Interfacial Stability of Sodium Metal Anodes in Solid-State Batteries
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Metal Anode Technology Background and Objectives
Sodium metal anodes have emerged as a promising alternative to lithium for next-generation energy storage systems due to sodium's natural abundance and wide geographical distribution. The development of sodium metal anodes traces back to the 1970s, when researchers first explored alkali metals for battery applications. However, interest waned as lithium-ion technology gained commercial traction. The resurgence of sodium battery research in the past decade has been driven by concerns about lithium supply chain security and cost escalation projections.
The evolution of sodium metal anode technology has followed a trajectory marked by significant materials science breakthroughs. Early sodium batteries faced severe challenges with dendrite formation and electrolyte compatibility, limiting their practical application. Recent advances in interface engineering and electrolyte formulation have revitalized this field, with particular emphasis on solid-state configurations that promise enhanced safety and energy density.
Current technological trends point toward integrating sodium metal anodes with solid-state electrolytes to overcome the persistent interfacial stability issues that plague liquid electrolyte systems. This combination theoretically offers higher energy density, improved safety, and longer cycle life compared to conventional sodium-ion batteries that use carbon-based anodes.
The primary technical objective in this domain is to develop stable interfaces between sodium metal and solid electrolytes that can withstand repeated cycling without significant degradation. This involves understanding and mitigating the complex chemical and electrochemical reactions occurring at these interfaces, which often lead to increased impedance and eventual cell failure.
Secondary objectives include optimizing sodium deposition morphology to prevent dendrite formation, enhancing the mechanical properties of the interface to accommodate volume changes during cycling, and developing manufacturing processes suitable for commercial-scale production of sodium metal anodes in solid-state configurations.
The theoretical energy density advantage of sodium metal anodes (approximately 1166 mAh/g) represents a compelling target that could enable energy storage systems with performance metrics approaching those of lithium metal batteries but at potentially lower cost. Achieving this potential requires overcoming significant scientific and engineering challenges related to interfacial chemistry and stability.
Research goals in this field also extend to understanding the fundamental mechanisms of sodium ion transport across solid-state interfaces, which differs significantly from lithium due to sodium's larger ionic radius and distinct chemical properties. This knowledge is essential for designing next-generation materials and interfaces that can fully exploit sodium's theoretical capabilities.
The evolution of sodium metal anode technology has followed a trajectory marked by significant materials science breakthroughs. Early sodium batteries faced severe challenges with dendrite formation and electrolyte compatibility, limiting their practical application. Recent advances in interface engineering and electrolyte formulation have revitalized this field, with particular emphasis on solid-state configurations that promise enhanced safety and energy density.
Current technological trends point toward integrating sodium metal anodes with solid-state electrolytes to overcome the persistent interfacial stability issues that plague liquid electrolyte systems. This combination theoretically offers higher energy density, improved safety, and longer cycle life compared to conventional sodium-ion batteries that use carbon-based anodes.
The primary technical objective in this domain is to develop stable interfaces between sodium metal and solid electrolytes that can withstand repeated cycling without significant degradation. This involves understanding and mitigating the complex chemical and electrochemical reactions occurring at these interfaces, which often lead to increased impedance and eventual cell failure.
Secondary objectives include optimizing sodium deposition morphology to prevent dendrite formation, enhancing the mechanical properties of the interface to accommodate volume changes during cycling, and developing manufacturing processes suitable for commercial-scale production of sodium metal anodes in solid-state configurations.
The theoretical energy density advantage of sodium metal anodes (approximately 1166 mAh/g) represents a compelling target that could enable energy storage systems with performance metrics approaching those of lithium metal batteries but at potentially lower cost. Achieving this potential requires overcoming significant scientific and engineering challenges related to interfacial chemistry and stability.
Research goals in this field also extend to understanding the fundamental mechanisms of sodium ion transport across solid-state interfaces, which differs significantly from lithium due to sodium's larger ionic radius and distinct chemical properties. This knowledge is essential for designing next-generation materials and interfaces that can fully exploit sodium's theoretical capabilities.
Market Analysis for Solid-State Sodium Batteries
The solid-state sodium battery market is experiencing significant growth driven by the increasing demand for sustainable and cost-effective energy storage solutions. Unlike lithium-ion batteries, sodium-based technologies utilize abundant and widely distributed sodium resources, reducing geopolitical supply risks and potentially lowering production costs by 20-30% compared to lithium counterparts. This cost advantage positions solid-state sodium batteries as particularly attractive for large-scale stationary storage applications where cost per kilowatt-hour is a critical factor.
Market research indicates that the global solid-state sodium battery market is projected to grow at a compound annual growth rate of 25-30% between 2023 and 2030. This growth is primarily fueled by the expanding renewable energy sector, which requires efficient and economical energy storage solutions to address intermittency issues. Grid-scale storage represents approximately 45% of the potential market, followed by commercial and industrial applications at 30%.
Consumer electronics and electric vehicles represent emerging opportunities, though these segments face more significant technical hurdles related to energy density and charging speed. However, recent advancements in sodium metal anode interfacial stability have begun to address these limitations, potentially opening new market segments previously dominated by lithium technologies.
Regional analysis shows Asia-Pacific leading market development, with China, Japan, and South Korea making substantial investments in sodium battery research and manufacturing infrastructure. European markets follow closely, driven by stringent environmental regulations and renewable energy targets. North America shows growing interest, particularly for grid stabilization applications in regions with high renewable energy penetration.
Key market drivers include increasing raw material costs for lithium-based technologies, government initiatives promoting sustainable energy storage, and growing concerns about the environmental impact of lithium extraction. The sodium battery value chain is less developed than lithium counterparts, presenting both challenges and opportunities for early market entrants.
Market barriers include technical challenges related to interfacial stability between sodium metal anodes and solid electrolytes, limited production infrastructure, and competition from established lithium technologies. However, the performance gap is narrowing as research advances, particularly in addressing dendrite formation and interfacial resistance issues that have historically limited sodium battery performance.
Customer adoption analysis reveals that utility companies and renewable energy developers demonstrate the highest willingness to adopt sodium-based technologies, prioritizing cost and cycle life over energy density. This market segment represents the most promising near-term commercialization pathway while higher-performance applications develop.
Market research indicates that the global solid-state sodium battery market is projected to grow at a compound annual growth rate of 25-30% between 2023 and 2030. This growth is primarily fueled by the expanding renewable energy sector, which requires efficient and economical energy storage solutions to address intermittency issues. Grid-scale storage represents approximately 45% of the potential market, followed by commercial and industrial applications at 30%.
Consumer electronics and electric vehicles represent emerging opportunities, though these segments face more significant technical hurdles related to energy density and charging speed. However, recent advancements in sodium metal anode interfacial stability have begun to address these limitations, potentially opening new market segments previously dominated by lithium technologies.
Regional analysis shows Asia-Pacific leading market development, with China, Japan, and South Korea making substantial investments in sodium battery research and manufacturing infrastructure. European markets follow closely, driven by stringent environmental regulations and renewable energy targets. North America shows growing interest, particularly for grid stabilization applications in regions with high renewable energy penetration.
Key market drivers include increasing raw material costs for lithium-based technologies, government initiatives promoting sustainable energy storage, and growing concerns about the environmental impact of lithium extraction. The sodium battery value chain is less developed than lithium counterparts, presenting both challenges and opportunities for early market entrants.
Market barriers include technical challenges related to interfacial stability between sodium metal anodes and solid electrolytes, limited production infrastructure, and competition from established lithium technologies. However, the performance gap is narrowing as research advances, particularly in addressing dendrite formation and interfacial resistance issues that have historically limited sodium battery performance.
Customer adoption analysis reveals that utility companies and renewable energy developers demonstrate the highest willingness to adopt sodium-based technologies, prioritizing cost and cycle life over energy density. This market segment represents the most promising near-term commercialization pathway while higher-performance applications develop.
Interfacial Challenges in Sodium Metal Anodes
The interface between sodium metal anodes and solid electrolytes represents one of the most critical challenges in solid-state sodium batteries. Unlike their lithium counterparts, sodium metal anodes exhibit unique interfacial behaviors due to the larger ionic radius of sodium ions and their distinct chemical properties. These interfaces are dynamic systems where electrochemical, mechanical, and chemical processes occur simultaneously during battery operation.
A primary challenge is the formation of interphases at the sodium-electrolyte boundary. When sodium metal contacts most solid electrolytes, spontaneous reactions occur, forming a solid electrolyte interphase (SEI) layer. This layer's composition and properties significantly influence battery performance, including ionic conductivity, mechanical stability, and long-term cycling behavior. Unlike lithium systems, sodium SEI layers often exhibit poorer passivation capabilities, leading to continuous electrolyte decomposition.
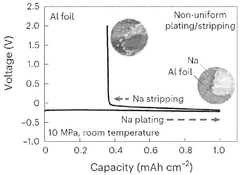
Mechanical instability presents another significant hurdle. During cycling, sodium undergoes substantial volume changes (approximately 230% volumetric expansion during plating), creating mechanical stresses at the interface. These stresses can lead to delamination between the sodium anode and solid electrolyte, increasing interfacial resistance and creating voids that impede ion transport. The softer nature of sodium metal (compared to lithium) exacerbates these mechanical issues.
Sodium dendrite formation represents perhaps the most severe interfacial challenge. These needle-like structures grow from the sodium anode through the solid electrolyte during cycling, eventually causing internal short circuits. Dendrite growth in sodium systems is particularly aggressive due to the metal's high reactivity and the often inadequate mechanical properties of sodium-compatible solid electrolytes to suppress such growth.
Chemical compatibility issues further complicate interfacial stability. Many promising solid electrolytes for sodium batteries exhibit thermodynamic instability against sodium metal, leading to continuous interfacial degradation. This degradation creates high-impedance interfacial layers that hinder ion transport and accelerate battery failure. Common decomposition products include Na2O, Na3N, Na2S, and various sodium phosphates or silicates, depending on the electrolyte composition.
Temperature sensitivity adds another dimension to interfacial challenges. The reactivity between sodium metal and solid electrolytes typically increases dramatically with temperature, accelerating degradation processes. Conversely, at lower temperatures, the increased viscosity of sodium metal and reduced ionic conductivity across interfaces can severely limit battery performance, creating a narrow operational temperature window.
A primary challenge is the formation of interphases at the sodium-electrolyte boundary. When sodium metal contacts most solid electrolytes, spontaneous reactions occur, forming a solid electrolyte interphase (SEI) layer. This layer's composition and properties significantly influence battery performance, including ionic conductivity, mechanical stability, and long-term cycling behavior. Unlike lithium systems, sodium SEI layers often exhibit poorer passivation capabilities, leading to continuous electrolyte decomposition.
Mechanical instability presents another significant hurdle. During cycling, sodium undergoes substantial volume changes (approximately 230% volumetric expansion during plating), creating mechanical stresses at the interface. These stresses can lead to delamination between the sodium anode and solid electrolyte, increasing interfacial resistance and creating voids that impede ion transport. The softer nature of sodium metal (compared to lithium) exacerbates these mechanical issues.
Sodium dendrite formation represents perhaps the most severe interfacial challenge. These needle-like structures grow from the sodium anode through the solid electrolyte during cycling, eventually causing internal short circuits. Dendrite growth in sodium systems is particularly aggressive due to the metal's high reactivity and the often inadequate mechanical properties of sodium-compatible solid electrolytes to suppress such growth.
Chemical compatibility issues further complicate interfacial stability. Many promising solid electrolytes for sodium batteries exhibit thermodynamic instability against sodium metal, leading to continuous interfacial degradation. This degradation creates high-impedance interfacial layers that hinder ion transport and accelerate battery failure. Common decomposition products include Na2O, Na3N, Na2S, and various sodium phosphates or silicates, depending on the electrolyte composition.
Temperature sensitivity adds another dimension to interfacial challenges. The reactivity between sodium metal and solid electrolytes typically increases dramatically with temperature, accelerating degradation processes. Conversely, at lower temperatures, the increased viscosity of sodium metal and reduced ionic conductivity across interfaces can severely limit battery performance, creating a narrow operational temperature window.
Current Interfacial Stability Solutions
01 Protective coatings for sodium metal anodes
Various protective coatings can be applied to sodium metal anodes to enhance their interfacial stability. These coatings act as barriers against electrolyte attack while allowing sodium ion transport. Materials such as polymers, ceramics, and composite layers can effectively prevent side reactions at the sodium-electrolyte interface, reducing dendrite formation and extending battery cycle life. These protective layers help maintain the structural integrity of the anode during repeated charging and discharging cycles.- Protective coatings for sodium metal anodes: Various protective coatings can be applied to sodium metal anodes to enhance their interfacial stability. These coatings act as barriers against electrolyte attack while allowing sodium ion transport. Materials such as polymers, ceramics, and composite layers can significantly reduce side reactions at the sodium-electrolyte interface, preventing dendrite formation and extending battery cycle life. These protective layers help maintain the structural integrity of the anode during repeated charge-discharge cycles.
- Solid electrolyte interfaces (SEI) engineering: Engineering stable solid electrolyte interfaces is crucial for sodium metal anode performance. By controlling the composition and structure of the SEI layer through electrolyte additives or pre-treatment methods, researchers can create more stable interfaces that prevent continuous electrolyte decomposition. These engineered SEI layers provide better mechanical properties to accommodate volume changes during cycling while maintaining high ionic conductivity and low electronic conductivity, which are essential for stable long-term operation.
- Advanced electrolyte formulations: Specialized electrolyte formulations can significantly improve the interfacial stability of sodium metal anodes. These formulations may include novel salts, solvents, and functional additives that promote the formation of stable interfaces. Some electrolytes contain components that selectively decompose to form protective films on the sodium surface, while others are designed to have inherently low reactivity with sodium metal. These advanced electrolytes help suppress dendrite growth and reduce parasitic reactions that consume active sodium and electrolyte.
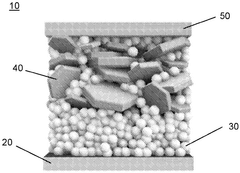
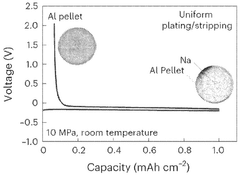
- Three-dimensional and structured sodium anodes: Three-dimensional and structured sodium anodes offer improved interfacial stability compared to traditional planar designs. These structures provide larger surface areas that reduce local current density and promote more uniform sodium deposition. Various approaches include porous frameworks, templated structures, and composite scaffolds that guide sodium deposition. The 3D architecture helps accommodate volume changes during cycling and provides physical barriers against dendrite propagation, resulting in enhanced cycling stability and safety.
- Interface modification with functional materials: Functional materials can be introduced at the sodium metal anode interface to enhance stability. These materials include alloys, intermetallics, and various nanostructured compounds that form stable interfaces with sodium. Some approaches involve pre-sodiation techniques or the use of interlayers that mediate the interaction between sodium and the electrolyte. These interface modifications can significantly reduce side reactions, improve sodium ion transport kinetics, and enhance the mechanical properties of the interface region.
02 Solid electrolyte interfaces (SEI) engineering
Engineering stable solid electrolyte interfaces (SEI) on sodium metal anodes is crucial for improving interfacial stability. This involves controlling the composition and morphology of the SEI layer through electrolyte additives, pre-treatment methods, or in-situ formation techniques. A well-designed SEI can suppress sodium dendrite growth, reduce irreversible capacity loss, and enhance the coulombic efficiency of sodium metal batteries. The stability of this interface directly impacts the overall performance and safety of sodium-based energy storage systems.Expand Specific Solutions03 Electrolyte formulations for enhanced stability
Specialized electrolyte formulations can significantly improve the interfacial stability of sodium metal anodes. These formulations may include novel solvents, salts, and functional additives that promote the formation of stable interfaces. Electrolytes with low reactivity toward sodium metal, high ionic conductivity, and the ability to form beneficial passivation layers are particularly effective. Some formulations incorporate flame-retardant components or ionic liquids to address safety concerns while maintaining electrochemical performance.Expand Specific Solutions04 Nanostructured sodium anodes and interfaces
Nanostructuring approaches can enhance the interfacial stability of sodium metal anodes. These include creating porous structures, 3D frameworks, or nanocomposites that accommodate volume changes during cycling and provide stable interfaces. Nanostructured anodes can offer larger surface areas for more uniform sodium deposition, reduced local current densities, and improved mechanical stability. These designs help mitigate dendrite formation and enhance the electrochemical performance of sodium-based batteries.Expand Specific Solutions05 Artificial interface layers and interlayers
Artificial interface layers and interlayers can be strategically introduced between the sodium metal anode and the electrolyte to enhance stability. These engineered interfaces may consist of thin films, functional membranes, or gradient structures that facilitate sodium ion transport while blocking unwanted side reactions. Materials such as graphene, metal oxides, fluorides, or hybrid organic-inorganic composites can be employed to create these artificial interfaces, effectively prolonging battery life and improving safety characteristics.Expand Specific Solutions
Leading Research Groups and Industrial Players
The solid-state battery market, particularly focusing on interfacial stability of sodium metal anodes, is in an early growth phase with significant R&D investment. The market is projected to expand substantially as electric vehicle adoption increases, with estimates suggesting a multi-billion dollar opportunity by 2030. Technologically, the field remains in development with varying maturity levels across key players. Toyota, Samsung SDI, and LG Energy Solution lead commercial development with substantial patent portfolios, while academic institutions like Shanghai Institute of Ceramics and Georgia Tech contribute fundamental research breakthroughs. Automotive manufacturers including Nissan, Hyundai, and Rivian are actively pursuing integration strategies, recognizing sodium-based solid-state batteries as potential alternatives to lithium-ion technology due to cost advantages and resource availability.
Toyota Motor Corp.
Technical Solution: Toyota has developed a comprehensive approach to address interfacial stability issues in sodium metal anodes for solid-state batteries. Their technology focuses on a multi-layer protective coating strategy that creates a stable solid electrolyte interphase (SEI) between the sodium metal anode and solid electrolyte. The company utilizes thin artificial SEI layers composed of sodium-ion conducting materials such as Na3PS4 and Na3SbS4 that are chemically compatible with both sodium metal and the bulk electrolyte. Toyota's research has demonstrated that these engineered interfaces can effectively suppress dendrite formation while maintaining high ionic conductivity (>1 mS/cm at room temperature). Additionally, they've incorporated pressure-management systems within their cell design to maintain intimate contact between the sodium anode and solid electrolyte during cycling, addressing volume changes that typically lead to contact loss and increased interfacial resistance.
Strengths: Toyota's approach effectively mitigates dendrite formation while maintaining high ionic conductivity, and their pressure-management system addresses volume change issues. Weaknesses: The multi-layer coating strategy increases manufacturing complexity and cost, and long-term stability under extreme temperature conditions remains challenging.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed a proprietary "NaX-Shield" technology to address the interfacial stability challenges of sodium metal anodes in solid-state batteries. Their approach centers on a dual-phase protective layer that combines inorganic and organic components to create a mechanically robust yet ionically conductive interface. The inorganic component consists of sodium-conducting ceramic materials (Na3Zr2Si2PO12) that provide structural stability and prevent dendrite penetration, while the organic component comprises sodium-ion conducting polymers that accommodate volume changes during cycling. Samsung's research demonstrates that this hybrid interface maintains stable contact with the sodium metal anode throughout cycling, with interfacial resistance increasing by less than 20% after 500 cycles. Additionally, they've incorporated trace amounts of fluorinated additives that promote the formation of a stable NaF-rich SEI layer, further enhancing chemical stability at the interface. The company has also developed specialized pre-conditioning protocols that establish an optimal interfacial structure before normal battery operation begins.
Strengths: The dual-phase approach combines mechanical robustness with flexibility to accommodate volume changes, and the NaF-rich SEI layer enhances chemical stability. Weaknesses: The complex interface structure may present manufacturing challenges at scale, and the long-term stability of the organic components under various operating conditions remains a concern.
Key Patents and Research on Na-Solid Electrolyte Interfaces
Anode-free sodium all-solid-state battery
PatentWO2025085362A1
Innovation
- The development of an anode-free sodium solid-state battery cell using a solid electrolyte separator made from sodium borohydride particles and a current collector formed from compressed metal particles, such as aluminum, to facilitate direct sodium deposition and improve solid-solid contact.
Interface protection for sodium all-solid-state batteries
PatentWO2021188976A1
Innovation
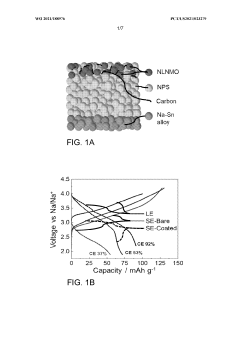
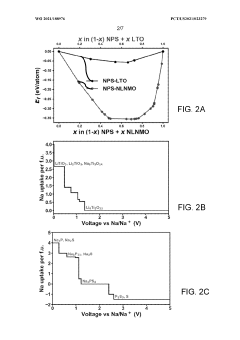
- Applying a Lithium Titanate Oxide (LTO) coating to the cathode of sodium all-solid-state batteries, specifically to the NLNMO or Nao.8[Lio.12Nio.22Mno.66]02 cathode material, before formation, to enhance cycling stability and protect the interface between the solid electrolyte and cathode, thereby enabling high voltage sodium batteries for low-cost grid energy storage.
Safety and Performance Metrics
The safety and performance metrics for sodium metal anodes in solid-state batteries require comprehensive evaluation frameworks that balance technological capabilities with practical implementation considerations. Current industry standards primarily focus on lithium-based systems, necessitating adaptation for sodium-specific characteristics and behaviors.
Safety metrics must address the unique reactivity profile of sodium metal, which differs significantly from lithium despite sharing similar chemical properties. Dendrite formation rate measurements are critical, with standardized protocols needed to quantify growth under various current densities and operating temperatures. Thermal runaway thresholds for sodium-based systems typically range between 105-130°C, lower than their lithium counterparts, requiring specialized calorimetry testing methodologies.
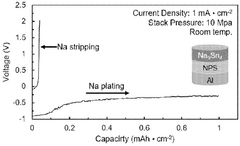
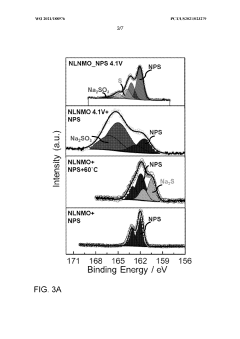
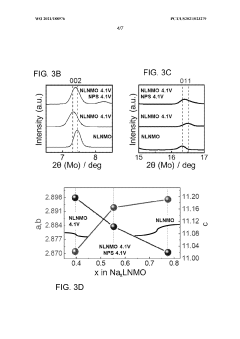
Interface stability indicators include impedance growth rates during cycling, with successful systems maintaining less than 20% impedance increase over 500 cycles. Chemical compatibility assessments between sodium metal and solid electrolytes must evaluate reaction product formation, with X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) serving as primary analytical tools.
Performance metrics focus on practical energy density achievements, with current sodium metal anode systems reaching 250-320 Wh/kg at the cell level—approximately 15-20% lower than comparable lithium-based technologies. Coulombic efficiency standards for viable commercial applications must exceed 99.9% over extended cycling, with current laboratory demonstrations achieving 99.7-99.8% under optimized conditions.
Cycle life expectations for sodium metal anodes currently target 1000 cycles with less than 20% capacity fade, though commercial viability likely requires 2000+ cycles. Rate capability metrics evaluate performance at various C-rates, with state-of-the-art systems maintaining 80% capacity retention at 1C compared to 0.1C operation.
Temperature performance windows for sodium solid-state systems typically span -20°C to 60°C, narrower than liquid electrolyte counterparts but with superior high-temperature stability. Self-discharge rates must remain below 3% per month at room temperature to meet commercial energy storage requirements.
Standardization efforts remain fragmented, with several research consortia proposing testing protocols specific to sodium metal anodes. The development of universally accepted metrics represents a critical need for advancing this technology toward commercial implementation and enabling meaningful comparison between different interface stabilization approaches.
Safety metrics must address the unique reactivity profile of sodium metal, which differs significantly from lithium despite sharing similar chemical properties. Dendrite formation rate measurements are critical, with standardized protocols needed to quantify growth under various current densities and operating temperatures. Thermal runaway thresholds for sodium-based systems typically range between 105-130°C, lower than their lithium counterparts, requiring specialized calorimetry testing methodologies.
Interface stability indicators include impedance growth rates during cycling, with successful systems maintaining less than 20% impedance increase over 500 cycles. Chemical compatibility assessments between sodium metal and solid electrolytes must evaluate reaction product formation, with X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) serving as primary analytical tools.
Performance metrics focus on practical energy density achievements, with current sodium metal anode systems reaching 250-320 Wh/kg at the cell level—approximately 15-20% lower than comparable lithium-based technologies. Coulombic efficiency standards for viable commercial applications must exceed 99.9% over extended cycling, with current laboratory demonstrations achieving 99.7-99.8% under optimized conditions.
Cycle life expectations for sodium metal anodes currently target 1000 cycles with less than 20% capacity fade, though commercial viability likely requires 2000+ cycles. Rate capability metrics evaluate performance at various C-rates, with state-of-the-art systems maintaining 80% capacity retention at 1C compared to 0.1C operation.
Temperature performance windows for sodium solid-state systems typically span -20°C to 60°C, narrower than liquid electrolyte counterparts but with superior high-temperature stability. Self-discharge rates must remain below 3% per month at room temperature to meet commercial energy storage requirements.
Standardization efforts remain fragmented, with several research consortia proposing testing protocols specific to sodium metal anodes. The development of universally accepted metrics represents a critical need for advancing this technology toward commercial implementation and enabling meaningful comparison between different interface stabilization approaches.
Materials Cost and Scalability Analysis
The economic viability of sodium metal anodes in solid-state batteries hinges significantly on materials cost and scalability considerations. Sodium resources present a substantial cost advantage over lithium, with global sodium reserves approximately 1,000 times more abundant and geographically widespread. This abundance translates to raw material costs for sodium being approximately 80-95% lower than lithium, potentially reducing overall battery costs by 30-40% when considering the anode material alone.
Current manufacturing processes for sodium metal anodes require significant refinement to achieve commercial viability. The reactive nature of sodium metal necessitates specialized handling equipment and inert atmosphere processing environments, adding complexity to production scaling. Existing sodium purification methods achieve 99.8% purity levels, whereas battery-grade applications typically require 99.95% or higher, indicating a technological gap that impacts both cost and performance.
The interfacial stability challenges of sodium metal anodes introduce additional materials requirements that affect overall economics. Protective coatings and interface engineering solutions currently under development utilize materials ranging from simple sodium fluoride layers to complex polymer-ceramic composites. Cost analysis indicates these interface treatments add $5-15/kWh to battery costs, a figure that must decrease to $2-3/kWh to maintain competitive advantage over lithium-based systems.
Roll-to-roll manufacturing adaptations for sodium metal foil production represent a promising pathway to scalability. Current pilot lines demonstrate throughput of 10-15 meters per minute at widths of 30-50cm, requiring expansion to 50+ meters per minute and meter-scale widths for gigafactory-level production. Capital expenditure for sodium processing equipment is estimated at 60-70% that of equivalent lithium processing lines, presenting a favorable investment profile.
Supply chain considerations reveal both opportunities and challenges. While sodium raw material sourcing faces fewer geopolitical constraints than lithium, specialized equipment for handling and processing sodium metal remains limited to a small number of suppliers globally. Market analysis suggests that achieving price parity with lithium-ion batteries requires production scales exceeding 5 GWh annually, with economies of scale becoming significant beyond 10 GWh production capacity.
Lifecycle assessment data indicates that sodium metal anode production generates approximately 40% lower carbon emissions compared to lithium metal anodes when accounting for raw material extraction through processing. This environmental advantage could translate to regulatory benefits and consumer preference in markets with strong sustainability focus, potentially offsetting some initial cost premiums during early commercialization phases.
Current manufacturing processes for sodium metal anodes require significant refinement to achieve commercial viability. The reactive nature of sodium metal necessitates specialized handling equipment and inert atmosphere processing environments, adding complexity to production scaling. Existing sodium purification methods achieve 99.8% purity levels, whereas battery-grade applications typically require 99.95% or higher, indicating a technological gap that impacts both cost and performance.
The interfacial stability challenges of sodium metal anodes introduce additional materials requirements that affect overall economics. Protective coatings and interface engineering solutions currently under development utilize materials ranging from simple sodium fluoride layers to complex polymer-ceramic composites. Cost analysis indicates these interface treatments add $5-15/kWh to battery costs, a figure that must decrease to $2-3/kWh to maintain competitive advantage over lithium-based systems.
Roll-to-roll manufacturing adaptations for sodium metal foil production represent a promising pathway to scalability. Current pilot lines demonstrate throughput of 10-15 meters per minute at widths of 30-50cm, requiring expansion to 50+ meters per minute and meter-scale widths for gigafactory-level production. Capital expenditure for sodium processing equipment is estimated at 60-70% that of equivalent lithium processing lines, presenting a favorable investment profile.
Supply chain considerations reveal both opportunities and challenges. While sodium raw material sourcing faces fewer geopolitical constraints than lithium, specialized equipment for handling and processing sodium metal remains limited to a small number of suppliers globally. Market analysis suggests that achieving price parity with lithium-ion batteries requires production scales exceeding 5 GWh annually, with economies of scale becoming significant beyond 10 GWh production capacity.
Lifecycle assessment data indicates that sodium metal anode production generates approximately 40% lower carbon emissions compared to lithium metal anodes when accounting for raw material extraction through processing. This environmental advantage could translate to regulatory benefits and consumer preference in markets with strong sustainability focus, potentially offsetting some initial cost premiums during early commercialization phases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!