Characterization Techniques For Living And Non-Living Components Of ELMs.
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ELM Characterization Background and Objectives
Engineered Living Materials (ELMs) represent a revolutionary frontier in materials science, combining biological components with non-living matrices to create functional materials with unprecedented capabilities. The characterization of these complex hybrid systems presents unique challenges that traditional analytical methods cannot fully address. This technical exploration aims to comprehensively examine the evolution of characterization techniques specifically developed for ELMs, tracing their historical development from early rudimentary approaches to current sophisticated methodologies.
The field of ELMs emerged at the intersection of synthetic biology, materials science, and bioengineering approximately two decades ago, with significant acceleration in research occurring within the last decade. Initial characterization efforts focused primarily on separate analysis of biological and material components, failing to capture the critical interactions at their interfaces. As the field matured, integrated approaches became necessary to understand the dynamic nature of these living-nonliving composites.
Current characterization objectives center on developing non-destructive, real-time monitoring techniques that can simultaneously assess biological viability, metabolic activity, and material properties. These techniques must function across multiple length scales—from molecular interactions to macroscopic material performance—while maintaining temporal resolution sufficient to capture dynamic biological processes. Additionally, standardization of characterization protocols represents a critical goal to enable meaningful comparison between different ELM systems developed across research groups worldwide.
The technical trajectory of ELM characterization has been shaped by advances in adjacent fields, particularly biomedical imaging, microfluidics, and sensor technology. Innovations such as fluorescence lifetime imaging microscopy (FLIM), surface-enhanced Raman spectroscopy (SERS), and embedded biosensors have been adapted specifically for ELM applications. These adaptations reflect the unique requirements of maintaining living component functionality while obtaining meaningful materials data.
Looking forward, characterization technology development aims to address several persistent challenges: minimizing perturbation of living components during measurement, achieving sufficient spatial and temporal resolution to capture heterogeneous biological activity, and developing predictive models that can correlate early-stage measurements with long-term material performance. The ultimate objective is to establish a comprehensive characterization framework that enables rational design of ELMs with predictable properties and behaviors.
This technical investigation will examine both established and emerging characterization methodologies, evaluating their capabilities, limitations, and potential for further development to meet the evolving needs of ELM research and applications. Particular attention will be given to techniques that bridge traditional disciplinary boundaries and offer insights into the unique emergent properties that arise from living-nonliving interfaces.
The field of ELMs emerged at the intersection of synthetic biology, materials science, and bioengineering approximately two decades ago, with significant acceleration in research occurring within the last decade. Initial characterization efforts focused primarily on separate analysis of biological and material components, failing to capture the critical interactions at their interfaces. As the field matured, integrated approaches became necessary to understand the dynamic nature of these living-nonliving composites.
Current characterization objectives center on developing non-destructive, real-time monitoring techniques that can simultaneously assess biological viability, metabolic activity, and material properties. These techniques must function across multiple length scales—from molecular interactions to macroscopic material performance—while maintaining temporal resolution sufficient to capture dynamic biological processes. Additionally, standardization of characterization protocols represents a critical goal to enable meaningful comparison between different ELM systems developed across research groups worldwide.
The technical trajectory of ELM characterization has been shaped by advances in adjacent fields, particularly biomedical imaging, microfluidics, and sensor technology. Innovations such as fluorescence lifetime imaging microscopy (FLIM), surface-enhanced Raman spectroscopy (SERS), and embedded biosensors have been adapted specifically for ELM applications. These adaptations reflect the unique requirements of maintaining living component functionality while obtaining meaningful materials data.
Looking forward, characterization technology development aims to address several persistent challenges: minimizing perturbation of living components during measurement, achieving sufficient spatial and temporal resolution to capture heterogeneous biological activity, and developing predictive models that can correlate early-stage measurements with long-term material performance. The ultimate objective is to establish a comprehensive characterization framework that enables rational design of ELMs with predictable properties and behaviors.
This technical investigation will examine both established and emerging characterization methodologies, evaluating their capabilities, limitations, and potential for further development to meet the evolving needs of ELM research and applications. Particular attention will be given to techniques that bridge traditional disciplinary boundaries and offer insights into the unique emergent properties that arise from living-nonliving interfaces.
Market Applications and Demand Analysis for ELM Technologies
The market for Extracellular-Like Materials (ELMs) characterization technologies is experiencing significant growth driven by advancements in biomaterials research, tissue engineering, and regenerative medicine. Current market analysis indicates strong demand across pharmaceutical, biotechnology, and medical device sectors, where precise characterization of both living and non-living ELM components is critical for product development and regulatory approval.
Healthcare applications represent the largest market segment, with hospitals and clinical research organizations increasingly adopting ELM characterization technologies for diagnostic purposes and personalized medicine approaches. The ability to accurately distinguish between living cellular components and non-living matrix elements provides valuable insights for disease modeling and therapeutic development.
The research and academic sector constitutes another substantial market, with universities and research institutions investing in advanced characterization equipment to further fundamental understanding of cell-matrix interactions. This segment is particularly focused on high-resolution imaging and spectroscopic techniques that can analyze ELM components without disrupting their native structure.
Industrial applications are emerging rapidly, especially in the biofabrication and bioprinting sectors. Companies developing artificial tissues and organs require sophisticated characterization methods to ensure proper integration of living and non-living components, driving demand for multi-modal analysis platforms that can provide comprehensive structural and functional information.
Geographically, North America leads the market due to substantial research funding and presence of major biotechnology companies, followed by Europe and Asia-Pacific. The latter region is experiencing the fastest growth rate as countries like China, Japan, and South Korea increase investments in biomedical research infrastructure.
Market forecasts suggest a compound annual growth rate exceeding the broader scientific instrumentation market, with particular acceleration in portable and automated characterization systems. This trend reflects the growing need for point-of-care applications and high-throughput screening capabilities in pharmaceutical development.
Customer requirements are evolving toward integrated solutions that combine multiple characterization modalities with advanced data analytics. End-users increasingly demand technologies that can perform real-time, non-destructive analysis of ELM components while maintaining sample viability for longitudinal studies.
Regulatory considerations are significantly influencing market dynamics, as agencies worldwide establish guidelines for characterizing biomaterials intended for clinical applications. This regulatory landscape is creating opportunities for technologies that can provide standardized, reproducible characterization data suitable for submission in approval processes.
Healthcare applications represent the largest market segment, with hospitals and clinical research organizations increasingly adopting ELM characterization technologies for diagnostic purposes and personalized medicine approaches. The ability to accurately distinguish between living cellular components and non-living matrix elements provides valuable insights for disease modeling and therapeutic development.
The research and academic sector constitutes another substantial market, with universities and research institutions investing in advanced characterization equipment to further fundamental understanding of cell-matrix interactions. This segment is particularly focused on high-resolution imaging and spectroscopic techniques that can analyze ELM components without disrupting their native structure.
Industrial applications are emerging rapidly, especially in the biofabrication and bioprinting sectors. Companies developing artificial tissues and organs require sophisticated characterization methods to ensure proper integration of living and non-living components, driving demand for multi-modal analysis platforms that can provide comprehensive structural and functional information.
Geographically, North America leads the market due to substantial research funding and presence of major biotechnology companies, followed by Europe and Asia-Pacific. The latter region is experiencing the fastest growth rate as countries like China, Japan, and South Korea increase investments in biomedical research infrastructure.
Market forecasts suggest a compound annual growth rate exceeding the broader scientific instrumentation market, with particular acceleration in portable and automated characterization systems. This trend reflects the growing need for point-of-care applications and high-throughput screening capabilities in pharmaceutical development.
Customer requirements are evolving toward integrated solutions that combine multiple characterization modalities with advanced data analytics. End-users increasingly demand technologies that can perform real-time, non-destructive analysis of ELM components while maintaining sample viability for longitudinal studies.
Regulatory considerations are significantly influencing market dynamics, as agencies worldwide establish guidelines for characterizing biomaterials intended for clinical applications. This regulatory landscape is creating opportunities for technologies that can provide standardized, reproducible characterization data suitable for submission in approval processes.
Current Challenges in Distinguishing ELM Components
Despite significant advancements in characterization techniques for Extracellular Liquid Matrices (ELMs), researchers continue to face substantial challenges in accurately distinguishing between living and non-living components. The fundamental difficulty lies in the complex, heterogeneous nature of ELMs, where biological and non-biological elements exist in intricate relationships that often defy simple categorization methods.
Current analytical approaches struggle with resolution limitations when examining nanoscale structures within ELMs. Conventional microscopy techniques, including scanning electron microscopy (SEM) and transmission electron microscopy (TEM), while powerful for structural analysis, frequently require sample preparation methods that can alter the native state of living components, leading to potential mischaracterization of the true biological status of ELM constituents.
Spectroscopic methods present another significant challenge area. While techniques such as Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) offer non-destructive analysis capabilities, they often lack the sensitivity required to differentiate between biomolecules in their active state versus those that have degraded or become non-functional. The spectral signatures of living versus recently deceased cellular components can be remarkably similar, creating ambiguity in interpretation.
Molecular probes designed to selectively bind to living components frequently encounter specificity issues when applied to complex ELM environments. Cross-reactivity with non-living components that retain certain structural features of their living counterparts leads to false positive results. Conversely, some viable but metabolically quiescent components may fail to interact with viability probes, resulting in false negatives.
The dynamic nature of the living-non-living continuum presents perhaps the most fundamental conceptual challenge. Many ELM components exist in intermediate states that defy binary classification. Extracellular vesicles, for instance, contain biological molecules and can mediate cellular processes despite lacking independent metabolic activity. Similarly, viral particles occupy a gray area between living and non-living classifications, further complicating characterization efforts.
Time-dependent degradation processes add another layer of complexity. Components that were once living undergo gradual transitions to non-living states, with different molecular constituents degrading at varying rates. This temporal heterogeneity means that a single ELM sample may contain components spanning the entire spectrum from fully viable to completely degraded, with numerous intermediate states represented simultaneously.
Emerging technologies like cryo-electron tomography and super-resolution microscopy offer promising avenues for addressing these challenges, but require significant methodological refinement before they can reliably distinguish the full spectrum of living and non-living ELM components in their native contexts.
Current analytical approaches struggle with resolution limitations when examining nanoscale structures within ELMs. Conventional microscopy techniques, including scanning electron microscopy (SEM) and transmission electron microscopy (TEM), while powerful for structural analysis, frequently require sample preparation methods that can alter the native state of living components, leading to potential mischaracterization of the true biological status of ELM constituents.
Spectroscopic methods present another significant challenge area. While techniques such as Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) offer non-destructive analysis capabilities, they often lack the sensitivity required to differentiate between biomolecules in their active state versus those that have degraded or become non-functional. The spectral signatures of living versus recently deceased cellular components can be remarkably similar, creating ambiguity in interpretation.
Molecular probes designed to selectively bind to living components frequently encounter specificity issues when applied to complex ELM environments. Cross-reactivity with non-living components that retain certain structural features of their living counterparts leads to false positive results. Conversely, some viable but metabolically quiescent components may fail to interact with viability probes, resulting in false negatives.
The dynamic nature of the living-non-living continuum presents perhaps the most fundamental conceptual challenge. Many ELM components exist in intermediate states that defy binary classification. Extracellular vesicles, for instance, contain biological molecules and can mediate cellular processes despite lacking independent metabolic activity. Similarly, viral particles occupy a gray area between living and non-living classifications, further complicating characterization efforts.
Time-dependent degradation processes add another layer of complexity. Components that were once living undergo gradual transitions to non-living states, with different molecular constituents degrading at varying rates. This temporal heterogeneity means that a single ELM sample may contain components spanning the entire spectrum from fully viable to completely degraded, with numerous intermediate states represented simultaneously.
Emerging technologies like cryo-electron tomography and super-resolution microscopy offer promising avenues for addressing these challenges, but require significant methodological refinement before they can reliably distinguish the full spectrum of living and non-living ELM components in their native contexts.
Leading Research Institutions and Companies in ELM Analysis
The characterization of Extracellular Large Molecules (ELMs) is currently in a growth phase, with the market expanding as researchers recognize the importance of understanding both living and non-living ELM components. The global market is estimated to reach significant value due to applications in pharmaceutical development, diagnostics, and materials science. Technologically, the field demonstrates varying maturity levels across different characterization approaches. Leading companies like FEI Co. offer advanced microscopy solutions for high-resolution imaging, while Cytokinetics and Millennium Pharmaceuticals (now Takeda Oncology) focus on biological applications. Academic institutions including Harvard, Oxford Nanoimaging, and Swiss Federal Institute of Technology are driving innovation through novel imaging techniques. Companies like ONI and GreenVision Systems are developing next-generation spectroscopic methods, indicating a competitive landscape balanced between established microscopy providers and emerging technology innovators.
FEI Co.
Technical Solution: FEI Co. has developed comprehensive characterization solutions for ELMs through their advanced electron microscopy platforms. Their technology combines cryo-electron microscopy (cryo-EM) with specialized sample preparation techniques to preserve the native structure of both living and non-living ELM components. FEI's Vitrobot system enables vitrification of biological samples without ice crystal formation, maintaining structural integrity at the molecular level. Their Titan Krios microscope platform achieves sub-angstrom resolution for detailed structural analysis of ELM components, while their correlative light and electron microscopy (CLEM) workflow allows researchers to bridge the gap between living cell dynamics and high-resolution structural information. FEI has also pioneered automated data collection and processing systems that enable high-throughput analysis of ELM samples, facilitating large-scale studies of matrix composition and organization. Their DualBeam systems combine focused ion beam (FIB) with scanning electron microscopy (SEM) to enable 3D reconstruction of ELM architectures with nanometer precision.
Strengths: Unparalleled resolution capabilities allow visualization of molecular-level details in ELM components; integrated workflows from sample preparation to analysis streamline research processes. Weaknesses: High equipment and maintenance costs; requires specialized facilities and technical expertise; sample preparation for electron microscopy can potentially alter native ELM structures.
Oxford Nanoimaging Ltd.
Technical Solution: Oxford Nanoimaging has developed advanced super-resolution microscopy techniques specifically designed for characterizing both living and non-living components of Extracellular Matrix-like Substances (ELMs). Their proprietary NanoImager platform utilizes Single-Molecule Localization Microscopy (SMLM) techniques including STORM and PALM to achieve resolutions down to 20nm, enabling detailed visualization of ELM components at the nanoscale level. The company has pioneered drift correction algorithms and specialized fluorophores that maintain viability in living samples while providing high photon yields for precise localization. Their technology incorporates multi-color imaging capabilities that allow simultaneous visualization of different ELM components, facilitating the study of interactions between living cells and their surrounding matrix. Recent developments include integration of machine learning algorithms for automated component identification and classification within complex ELM structures.
Strengths: Superior spatial resolution (20nm) allows visualization of nanoscale ELM structures impossible with conventional microscopy; specialized fluorophores maintain cell viability during extended imaging sessions. Weaknesses: High equipment costs limit accessibility; requires specialized training for operation and data interpretation; imaging depth remains limited compared to other techniques.
Key Scientific Breakthroughs in ELM Component Differentiation
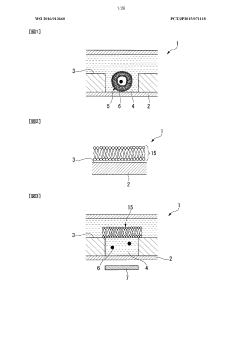
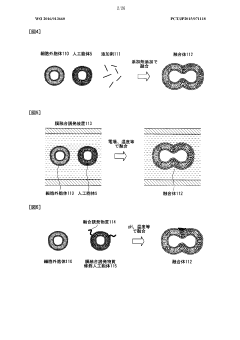
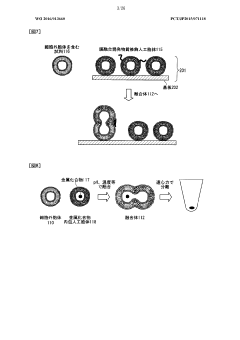
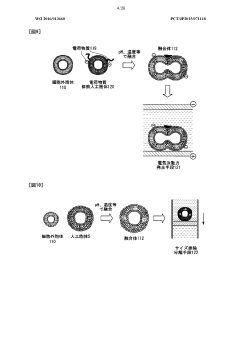
Lipid membrane structure, lipid-membrane-structure-immobilization carrier, and method for fusing cells
PatentWO2016013660A1
Innovation
- A lipid membrane structure containing a fusogenic lipid that can fuse with vesicles having a lipid bilayer membrane, and a lipid membrane structure-immobilized carrier, which enables simpler and more efficient fusion, separation, detection, and transfer of cell bodies by membrane fusion, using techniques like external forces, size, weight, and affinity.
Standardization and Validation Protocols for ELM Analysis
The standardization and validation of characterization techniques for Engineered Living Materials (ELMs) represents a critical challenge in advancing this interdisciplinary field. Current analytical approaches often suffer from inconsistency across laboratories, making reproducibility and comparative analysis difficult. Establishing robust protocols requires consideration of both living components (cells, microorganisms) and non-living matrices (scaffolds, substrates) that comprise these complex materials.
A comprehensive standardization framework must address sample preparation methods that preserve biological activity while enabling accurate physical characterization. This includes protocols for fixation, sectioning, and staining that minimize artifacts while maintaining cellular viability assessment capabilities. Standardized approaches for quantifying cell distribution, density, and metabolic activity within ELM structures are particularly important for ensuring consistent performance evaluation.
For non-living components, standardized protocols should include mechanical testing parameters (compression, tension, shear), degradation assessment methods, and surface characterization techniques. These must account for the dynamic nature of ELMs, where material properties may change over time due to cellular activity and environmental interactions. Validation methods should incorporate reference materials with known properties to calibrate instruments and verify measurement accuracy.
Interlaboratory validation studies represent a crucial step toward establishing reliable analytical methods. These collaborative efforts should involve multiple research groups performing identical characterization procedures on standardized ELM samples, with statistical analysis of variability to identify protocol weaknesses. Such studies have successfully improved standardization in related fields like tissue engineering and biomaterials science.
Quality control metrics must be developed specifically for ELMs, including acceptance criteria for cellular viability, material homogeneity, and functional performance. These metrics should be adaptable to different ELM applications while maintaining core validation principles. Documentation standards for reporting characterization results are equally important, ensuring that published data includes all parameters necessary for reproduction.
Regulatory considerations must also be integrated into validation protocols, particularly for ELMs intended for biomedical applications. This includes alignment with existing standards from organizations like ASTM International and ISO, while acknowledging the unique challenges posed by living-non-living interfaces in these materials. Developing consensus standards through collaborative efforts between academic, industrial, and regulatory stakeholders will accelerate the translation of ELM technologies.
A comprehensive standardization framework must address sample preparation methods that preserve biological activity while enabling accurate physical characterization. This includes protocols for fixation, sectioning, and staining that minimize artifacts while maintaining cellular viability assessment capabilities. Standardized approaches for quantifying cell distribution, density, and metabolic activity within ELM structures are particularly important for ensuring consistent performance evaluation.
For non-living components, standardized protocols should include mechanical testing parameters (compression, tension, shear), degradation assessment methods, and surface characterization techniques. These must account for the dynamic nature of ELMs, where material properties may change over time due to cellular activity and environmental interactions. Validation methods should incorporate reference materials with known properties to calibrate instruments and verify measurement accuracy.
Interlaboratory validation studies represent a crucial step toward establishing reliable analytical methods. These collaborative efforts should involve multiple research groups performing identical characterization procedures on standardized ELM samples, with statistical analysis of variability to identify protocol weaknesses. Such studies have successfully improved standardization in related fields like tissue engineering and biomaterials science.
Quality control metrics must be developed specifically for ELMs, including acceptance criteria for cellular viability, material homogeneity, and functional performance. These metrics should be adaptable to different ELM applications while maintaining core validation principles. Documentation standards for reporting characterization results are equally important, ensuring that published data includes all parameters necessary for reproduction.
Regulatory considerations must also be integrated into validation protocols, particularly for ELMs intended for biomedical applications. This includes alignment with existing standards from organizations like ASTM International and ISO, while acknowledging the unique challenges posed by living-non-living interfaces in these materials. Developing consensus standards through collaborative efforts between academic, industrial, and regulatory stakeholders will accelerate the translation of ELM technologies.
Environmental Impact and Sustainability of ELM Characterization Methods
The environmental impact of characterization techniques for Engineered Living Materials (ELMs) represents a critical consideration as these technologies advance toward commercial applications. Current characterization methods often employ hazardous chemicals, energy-intensive processes, and generate significant waste streams. Microscopy techniques such as SEM and TEM require heavy metal staining agents including uranium acetate and lead citrate, which pose environmental risks during both application and disposal phases. Similarly, spectroscopic methods frequently utilize organic solvents that contribute to air pollution and potential groundwater contamination.
Energy consumption presents another substantial environmental concern, particularly with advanced imaging systems that require continuous power for cryogenic cooling and vacuum maintenance. The carbon footprint of these characterization facilities can be considerable, especially when factoring in the embodied energy of specialized equipment manufacturing and transportation. Recent life cycle assessments indicate that a typical research laboratory utilizing comprehensive ELM characterization protocols may consume energy equivalent to 3-5 residential households.
Waste management challenges are particularly pronounced with biological components of ELMs. Characterization procedures often generate biohazardous materials requiring specialized disposal protocols, including inactivation treatments that themselves carry environmental implications. The intersection of living and non-living components in ELMs creates unique waste streams that existing laboratory waste management systems may not be optimally designed to handle.
Encouragingly, sustainable alternatives are emerging across the characterization landscape. Green chemistry principles are being applied to develop less toxic staining agents and reagents, while equipment manufacturers are increasingly focusing on energy efficiency improvements. Multi-modal characterization approaches that maximize information yield per sample are reducing overall material consumption and waste generation. Several research institutions have pioneered shared instrumentation facilities that optimize equipment utilization and reduce redundant resource consumption.
Looking forward, sustainability metrics for ELM characterization should be standardized and incorporated into research protocols and regulatory frameworks. The development of in-line, non-destructive characterization methods represents a promising direction for minimizing environmental impact while maintaining analytical rigor. As ELMs transition from laboratory curiosities to industrial applications, the environmental footprint of their characterization must be considered alongside their potential environmental benefits.
Energy consumption presents another substantial environmental concern, particularly with advanced imaging systems that require continuous power for cryogenic cooling and vacuum maintenance. The carbon footprint of these characterization facilities can be considerable, especially when factoring in the embodied energy of specialized equipment manufacturing and transportation. Recent life cycle assessments indicate that a typical research laboratory utilizing comprehensive ELM characterization protocols may consume energy equivalent to 3-5 residential households.
Waste management challenges are particularly pronounced with biological components of ELMs. Characterization procedures often generate biohazardous materials requiring specialized disposal protocols, including inactivation treatments that themselves carry environmental implications. The intersection of living and non-living components in ELMs creates unique waste streams that existing laboratory waste management systems may not be optimally designed to handle.
Encouragingly, sustainable alternatives are emerging across the characterization landscape. Green chemistry principles are being applied to develop less toxic staining agents and reagents, while equipment manufacturers are increasingly focusing on energy efficiency improvements. Multi-modal characterization approaches that maximize information yield per sample are reducing overall material consumption and waste generation. Several research institutions have pioneered shared instrumentation facilities that optimize equipment utilization and reduce redundant resource consumption.
Looking forward, sustainability metrics for ELM characterization should be standardized and incorporated into research protocols and regulatory frameworks. The development of in-line, non-destructive characterization methods represents a promising direction for minimizing environmental impact while maintaining analytical rigor. As ELMs transition from laboratory curiosities to industrial applications, the environmental footprint of their characterization must be considered alongside their potential environmental benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!