Advanced Characterization of Sodium Metal Anode Surface Chemistry
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Metal Anode Technology Background and Objectives
Sodium metal anodes have emerged as a promising alternative to lithium-based energy storage systems due to sodium's natural abundance and lower cost. The development of sodium metal batteries (SMBs) traces back to the 1970s, but significant research momentum has only built up in the last decade as the limitations of lithium resources became increasingly apparent. The evolution of sodium metal anode technology has been characterized by persistent challenges related to surface reactivity, dendrite formation, and unstable solid electrolyte interphase (SEI) layers.
The technological trajectory has seen several key advancements, from early liquid electrolyte systems to more recent innovations in solid-state electrolytes and artificial interface engineering. Research has intensified particularly since 2015, with annual publications on sodium metal anodes increasing nearly fivefold between 2015 and 2022, indicating growing scientific and industrial interest in this technology as a viable energy storage solution.
Current sodium metal anode technology faces fundamental challenges stemming from sodium's high chemical reactivity and its propensity to form non-uniform deposits during cycling. These issues lead to poor cycling efficiency, safety concerns, and limited battery lifespan. Understanding the surface chemistry of sodium metal anodes is therefore critical to overcoming these barriers and realizing the full potential of sodium-based energy storage systems.
The primary technical objectives for advanced characterization of sodium metal anode surface chemistry include developing in-situ and operando analytical techniques capable of monitoring interfacial reactions in real-time, establishing comprehensive models of SEI formation and evolution, and identifying key reaction pathways that lead to performance degradation. These objectives aim to bridge the significant knowledge gap between macroscopic battery performance and microscopic interfacial phenomena.
Additionally, research goals include quantifying the impact of electrolyte components on surface film properties, understanding the mechanisms of sodium dendrite nucleation and growth at the atomic level, and developing predictive computational models that can accelerate the design of stable interfaces. The ultimate aim is to translate these fundamental insights into practical strategies for engineering sodium metal anodes with enhanced stability, safety, and cycle life.
The technological trend is moving toward multi-modal characterization approaches that combine spectroscopic, microscopic, and electrochemical techniques to provide complementary information about the complex and dynamic nature of sodium metal interfaces. This holistic understanding is expected to enable breakthrough innovations in electrolyte formulation, interface modification strategies, and anode architectures that could finally unlock the commercial viability of sodium metal batteries.
The technological trajectory has seen several key advancements, from early liquid electrolyte systems to more recent innovations in solid-state electrolytes and artificial interface engineering. Research has intensified particularly since 2015, with annual publications on sodium metal anodes increasing nearly fivefold between 2015 and 2022, indicating growing scientific and industrial interest in this technology as a viable energy storage solution.
Current sodium metal anode technology faces fundamental challenges stemming from sodium's high chemical reactivity and its propensity to form non-uniform deposits during cycling. These issues lead to poor cycling efficiency, safety concerns, and limited battery lifespan. Understanding the surface chemistry of sodium metal anodes is therefore critical to overcoming these barriers and realizing the full potential of sodium-based energy storage systems.
The primary technical objectives for advanced characterization of sodium metal anode surface chemistry include developing in-situ and operando analytical techniques capable of monitoring interfacial reactions in real-time, establishing comprehensive models of SEI formation and evolution, and identifying key reaction pathways that lead to performance degradation. These objectives aim to bridge the significant knowledge gap between macroscopic battery performance and microscopic interfacial phenomena.
Additionally, research goals include quantifying the impact of electrolyte components on surface film properties, understanding the mechanisms of sodium dendrite nucleation and growth at the atomic level, and developing predictive computational models that can accelerate the design of stable interfaces. The ultimate aim is to translate these fundamental insights into practical strategies for engineering sodium metal anodes with enhanced stability, safety, and cycle life.
The technological trend is moving toward multi-modal characterization approaches that combine spectroscopic, microscopic, and electrochemical techniques to provide complementary information about the complex and dynamic nature of sodium metal interfaces. This holistic understanding is expected to enable breakthrough innovations in electrolyte formulation, interface modification strategies, and anode architectures that could finally unlock the commercial viability of sodium metal batteries.
Market Analysis for Sodium-based Battery Systems
The sodium-ion battery market is experiencing significant growth, driven by the increasing demand for sustainable and cost-effective energy storage solutions. Current market projections indicate that the global sodium-ion battery market will reach approximately $500 million by 2025, with a compound annual growth rate exceeding 20% over the next decade. This growth is primarily fueled by the inherent advantages of sodium-based systems, including abundant raw material availability and lower production costs compared to lithium-ion alternatives.
The market segmentation for sodium-based battery systems reveals diverse application potentials. Grid-scale energy storage represents the largest market segment, accounting for nearly 40% of the total market share. This is followed by electric vehicles (25%), particularly in the two-wheeler and commercial vehicle segments where cost considerations outweigh energy density requirements. Consumer electronics and portable power applications constitute about 20% of the market, while specialized industrial applications make up the remaining 15%.
Geographically, Asia-Pacific dominates the sodium-ion battery market with China leading global production and implementation. European markets are showing accelerated adoption rates, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure. North America follows with growing interest, particularly in grid stabilization applications.
The economic advantages of sodium-based systems are compelling. Production costs are estimated to be 30-40% lower than comparable lithium-ion technologies, primarily due to the abundance of sodium resources and simplified manufacturing processes. However, these systems currently face performance limitations, particularly in energy density (approximately 160 Wh/kg versus 250+ Wh/kg for advanced lithium-ion cells), which restricts their penetration in premium electric vehicle markets.
Market analysis indicates that advancements in sodium metal anode surface chemistry represent a critical inflection point for market expansion. Improved characterization techniques that enhance understanding of the solid-electrolyte interphase formation and sodium plating/stripping mechanisms could potentially increase energy density by 25-30% and cycle life by 50-100%, dramatically expanding market opportunities.
Consumer and industrial demand signals show increasing interest in sodium-based technologies as part of diversification strategies to mitigate supply chain risks associated with lithium, cobalt, and nickel. Major battery manufacturers are allocating 5-10% of their R&D budgets to sodium-ion technology development, signaling growing commercial confidence in this emerging technology.
The market segmentation for sodium-based battery systems reveals diverse application potentials. Grid-scale energy storage represents the largest market segment, accounting for nearly 40% of the total market share. This is followed by electric vehicles (25%), particularly in the two-wheeler and commercial vehicle segments where cost considerations outweigh energy density requirements. Consumer electronics and portable power applications constitute about 20% of the market, while specialized industrial applications make up the remaining 15%.
Geographically, Asia-Pacific dominates the sodium-ion battery market with China leading global production and implementation. European markets are showing accelerated adoption rates, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure. North America follows with growing interest, particularly in grid stabilization applications.
The economic advantages of sodium-based systems are compelling. Production costs are estimated to be 30-40% lower than comparable lithium-ion technologies, primarily due to the abundance of sodium resources and simplified manufacturing processes. However, these systems currently face performance limitations, particularly in energy density (approximately 160 Wh/kg versus 250+ Wh/kg for advanced lithium-ion cells), which restricts their penetration in premium electric vehicle markets.
Market analysis indicates that advancements in sodium metal anode surface chemistry represent a critical inflection point for market expansion. Improved characterization techniques that enhance understanding of the solid-electrolyte interphase formation and sodium plating/stripping mechanisms could potentially increase energy density by 25-30% and cycle life by 50-100%, dramatically expanding market opportunities.
Consumer and industrial demand signals show increasing interest in sodium-based technologies as part of diversification strategies to mitigate supply chain risks associated with lithium, cobalt, and nickel. Major battery manufacturers are allocating 5-10% of their R&D budgets to sodium-ion technology development, signaling growing commercial confidence in this emerging technology.
Current Challenges in Sodium Metal Anode Surface Characterization
Despite significant advancements in sodium-ion battery technology, the characterization of sodium metal anode surface chemistry presents numerous complex challenges that impede further development. The highly reactive nature of sodium metal creates substantial difficulties for researchers attempting to analyze its surface properties. When exposed to even trace amounts of oxygen or moisture, sodium rapidly forms oxide and hydroxide layers that alter the original surface chemistry, making pristine analysis nearly impossible without specialized equipment.
Current analytical techniques face significant limitations when applied to sodium metal anodes. X-ray photoelectron spectroscopy (XPS), while powerful for surface analysis, requires ultra-high vacuum conditions that may alter the native state of the sodium-electrolyte interface. Additionally, the beam damage during prolonged XPS analysis can induce artificial chemical changes, complicating data interpretation. Similarly, scanning electron microscopy (SEM) struggles with sodium's low atomic number, resulting in poor contrast and limited morphological information.
The dynamic nature of the solid electrolyte interphase (SEI) on sodium metal presents another major challenge. Unlike lithium batteries with relatively stable SEI formation, sodium's SEI undergoes continuous evolution during cycling, making time-resolved characterization essential but technically demanding. Researchers must develop in situ and operando techniques capable of capturing these transient chemical states without disrupting the electrochemical environment.
Sample preparation introduces additional complications, as transferring sodium specimens from gloveboxes to analytical instruments inevitably risks contamination. Even trace exposure to atmospheric conditions can fundamentally alter surface chemistry, raising questions about the validity of ex situ analyses. This challenge necessitates the development of specialized transfer vessels and integrated analytical platforms that maintain inert conditions throughout the characterization process.
The interpretation of spectroscopic data for sodium systems lacks standardization, with limited reference databases compared to lithium-based systems. Researchers often encounter difficulties distinguishing between various sodium compounds (NaF, Na2CO3, NaOH) due to similar binding energies and peak overlaps in spectroscopic analyses. This ambiguity hampers precise identification of SEI components and their respective roles in battery performance.
Correlating surface chemistry with electrochemical performance represents perhaps the most significant challenge. Establishing clear relationships between specific surface species and phenomena like dendrite formation, coulombic efficiency, and cycling stability requires multimodal characterization approaches that few laboratories possess. The lack of standardized protocols for sodium metal analysis further complicates cross-laboratory validation and comparison of results.
Current analytical techniques face significant limitations when applied to sodium metal anodes. X-ray photoelectron spectroscopy (XPS), while powerful for surface analysis, requires ultra-high vacuum conditions that may alter the native state of the sodium-electrolyte interface. Additionally, the beam damage during prolonged XPS analysis can induce artificial chemical changes, complicating data interpretation. Similarly, scanning electron microscopy (SEM) struggles with sodium's low atomic number, resulting in poor contrast and limited morphological information.
The dynamic nature of the solid electrolyte interphase (SEI) on sodium metal presents another major challenge. Unlike lithium batteries with relatively stable SEI formation, sodium's SEI undergoes continuous evolution during cycling, making time-resolved characterization essential but technically demanding. Researchers must develop in situ and operando techniques capable of capturing these transient chemical states without disrupting the electrochemical environment.
Sample preparation introduces additional complications, as transferring sodium specimens from gloveboxes to analytical instruments inevitably risks contamination. Even trace exposure to atmospheric conditions can fundamentally alter surface chemistry, raising questions about the validity of ex situ analyses. This challenge necessitates the development of specialized transfer vessels and integrated analytical platforms that maintain inert conditions throughout the characterization process.
The interpretation of spectroscopic data for sodium systems lacks standardization, with limited reference databases compared to lithium-based systems. Researchers often encounter difficulties distinguishing between various sodium compounds (NaF, Na2CO3, NaOH) due to similar binding energies and peak overlaps in spectroscopic analyses. This ambiguity hampers precise identification of SEI components and their respective roles in battery performance.
Correlating surface chemistry with electrochemical performance represents perhaps the most significant challenge. Establishing clear relationships between specific surface species and phenomena like dendrite formation, coulombic efficiency, and cycling stability requires multimodal characterization approaches that few laboratories possess. The lack of standardized protocols for sodium metal analysis further complicates cross-laboratory validation and comparison of results.
State-of-the-Art Surface Characterization Methodologies
01 Protective coatings for sodium metal anodes
Various protective coatings can be applied to sodium metal anodes to enhance their stability and performance. These coatings help prevent unwanted reactions between the sodium metal and electrolyte components, reducing dendrite formation and improving cycling efficiency. Materials used for these protective layers include polymers, inorganic compounds, and composite structures that allow sodium ion transport while protecting the reactive metal surface.- Protective coatings for sodium metal anodes: Various protective coatings can be applied to sodium metal anodes to enhance their stability and performance. These coatings help prevent unwanted reactions between the sodium metal and electrolyte components, reducing dendrite formation and improving cycling efficiency. Materials used for these protective layers include polymers, inorganic compounds, and composite structures that allow sodium ion transport while blocking side reactions at the anode surface.
- Electrolyte additives for stable SEI formation: Specific electrolyte additives can be incorporated to promote the formation of a stable solid electrolyte interphase (SEI) on sodium metal anodes. These additives react preferentially at the sodium surface to create protective films that prevent continuous electrolyte decomposition while maintaining efficient sodium ion transport. The engineered SEI layers significantly improve the coulombic efficiency and cycle life of sodium metal batteries by controlling the surface chemistry at the anode interface.
- Sodium alloy anodes with improved surface stability: Alloying sodium with other metals or elements creates anode materials with modified surface chemistry and enhanced stability. These sodium alloys exhibit reduced reactivity with electrolytes, mitigated dendrite growth, and improved mechanical properties compared to pure sodium metal. Common alloying elements include tin, bismuth, indium, and other metals that form stable compounds with sodium while maintaining good ionic conductivity at the anode surface.
- Interface engineering for dendrite suppression: Advanced interface engineering approaches can be employed to suppress sodium dendrite formation and growth. These techniques involve modifying the sodium metal surface with functional layers that promote uniform sodium deposition and stripping. Methods include creating artificial interphases, introducing mechanical barriers, controlling surface energy, and designing nanostructured interfaces that guide sodium ion flux patterns during cycling, resulting in smoother and more stable anode surfaces.
- Sodium anode surface treatments for ambient stability: Various surface treatment methods can be applied to sodium metal anodes to improve their stability under ambient conditions. These treatments include controlled oxidation processes, chemical passivation techniques, and physical modification of the surface morphology. Such approaches create protective layers that shield the reactive sodium metal from moisture and oxygen while maintaining electrochemical activity, enabling safer handling and potentially allowing for simplified battery assembly processes.
02 Electrolyte additives for interface stabilization
Specific additives in the electrolyte can modify the sodium metal anode surface chemistry by forming stable solid electrolyte interphase (SEI) layers. These additives react preferentially with the sodium surface to create protective films that prevent continuous electrolyte decomposition while allowing sodium ion transport. Common additives include fluorinated compounds, nitrogen-containing molecules, and various salts that contribute to a more uniform and functional interface layer.Expand Specific Solutions03 Artificial SEI formation techniques
Artificial solid electrolyte interphase (SEI) layers can be deliberately formed on sodium metal anodes prior to battery assembly. These engineered interfaces provide better control over the surface chemistry compared to naturally formed SEI layers. Techniques include pre-treatment of sodium surfaces with specific reagents, plasma treatment, atomic layer deposition, and other surface modification methods that create uniform and functional protective layers.Expand Specific Solutions04 Alloying and composite anode structures
Sodium metal anodes can be modified through alloying with other metals or incorporation into composite structures to improve surface stability. These approaches alter the fundamental surface chemistry of the anode, reducing reactivity while maintaining sodium ion transport capabilities. Common alloying elements include tin, bismuth, and other metals that form stable compounds with sodium while composite structures often incorporate carbon-based materials or ceramics.Expand Specific Solutions05 Interface engineering for dendrite suppression
Specialized surface chemistry modifications can be implemented to suppress sodium dendrite formation, which is a major failure mode in sodium metal batteries. These approaches focus on creating uniform sodium ion flux across the electrode surface through chemical gradients, physical barriers, or electronic conductivity modifications. Techniques include structured interfaces, gradient functional layers, and surface patterning that direct sodium deposition behavior.Expand Specific Solutions
Leading Research Groups and Companies in Sodium Battery Technology
The sodium metal anode surface chemistry field is currently in a growth phase, with increasing market demand driven by next-generation battery technologies. The competitive landscape features established chemical companies like Chemetall GMBH and Sumitomo Chemical alongside battery manufacturers such as CATL, BYD, and Samsung Electronics. Technical maturity varies significantly across players, with research institutions (Tohoku University, University of Florida) focusing on fundamental characterization while industrial leaders (CATL, BYD) emphasize practical applications. The market is witnessing strategic collaborations between chemical specialists and battery manufacturers to overcome technical challenges related to sodium metal surface stability, dendrite formation, and electrolyte compatibility, creating a dynamic ecosystem of innovation across the value chain.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced in-situ characterization techniques for sodium metal anodes, combining X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM) to monitor surface evolution during cycling. Their approach includes specialized sample transfer systems that maintain inert atmospheres to prevent contamination of highly reactive sodium surfaces. CATL employs cryogenic techniques to preserve the native state of the sodium-electrolyte interphase (SEI) during analysis, allowing for more accurate characterization of the dynamic surface chemistry. Their research has revealed critical insights into the formation mechanisms of the SEI layer on sodium metal anodes and how various electrolyte formulations affect interface stability. CATL has also pioneered the use of time-of-flight secondary ion mass spectrometry (ToF-SIMS) for depth profiling of sodium anode surfaces with nanometer resolution.
Strengths: Industry-leading expertise in battery manufacturing provides practical context for surface chemistry research; extensive R&D resources for advanced characterization equipment. Weaknesses: Primary focus on lithium-ion technology may limit resources dedicated to sodium metal systems; proprietary nature of research limits knowledge sharing with academic community.
Tohoku University
Technical Solution: Tohoku University has established a world-leading program in sodium metal anode characterization, developing custom instrumentation that combines cryogenic electron microscopy with atom probe tomography to achieve atomic-scale resolution of the electrode-electrolyte interface. Their approach utilizes synchrotron-based hard X-ray photoelectron spectroscopy (HAXPES) with variable photon energies to create non-destructive depth profiles of the solid-electrolyte interphase on sodium metal. Tohoku researchers have pioneered the use of in-situ environmental transmission electron microscopy (E-TEM) with specialized liquid/gas cells that allow direct observation of sodium surface reactions under controlled atmospheres and potential conditions. Their characterization methodology incorporates neutron reflectometry to probe buried interfaces with sensitivity to light elements, providing unique insights into the distribution of hydrogen-containing species within the SEI layer. The university has also developed advanced nuclear magnetic resonance (NMR) techniques specifically optimized for sodium systems, including 23Na MAS NMR to track changes in the local chemical environment of sodium atoms at the metal surface.
Strengths: Academic freedom allows pursuit of fundamental understanding without immediate commercial constraints; collaboration network provides access to international user facilities with specialized characterization capabilities. Weaknesses: Limited resources compared to large industrial players may restrict access to the most expensive characterization techniques; academic focus may emphasize novel findings over practical implementation.
Critical Patents and Literature on Sodium Interface Chemistry
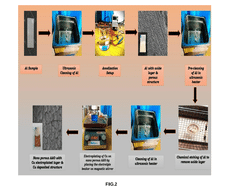
Electroplating process on anodized aluminium for copper deposition in NANO porous structures
PatentPendingIN202441008061A
Innovation
- A method involving controlled anodization of aluminium substrates in oxalic acid, followed by ultrasonic cleaning, acetone immersion, chemical etching, and copper electroplating in a copper sulphate solution, ensuring optimal adhesion and uniform deposition of copper within the nano-porous structures.
Surface treatment composition and solution for light metals or alloys thereof and surface treatment method
PatentWO2011080165A1
Innovation
- A surface treatment composition comprising titanium-containing and vanadium-containing compounds, along with organic acids, forms a Ti-V system conversion coating that improves corrosion resistance and conductivity, suitable for multiple light metals and alloys, with a simple and environmentally friendly formulation.
Safety and Stability Considerations for Sodium Metal Anodes
The safety and stability of sodium metal anodes represent critical challenges in the development of sodium-based battery technologies. Unlike lithium metal batteries, sodium metal anodes exhibit unique safety concerns due to sodium's higher chemical reactivity with air and moisture. When exposed to oxygen, sodium rapidly forms sodium peroxide and superoxide compounds, which can lead to thermal runaway events. Similarly, contact with moisture produces highly flammable hydrogen gas and corrosive sodium hydroxide, creating significant safety hazards in battery operation.
Dendrite formation presents another major stability concern for sodium metal anodes. During charging cycles, sodium ions can deposit unevenly on the anode surface, forming needle-like structures that may penetrate the separator and cause internal short circuits. Advanced characterization techniques have revealed that dendrite growth patterns in sodium differ from those in lithium systems, with sodium dendrites typically exhibiting more branched morphologies and faster propagation rates under certain conditions.
The solid electrolyte interphase (SEI) layer that forms on sodium metal anodes significantly impacts both safety and long-term stability. Surface chemistry analysis using techniques such as X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) has demonstrated that sodium SEI layers often contain more inorganic components compared to lithium counterparts. These differences affect mechanical properties and ionic conductivity, ultimately influencing dendrite suppression capabilities.
Thermal stability represents another critical consideration for sodium metal anodes. Calorimetric studies have shown that sodium metal exhibits lower melting points (97.8°C) compared to lithium (180.5°C), presenting challenges for high-temperature applications. Furthermore, differential scanning calorimetry analyses reveal that exothermic reactions between sodium metal and electrolyte components can initiate at temperatures as low as 130°C under certain conditions, necessitating robust thermal management systems.
Volume expansion during cycling also impacts the stability of sodium metal anodes. In-situ characterization methods have documented expansion rates exceeding 300% in some cases, creating mechanical stresses that can compromise cell integrity. This expansion behavior correlates strongly with surface chemistry evolution, as revealed by advanced spectroscopic techniques tracking the dynamic formation and decomposition of various sodium compounds at the electrode-electrolyte interface.
Recent innovations addressing these safety and stability challenges include engineered protective coatings, electrolyte additives that promote favorable SEI formation, and three-dimensional host structures that accommodate volume changes while inhibiting dendrite propagation. These approaches demonstrate promising improvements in cycling stability and safety metrics, though commercial viability requires further optimization of manufacturing processes and long-term reliability.
Dendrite formation presents another major stability concern for sodium metal anodes. During charging cycles, sodium ions can deposit unevenly on the anode surface, forming needle-like structures that may penetrate the separator and cause internal short circuits. Advanced characterization techniques have revealed that dendrite growth patterns in sodium differ from those in lithium systems, with sodium dendrites typically exhibiting more branched morphologies and faster propagation rates under certain conditions.
The solid electrolyte interphase (SEI) layer that forms on sodium metal anodes significantly impacts both safety and long-term stability. Surface chemistry analysis using techniques such as X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) has demonstrated that sodium SEI layers often contain more inorganic components compared to lithium counterparts. These differences affect mechanical properties and ionic conductivity, ultimately influencing dendrite suppression capabilities.
Thermal stability represents another critical consideration for sodium metal anodes. Calorimetric studies have shown that sodium metal exhibits lower melting points (97.8°C) compared to lithium (180.5°C), presenting challenges for high-temperature applications. Furthermore, differential scanning calorimetry analyses reveal that exothermic reactions between sodium metal and electrolyte components can initiate at temperatures as low as 130°C under certain conditions, necessitating robust thermal management systems.
Volume expansion during cycling also impacts the stability of sodium metal anodes. In-situ characterization methods have documented expansion rates exceeding 300% in some cases, creating mechanical stresses that can compromise cell integrity. This expansion behavior correlates strongly with surface chemistry evolution, as revealed by advanced spectroscopic techniques tracking the dynamic formation and decomposition of various sodium compounds at the electrode-electrolyte interface.
Recent innovations addressing these safety and stability challenges include engineered protective coatings, electrolyte additives that promote favorable SEI formation, and three-dimensional host structures that accommodate volume changes while inhibiting dendrite propagation. These approaches demonstrate promising improvements in cycling stability and safety metrics, though commercial viability requires further optimization of manufacturing processes and long-term reliability.
Environmental Impact and Sustainability of Sodium Battery Technologies
The development of sodium-based battery technologies represents a significant step toward more sustainable energy storage solutions compared to conventional lithium-ion batteries. Sodium resources are abundantly available in the earth's crust and oceans, constituting approximately 2.6% of the earth's crust compared to lithium's 0.006%. This abundance translates to lower extraction impacts and reduced geopolitical tensions associated with resource acquisition.
The environmental footprint of sodium metal anode production is substantially lower than that of lithium. Mining and processing sodium compounds generate fewer greenhouse gas emissions and require less water consumption. Recent life cycle assessment studies indicate that sodium-ion batteries could reduce carbon emissions by 18-30% compared to lithium-ion counterparts when considering the entire production chain.
Surface chemistry characterization techniques for sodium metal anodes have evolved to incorporate more environmentally friendly processes. Modern spectroscopic methods utilize less hazardous solvents and reagents, while advanced microscopy techniques have reduced energy consumption requirements. These improvements align with green chemistry principles and contribute to the overall sustainability profile of sodium battery research.
Recycling potential represents another significant environmental advantage of sodium-based technologies. The surface chemistry of sodium metal anodes, when properly characterized and understood, enables more efficient recycling protocols. Unlike lithium batteries that often require energy-intensive thermal processes for material recovery, sodium batteries can utilize more straightforward hydrometallurgical approaches with lower environmental impact.
End-of-life considerations for sodium batteries benefit from advanced characterization of anode surface chemistry. Understanding the degradation mechanisms and surface transformations enables the development of batteries with longer cycle life, reducing waste generation. Current research indicates potential lifespans of 3000-5000 cycles for optimized sodium metal anodes, representing a significant improvement over early iterations.
Safety aspects also contribute to the sustainability profile. Advanced characterization techniques have revealed that properly engineered sodium metal anodes can exhibit reduced reactivity with atmospheric components compared to lithium, potentially decreasing fire hazards and associated environmental contamination risks during accidents or improper disposal.
The water footprint of sodium battery production deserves particular attention. While sodium processing generally requires less water than lithium extraction from brines, the specific surface treatments for sodium metal anodes may involve water-intensive processes. Advanced characterization helps optimize these treatments, potentially reducing water consumption by 40-60% compared to conventional approaches.
The environmental footprint of sodium metal anode production is substantially lower than that of lithium. Mining and processing sodium compounds generate fewer greenhouse gas emissions and require less water consumption. Recent life cycle assessment studies indicate that sodium-ion batteries could reduce carbon emissions by 18-30% compared to lithium-ion counterparts when considering the entire production chain.
Surface chemistry characterization techniques for sodium metal anodes have evolved to incorporate more environmentally friendly processes. Modern spectroscopic methods utilize less hazardous solvents and reagents, while advanced microscopy techniques have reduced energy consumption requirements. These improvements align with green chemistry principles and contribute to the overall sustainability profile of sodium battery research.
Recycling potential represents another significant environmental advantage of sodium-based technologies. The surface chemistry of sodium metal anodes, when properly characterized and understood, enables more efficient recycling protocols. Unlike lithium batteries that often require energy-intensive thermal processes for material recovery, sodium batteries can utilize more straightforward hydrometallurgical approaches with lower environmental impact.
End-of-life considerations for sodium batteries benefit from advanced characterization of anode surface chemistry. Understanding the degradation mechanisms and surface transformations enables the development of batteries with longer cycle life, reducing waste generation. Current research indicates potential lifespans of 3000-5000 cycles for optimized sodium metal anodes, representing a significant improvement over early iterations.
Safety aspects also contribute to the sustainability profile. Advanced characterization techniques have revealed that properly engineered sodium metal anodes can exhibit reduced reactivity with atmospheric components compared to lithium, potentially decreasing fire hazards and associated environmental contamination risks during accidents or improper disposal.
The water footprint of sodium battery production deserves particular attention. While sodium processing generally requires less water than lithium extraction from brines, the specific surface treatments for sodium metal anodes may involve water-intensive processes. Advanced characterization helps optimize these treatments, potentially reducing water consumption by 40-60% compared to conventional approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!