Influence of Current Density on Sodium Metal Deposition Morphology
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Battery Technology Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The global lithium-ion battery market has been experiencing exponential growth, projected to reach $129.3 billion by 2027, yet concerns about lithium supply constraints and geopolitical distribution have intensified the search for alternative energy storage technologies. Sodium, being the sixth most abundant element in the Earth's crust and widely distributed geographically, presents a sustainable solution to these challenges.
The evolution of sodium battery technology can be traced back to the 1970s when initial research was conducted alongside lithium-ion batteries. However, the commercial success of lithium-ion batteries led to a temporary decline in sodium battery research. The resurgence of interest in sodium-ion technology began around 2010, driven by increasing concerns about lithium resource limitations and cost escalations.
Current density plays a critical role in sodium metal deposition morphology, which directly impacts the performance, safety, and lifespan of sodium-based batteries. Unlike lithium, sodium has distinct electrochemical properties, including a lower melting point (97.7°C vs. 180.5°C for lithium), larger ionic radius (1.02Å vs. 0.76Å for lithium), and different coordination preferences with electrolyte molecules. These intrinsic properties result in unique deposition behaviors that require specialized understanding and engineering approaches.
The technical objective of this research is to comprehensively understand how current density influences sodium metal deposition patterns, dendrite formation mechanisms, and overall electrochemical performance. This understanding is crucial for developing strategies to achieve uniform sodium deposition, mitigate dendrite growth, and enhance the cycling stability of sodium metal anodes.
Recent advancements in characterization techniques, including in-situ transmission electron microscopy, atomic force microscopy, and synchrotron-based X-ray techniques, have enabled researchers to observe sodium deposition processes at unprecedented resolution. These tools provide valuable insights into the nucleation, growth, and morphological evolution of sodium deposits under various current density conditions.
The technology trajectory indicates a shift from empirical approaches to mechanism-based strategies for controlling sodium deposition. Early research focused primarily on electrolyte formulations, while recent efforts have expanded to include advanced electrode architectures, artificial solid-electrolyte interphases, and intelligent charging protocols that adapt to the unique properties of sodium metal anodes.
Understanding and controlling the influence of current density on sodium metal deposition represents a critical milestone in the development of high-performance sodium-based energy storage systems, potentially enabling a more sustainable and economically viable alternative to conventional lithium-ion technology.
The evolution of sodium battery technology can be traced back to the 1970s when initial research was conducted alongside lithium-ion batteries. However, the commercial success of lithium-ion batteries led to a temporary decline in sodium battery research. The resurgence of interest in sodium-ion technology began around 2010, driven by increasing concerns about lithium resource limitations and cost escalations.
Current density plays a critical role in sodium metal deposition morphology, which directly impacts the performance, safety, and lifespan of sodium-based batteries. Unlike lithium, sodium has distinct electrochemical properties, including a lower melting point (97.7°C vs. 180.5°C for lithium), larger ionic radius (1.02Å vs. 0.76Å for lithium), and different coordination preferences with electrolyte molecules. These intrinsic properties result in unique deposition behaviors that require specialized understanding and engineering approaches.
The technical objective of this research is to comprehensively understand how current density influences sodium metal deposition patterns, dendrite formation mechanisms, and overall electrochemical performance. This understanding is crucial for developing strategies to achieve uniform sodium deposition, mitigate dendrite growth, and enhance the cycling stability of sodium metal anodes.
Recent advancements in characterization techniques, including in-situ transmission electron microscopy, atomic force microscopy, and synchrotron-based X-ray techniques, have enabled researchers to observe sodium deposition processes at unprecedented resolution. These tools provide valuable insights into the nucleation, growth, and morphological evolution of sodium deposits under various current density conditions.
The technology trajectory indicates a shift from empirical approaches to mechanism-based strategies for controlling sodium deposition. Early research focused primarily on electrolyte formulations, while recent efforts have expanded to include advanced electrode architectures, artificial solid-electrolyte interphases, and intelligent charging protocols that adapt to the unique properties of sodium metal anodes.
Understanding and controlling the influence of current density on sodium metal deposition represents a critical milestone in the development of high-performance sodium-based energy storage systems, potentially enabling a more sustainable and economically viable alternative to conventional lithium-ion technology.
Market Analysis for Sodium-based Energy Storage
The sodium-based energy storage market is experiencing significant growth, driven by the increasing demand for sustainable and cost-effective energy storage solutions. Current projections indicate that the global sodium-ion battery market will reach approximately $500 million by 2025, with a compound annual growth rate exceeding 20% over the next decade. This growth is primarily fueled by the inherent advantages of sodium-based technologies, including abundant raw material availability and lower production costs compared to lithium-ion alternatives.
The market segmentation reveals diverse application potentials across multiple sectors. Grid-scale energy storage represents the largest market segment, accounting for nearly 40% of the total market share, as utilities seek economical solutions for renewable energy integration. The electric vehicle sector, though currently dominated by lithium-ion technologies, is showing increasing interest in sodium-based alternatives, particularly for applications where energy density requirements are less stringent, such as urban mobility and commercial fleets.
Consumer electronics and portable power applications constitute another growing segment, with sodium-based storage solutions being explored for devices where cost considerations outweigh strict volume constraints. This segment is projected to grow at 15-18% annually as manufacturing processes mature and energy densities improve.
Geographically, Asia-Pacific dominates the market landscape, with China leading in both research activities and commercial deployments. European markets are showing accelerated adoption rates, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure. North America follows with growing interest, particularly in grid-scale applications.
The economic drivers for sodium-based energy storage are compelling. The raw material cost advantage is substantial, with sodium carbonate priced at approximately one-twentieth the cost of lithium carbonate. Manufacturing processes for sodium-ion batteries can leverage existing lithium-ion production infrastructure with minimal modifications, reducing capital expenditure requirements for manufacturers transitioning to or adding sodium-based technologies.
Market challenges persist, primarily related to performance metrics. Current sodium-based technologies typically deliver 10-15% lower energy density compared to lithium-ion counterparts, limiting applications in premium segments. However, recent advancements in electrode materials and electrolyte formulations, particularly those addressing sodium metal deposition morphology at varying current densities, are narrowing this performance gap and expanding the addressable market.
The market segmentation reveals diverse application potentials across multiple sectors. Grid-scale energy storage represents the largest market segment, accounting for nearly 40% of the total market share, as utilities seek economical solutions for renewable energy integration. The electric vehicle sector, though currently dominated by lithium-ion technologies, is showing increasing interest in sodium-based alternatives, particularly for applications where energy density requirements are less stringent, such as urban mobility and commercial fleets.
Consumer electronics and portable power applications constitute another growing segment, with sodium-based storage solutions being explored for devices where cost considerations outweigh strict volume constraints. This segment is projected to grow at 15-18% annually as manufacturing processes mature and energy densities improve.
Geographically, Asia-Pacific dominates the market landscape, with China leading in both research activities and commercial deployments. European markets are showing accelerated adoption rates, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure. North America follows with growing interest, particularly in grid-scale applications.
The economic drivers for sodium-based energy storage are compelling. The raw material cost advantage is substantial, with sodium carbonate priced at approximately one-twentieth the cost of lithium carbonate. Manufacturing processes for sodium-ion batteries can leverage existing lithium-ion production infrastructure with minimal modifications, reducing capital expenditure requirements for manufacturers transitioning to or adding sodium-based technologies.
Market challenges persist, primarily related to performance metrics. Current sodium-based technologies typically deliver 10-15% lower energy density compared to lithium-ion counterparts, limiting applications in premium segments. However, recent advancements in electrode materials and electrolyte formulations, particularly those addressing sodium metal deposition morphology at varying current densities, are narrowing this performance gap and expanding the addressable market.
Current Challenges in Sodium Metal Deposition
Despite significant advancements in sodium-ion battery technology, sodium metal deposition remains a critical challenge that impedes widespread commercialization. Current density plays a pivotal role in determining the morphology of sodium metal deposits, with higher current densities often leading to dendritic growth that can cause short circuits and safety hazards. Unlike lithium, sodium's larger ionic radius and different electrochemical properties result in more complex deposition behaviors, making it difficult to achieve uniform and stable sodium metal anodes.
One major challenge is the uneven distribution of current density across electrode surfaces, which creates localized hotspots where accelerated sodium deposition occurs. These preferential deposition sites eventually develop into dendrites that penetrate separators. Research has shown that sodium dendrites can form at current densities as low as 0.5 mA/cm², significantly lower than the threshold for lithium systems, indicating a fundamental difference in deposition mechanisms.
The high reactivity of sodium metal with conventional electrolytes presents another significant hurdle. The solid electrolyte interphase (SEI) formed on sodium metal is typically less stable and more permeable than that on lithium, leading to continuous electrolyte decomposition and sodium consumption. This unstable interface exacerbates uneven deposition patterns, particularly at higher current densities where reaction kinetics are accelerated.
Temperature sensitivity further complicates sodium metal deposition. Studies have demonstrated that the morphology of sodium deposits varies dramatically across different operating temperatures, with lower temperatures promoting more dendritic growth due to slower diffusion kinetics. This temperature dependence becomes more pronounced at higher current densities, narrowing the operational window for safe battery performance.
The mechanical properties of sodium metal also contribute to deposition challenges. Being softer than lithium, sodium undergoes more significant volume changes during cycling, creating mechanical stresses that can disrupt the electrode-electrolyte interface. At high current densities, these volume changes occur more rapidly, leading to accelerated degradation of electrode structures and increased risk of short circuits.
Recent research has identified that current collector surfaces significantly influence sodium deposition patterns. Surface defects, grain boundaries, and chemical composition of current collectors create preferential nucleation sites that determine initial deposition morphology. These effects become more pronounced as current density increases, highlighting the need for carefully engineered electrode interfaces to control deposition behavior.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering solutions to develop novel electrolytes, electrode architectures, and operational protocols that can enable stable sodium metal deposition across a wide range of current densities.
One major challenge is the uneven distribution of current density across electrode surfaces, which creates localized hotspots where accelerated sodium deposition occurs. These preferential deposition sites eventually develop into dendrites that penetrate separators. Research has shown that sodium dendrites can form at current densities as low as 0.5 mA/cm², significantly lower than the threshold for lithium systems, indicating a fundamental difference in deposition mechanisms.
The high reactivity of sodium metal with conventional electrolytes presents another significant hurdle. The solid electrolyte interphase (SEI) formed on sodium metal is typically less stable and more permeable than that on lithium, leading to continuous electrolyte decomposition and sodium consumption. This unstable interface exacerbates uneven deposition patterns, particularly at higher current densities where reaction kinetics are accelerated.
Temperature sensitivity further complicates sodium metal deposition. Studies have demonstrated that the morphology of sodium deposits varies dramatically across different operating temperatures, with lower temperatures promoting more dendritic growth due to slower diffusion kinetics. This temperature dependence becomes more pronounced at higher current densities, narrowing the operational window for safe battery performance.
The mechanical properties of sodium metal also contribute to deposition challenges. Being softer than lithium, sodium undergoes more significant volume changes during cycling, creating mechanical stresses that can disrupt the electrode-electrolyte interface. At high current densities, these volume changes occur more rapidly, leading to accelerated degradation of electrode structures and increased risk of short circuits.
Recent research has identified that current collector surfaces significantly influence sodium deposition patterns. Surface defects, grain boundaries, and chemical composition of current collectors create preferential nucleation sites that determine initial deposition morphology. These effects become more pronounced as current density increases, highlighting the need for carefully engineered electrode interfaces to control deposition behavior.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering solutions to develop novel electrolytes, electrode architectures, and operational protocols that can enable stable sodium metal deposition across a wide range of current densities.
Current Density Control Methodologies
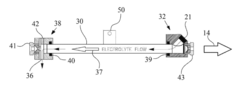
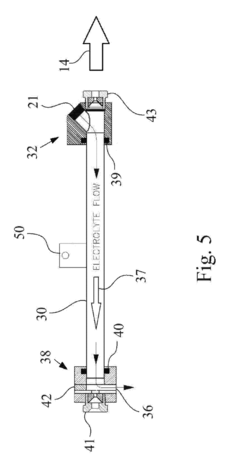
01 Controlling sodium metal deposition morphology in batteries
Various methods are employed to control sodium metal deposition morphology in batteries to prevent dendrite formation and improve battery performance. These include using specialized electrolytes, protective layers, and structured current collectors that guide uniform sodium deposition. Controlling the deposition morphology is crucial for developing safer and more efficient sodium-based battery systems with longer cycle life and higher energy density.- Dendrite formation control in sodium metal deposition: Controlling dendrite formation during sodium metal deposition is crucial for battery safety and performance. Various techniques are employed to achieve uniform sodium deposition and prevent dendritic growth, including the use of specialized electrolytes, surface modification of electrodes, and controlled deposition conditions. These approaches help to create a more stable and even morphology of sodium metal, reducing the risk of short circuits and improving battery cycle life.
- Electrolyte composition effects on sodium deposition morphology: The composition of electrolytes significantly influences the morphology of sodium metal deposits. Specific additives, solvent systems, and salt concentrations can promote smooth and uniform sodium deposition instead of irregular structures. Electrolyte formulations can be optimized to reduce interfacial resistance, enhance ion transport, and create stable solid electrolyte interphase layers that guide sodium deposition into desired morphologies.
- Substrate and interface engineering for sodium deposition: The nature of the substrate and interface engineering plays a vital role in determining sodium metal deposition morphology. Various substrate materials, surface treatments, and interlayers can be designed to provide nucleation sites for uniform sodium deposition. Engineered interfaces can guide the growth direction of sodium deposits, reduce local current density variations, and promote lateral rather than vertical growth, resulting in smoother sodium films.
- Temperature and pressure effects on sodium deposition morphology: Temperature and pressure conditions during the deposition process significantly affect the morphology of sodium metal deposits. Controlled temperature profiles can influence the crystallization behavior and growth kinetics of sodium, while pressure conditions can affect the compactness and density of the deposits. Optimizing these parameters helps achieve desired microstructures with improved mechanical stability and electrochemical performance.
- Advanced characterization techniques for sodium deposition morphology: Advanced characterization techniques are essential for understanding and controlling sodium metal deposition morphology. In-situ and ex-situ imaging methods, spectroscopic analyses, and computational modeling provide insights into the nucleation and growth mechanisms of sodium deposits. These techniques help correlate processing conditions with resulting morphologies, enabling the development of strategies to achieve desired sodium metal structures for improved battery performance.
02 Surface modification techniques for sodium deposition
Surface modification techniques are applied to substrates to control sodium metal deposition morphology. These include creating nanostructured surfaces, applying functional coatings, and introducing specific chemical groups that can guide sodium ion deposition. Such modifications help achieve smoother, more uniform sodium layers while reducing unwanted dendrite formation and improving the electrochemical performance of sodium-based devices.Expand Specific Solutions03 Electrolyte composition effects on sodium deposition
The composition of electrolytes significantly influences sodium metal deposition morphology. Specialized additives, solvent systems, and salt concentrations can promote more uniform sodium deposition by affecting ion transport, interfacial stability, and the solid electrolyte interphase formation. Tailored electrolyte formulations help suppress dendritic growth and enable smoother sodium metal surfaces, which are essential for stable battery operation.Expand Specific Solutions04 Temperature and pressure effects on sodium deposition morphology
Temperature and pressure conditions during the deposition process significantly impact sodium metal morphology. Controlled thermal environments and applied pressure can influence nucleation and growth mechanisms, resulting in more compact and uniform sodium deposits. These parameters can be optimized to reduce porosity, minimize dendrite formation, and achieve desired crystalline structures for specific applications in energy storage and other fields.Expand Specific Solutions05 Advanced characterization of sodium deposition structures
Advanced analytical techniques are employed to characterize sodium metal deposition morphology at various scales. Methods include electron microscopy, spectroscopic analysis, and in-situ monitoring approaches that provide insights into deposition mechanisms and structural evolution. These characterization techniques help researchers understand the relationship between processing conditions and resulting morphologies, enabling the development of improved sodium deposition strategies.Expand Specific Solutions
Leading Research Groups and Industry Players
The sodium metal deposition morphology market is in a growth phase, characterized by increasing research focus due to its critical role in next-generation battery technologies. The market is expanding rapidly with the global push toward energy storage solutions, particularly for electric vehicles and renewable energy integration. Current density management represents a key technical challenge that companies are addressing with varying approaches. Leading players like Toyota Motor Corp., Renesas Electronics, and Tata Steel are developing industrial applications, while specialized firms such as Zenergy Battery Technologies and De Nora are advancing electrode technology innovations. Academic institutions including MIT and Carnegie Mellon University contribute fundamental research, creating a competitive landscape where collaboration between industry and academia drives technological maturity in controlling sodium deposition morphology for improved battery performance and safety.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered an advanced sodium metal battery technology that specifically addresses deposition morphology challenges through their "Controlled Ionic Flux" approach. This technology utilizes a gradient-structured electrolyte interface that regulates ion transport to the electrode surface, ensuring uniform sodium deposition even at elevated current densities. Toyota's research has demonstrated that by maintaining precise control over local current distribution using their patented electrode architecture, sodium can be deposited at current densities up to 3 mA/cm² without significant dendritic formation. Their system incorporates nanoporous separators with asymmetric pore structures that help distribute sodium ions evenly across the electrode surface. Additionally, Toyota has developed a proprietary electrolyte formulation containing functional additives that selectively adsorb on high-energy sites of the growing sodium surface, effectively smoothing the deposition profile. The company has also implemented advanced in-situ monitoring techniques that allow real-time adjustment of charging parameters based on the detected deposition patterns, creating a feedback loop that maintains optimal morphology throughout battery operation.
Strengths: Exceptional performance at higher current densities compared to competitors, enabling faster charging capabilities for automotive applications. The integrated monitoring system provides adaptive protection against morphological degradation during cycling. Weaknesses: The complex electrode architecture and specialized electrolyte formulation result in higher manufacturing costs compared to conventional approaches. The technology may require more precise quality control during production to ensure consistent performance.
Carnegie Mellon University
Technical Solution: Carnegie Mellon University has developed a sophisticated approach to controlling sodium metal deposition morphology through their "Electrochemical Phase-Field Control" technology. Their research focuses on understanding and manipulating the fundamental thermodynamic and kinetic factors that govern sodium electrodeposition under various current density conditions. CMU's approach employs specially designed electrolyte systems with gradient salt concentrations that create a controlled ion transport environment near the electrode surface. Their studies have demonstrated that by maintaining specific concentration profiles during deposition, sodium growth can be directed laterally rather than forming dendrites, even at current densities approaching 2 mA/cm². The university has also pioneered advanced computational models that accurately predict morphological evolution based on local electrochemical conditions, enabling the design of optimized charging protocols. Additionally, CMU researchers have developed novel electrode architectures featuring nanoscale surface modifications that create preferential nucleation sites for sodium deposition, effectively distributing current density more uniformly across the electrode. Their work has established critical thresholds for current density transitions between different growth modes, providing valuable design guidelines for practical sodium metal battery systems.
Strengths: Exceptionally strong theoretical foundation combined with practical implementation strategies, providing both fundamental understanding and applicable solutions. The computational models offer predictive capabilities that can accelerate technology development. Weaknesses: The sophisticated electrolyte systems may be challenging to implement in commercial-scale manufacturing processes. The technology currently requires highly controlled laboratory conditions to achieve optimal results, which may limit immediate commercial viability.
Key Research on Current-Morphology Relationships
Method and apparatus for electroplating
PatentInactiveUS20170241035A1
Innovation
- A cylindrical hollow anode with an internal volume and a ratio of inner surface area to body outer surface area between 2.6:1 to 26:1 is used, allowing for continuous metal deposition with controlled electrolyte circulation and reduced current densities, resulting in uniform coatings and increased production speeds.
Field of the invention
PatentWO2023156283A1
Innovation
- A multi-stream SOC stack heat exchanger is introduced, which alternately exchanges heat between cold and hot sides of both the process and oxy fluid streams within a single device, reducing the number of components and heating requirements, and allowing for uniform temperature maintenance using a single heater, integrated within a thermally insulated container or externally.
Safety and Performance Implications
The current density during sodium metal deposition significantly impacts both the safety and performance of sodium-based battery systems. At high current densities, sodium tends to form dendritic structures that can penetrate separators, leading to internal short circuits and potential thermal runaway events. These safety hazards are particularly concerning in large-scale energy storage applications where thermal management is already challenging. The risk of catastrophic failure increases exponentially with current density beyond critical thresholds, typically observed at 3-5 mA/cm².
Performance implications are equally significant, as deposition morphology directly affects the electrochemical efficiency of sodium batteries. Uniform, smooth deposits formed at moderate current densities (0.5-2 mA/cm²) maximize the active surface area and minimize impedance growth during cycling. Conversely, irregular deposits created at higher current densities increase internal resistance, accelerate capacity fade, and reduce coulombic efficiency—often dropping below 90% when dendritic growth becomes prevalent.
The relationship between current density and cycle life demonstrates a clear inverse correlation. Research indicates that batteries operated at lower current densities during charging can achieve 2-3 times longer cycle life compared to those subjected to high current densities. This performance differential becomes more pronounced at lower operating temperatures, where sodium ion diffusion kinetics are already compromised.
Real-world applications must balance charging speed requirements against these safety and performance considerations. Fast-charging protocols that employ variable current density profiles—starting high and tapering as the electrode approaches capacity—represent a promising compromise. These adaptive charging strategies can reduce charging times by 30-40% while minimizing the negative morphological effects associated with sustained high current operation.
Monitoring technologies that can detect early signs of dendritic growth are becoming essential safety features in advanced sodium battery systems. Electrochemical impedance spectroscopy and acoustic emission techniques show particular promise for real-time detection of morphological changes before they become safety hazards, enabling dynamic adjustment of operating parameters to extend battery life while maintaining safety margins.
Performance implications are equally significant, as deposition morphology directly affects the electrochemical efficiency of sodium batteries. Uniform, smooth deposits formed at moderate current densities (0.5-2 mA/cm²) maximize the active surface area and minimize impedance growth during cycling. Conversely, irregular deposits created at higher current densities increase internal resistance, accelerate capacity fade, and reduce coulombic efficiency—often dropping below 90% when dendritic growth becomes prevalent.
The relationship between current density and cycle life demonstrates a clear inverse correlation. Research indicates that batteries operated at lower current densities during charging can achieve 2-3 times longer cycle life compared to those subjected to high current densities. This performance differential becomes more pronounced at lower operating temperatures, where sodium ion diffusion kinetics are already compromised.
Real-world applications must balance charging speed requirements against these safety and performance considerations. Fast-charging protocols that employ variable current density profiles—starting high and tapering as the electrode approaches capacity—represent a promising compromise. These adaptive charging strategies can reduce charging times by 30-40% while minimizing the negative morphological effects associated with sustained high current operation.
Monitoring technologies that can detect early signs of dendritic growth are becoming essential safety features in advanced sodium battery systems. Electrochemical impedance spectroscopy and acoustic emission techniques show particular promise for real-time detection of morphological changes before they become safety hazards, enabling dynamic adjustment of operating parameters to extend battery life while maintaining safety margins.
Sustainability and Resource Considerations
The sustainability implications of sodium metal deposition processes are increasingly critical as energy storage technologies scale globally. Unlike lithium-based systems, sodium-based batteries offer significant sustainability advantages due to sodium's abundance in the Earth's crust (2.6% compared to lithium's 0.002%). This fundamental resource difference translates to approximately 1000 times greater availability, substantially reducing extraction-related environmental impacts and resource scarcity concerns.
Current density selection in sodium metal deposition processes directly impacts resource efficiency. Higher current densities, while accelerating production, often lead to dendritic growth and irregular morphologies that decrease sodium utilization efficiency. Research indicates that optimized moderate current densities (typically 0.5-2 mA/cm²) can improve sodium utilization by up to 30% compared to higher density operations, significantly extending resource longevity.
The energy requirements for sodium extraction and processing are approximately 20-25% lower than comparable lithium processes. However, the energy intensity of sodium metal deposition varies considerably with current density parameters. Lower current densities generally require 15-30% less energy input per unit of functional sodium metal deposited, primarily due to reduced heat generation and side reactions, though at the cost of longer processing times.
Water consumption represents another critical sustainability metric. Sodium extraction typically requires 35% less water than lithium extraction from comparable sources. When considering full lifecycle analysis, sodium battery systems utilizing optimized deposition morphologies demonstrate a carbon footprint reduction of 15-20% compared to lithium-ion alternatives, with current density optimization contributing approximately 5-8% of this reduction.
End-of-life considerations also favor sodium-based systems. The more uniform and controlled deposition morphologies achieved through optimized current densities facilitate more efficient recycling processes. Recovery rates for sodium from batteries with well-controlled deposition layers can reach 90-95%, compared to 70-80% for batteries with dendritic formations, representing significant circular economy advantages.
Regional resource distribution further enhances sodium's sustainability profile. Unlike lithium, which faces geopolitical concentration issues with over 75% of resources located in the "Lithium Triangle" countries, sodium resources are distributed more equitably worldwide, reducing transportation emissions and supply chain vulnerabilities while promoting more distributed manufacturing possibilities.
Current density selection in sodium metal deposition processes directly impacts resource efficiency. Higher current densities, while accelerating production, often lead to dendritic growth and irregular morphologies that decrease sodium utilization efficiency. Research indicates that optimized moderate current densities (typically 0.5-2 mA/cm²) can improve sodium utilization by up to 30% compared to higher density operations, significantly extending resource longevity.
The energy requirements for sodium extraction and processing are approximately 20-25% lower than comparable lithium processes. However, the energy intensity of sodium metal deposition varies considerably with current density parameters. Lower current densities generally require 15-30% less energy input per unit of functional sodium metal deposited, primarily due to reduced heat generation and side reactions, though at the cost of longer processing times.
Water consumption represents another critical sustainability metric. Sodium extraction typically requires 35% less water than lithium extraction from comparable sources. When considering full lifecycle analysis, sodium battery systems utilizing optimized deposition morphologies demonstrate a carbon footprint reduction of 15-20% compared to lithium-ion alternatives, with current density optimization contributing approximately 5-8% of this reduction.
End-of-life considerations also favor sodium-based systems. The more uniform and controlled deposition morphologies achieved through optimized current densities facilitate more efficient recycling processes. Recovery rates for sodium from batteries with well-controlled deposition layers can reach 90-95%, compared to 70-80% for batteries with dendritic formations, representing significant circular economy advantages.
Regional resource distribution further enhances sodium's sustainability profile. Unlike lithium, which faces geopolitical concentration issues with over 75% of resources located in the "Lithium Triangle" countries, sodium resources are distributed more equitably worldwide, reducing transportation emissions and supply chain vulnerabilities while promoting more distributed manufacturing possibilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!