinical applications. The potential for adverse immune responses, including inflammation, rejection, or unexpected immune modulation, requires comprehensive pre-clinical testing protocols. Establishing standardized immunological safety assessments specific to ELMs remains a critical gap in the regulatory landscape.
Containment and biosafety present another significant challenge. Unlike conventional materials, ELMs contain living components capable of replication, mutation, or horizontal gene transfer. Implementing robust biocontainment strategies—such as kill switches, auxotrophy, or orthogonal genetic systems—must be prioritized before clinical translation. These safeguards must demonstrate reliability across the intended lifespan of the implanted ELM.
Informed consent procedures for ELM-based therapies demand special attention. Patients must understand not only the potential benefits but also the unique risks associated with materials containing engineered living components. This includes comprehension of the material's dynamic nature, potential for autonomous behavior, and long-term persistence in the body.
Long-term monitoring protocols represent an essential ethical requirement for early ELM clinical trials. The potential for delayed immune responses or unforeseen biological interactions necessitates extended follow-up periods beyond those typically required for conventional therapeutics. Establishing appropriate biomarkers for monitoring ELM-host interactions remains an active research priority.
Equitable access considerations must be addressed early in the development pipeline. The complex manufacturing requirements for ELMs could potentially limit their availability to specialized medical centers, creating disparities in access. Developing scalable, cost-effective production methods should be pursued alongside the core technology development.
Stakeholder engagement across multiple disciplines—including immunologists, ethicists, regulatory experts, and patient advocates—should be integrated throughout the development process. This multidisciplinary approach ensures that ethical and safety considerations are not merely regulatory hurdles to overcome but fundamental design parameters that shape the development trajectory of clinically viable ELMs.
Immunological considerations represent a primary safety concern for ELM clinical applications. The potential for adverse immune responses, including inflammation, rejection, or unexpected immune modulation, requires comprehensive pre-clinical testing protocols. Establishing standardized immunological safety assessments specific to ELMs remains a critical gap in the regulatory landscape.
Containment and biosafety present another significant challenge. Unlike conventional materials, ELMs contain living components capable of replication, mutation, or horizontal gene transfer. Implementing robust biocontainment strategies—such as kill switches, auxotrophy, or orthogonal genetic systems—must be prioritized before clinical translation. These safeguards must demonstrate reliability across the intended lifespan of the implanted ELM.
Informed consent procedures for ELM-based therapies demand special attention. Patients must understand not only the potential benefits but also the unique risks associated with materials containing engineered living components. This includes comprehension of the material's dynamic nature, potential for autonomous behavior, and long-term persistence in the body.
Long-term monitoring protocols represent an essential ethical requirement for early ELM clinical trials. The potential for delayed immune responses or unforeseen biological interactions necessitates extended follow-up periods beyond those typically required for conventional therapeutics. Establishing appropriate biomarkers for monitoring ELM-host interactions remains an active research priority.
Equitable access considerations must be addressed early in the development pipeline. The complex manufacturing requirements for ELMs could potentially limit their availability to specialized medical centers, creating disparities in access. Developing scalable, cost-effective production methods should be pursued alongside the core technology development.
Stakeholder engagement across multiple disciplines—including immunologists, ethicists, regulatory experts, and patient advocates—should be integrated throughout the development process. This multidisciplinary approach ensures that ethical and safety considerations are not merely regulatory hurdles to overcome but fundamental design parameters that shape the development trajectory of clinically viable ELMs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!